Abstract
Background
Detailed knowledge of the dimensions and shape of the main arteries of the body and how they change with age and disease is important for understanding arterial pathophysiology and improving minimally invasive devices to treat arterial diseases. Our goal was to describe and compare geometric remodeling of the aorta and peripheral arteries in the context of patient demographics and cardiovascular risk factors.
Methods
3D reconstructions of Computerized Tomography Angiography scans were performed in n=122 subjects 5-93 years old (mean 47±24 years, 64M/58F). Best-fit arterial diameters, lengths and tortuosity for the principle named arteries in the chest, abdomen, pelvis and upper thigh were measured, and multiple linear regression analysis was performed to examine how these morphological parameters associate with patient demographics and risk factors.
Results
Large elastic arteries increased their diameter, length and tortuosity with age, while muscular arteries primarily became more tortuous. Demographics and risk factors explained >70% of the variation in diameters of the abdominal aorta, paravisceral aorta, and the aortic arch; and >75% of variation in tortuosity from the profunda femoris to the brachiocephalic artery. Male gender, larger body mass index and hypertension contributed to larger diameters, while presence of diabetes was associated with somewhat straighter arteries. Overall the effects of cardiovascular risk factors on geometric remodeling were small compared to those of demographics.
Conclusions
The geometry of the vascular tree is significantly affected by aging, demographics and some risk factors. Elastic and muscular arteries remodel differently, possibly due to differences in their microstructure.
Keywords: geometric remodeling, elastic artery, muscular artery, widening, tortuosity, aging
INTRODUCTION
The structure and function of arteries differ based on anatomic location within the arterial tree. Arteries more proximal to the heart demonstrate increased proportions of elastin, while smooth muscle cells comprise the majority of the artery wall in the distal peripheral arteries, such as the femoral artery(1-3). The structural composition of the artery wall influences its mechanical properties, function, and its interaction with surgical and endovascular repairs. Due to improved short-term outcomes of endovascular surgery compared to open surgery(4,5), minimally invasive techniques for aortic and peripheral arterial reconstruction in patients of all ages have become common. As the indications and complexity of endovascular operations have increased, the need for detailed knowledge of the dimensions and shape of the main arteries of the body and how they change with age and disease has become a key consideration in the development of minimally invasive devices and techniques to treat each of the arterial territories. Currently, most endovascular devices have been designed to treat vascular disease in the elderly, but frequently these devices are also used to treat traumatic injuries in young, otherwise healthy patients(6-9). Translation of these devices and methods to new patient populations raises concerns regarding the long-term integrity of endovascular repairs in these groups, as young arteries grow and remodel over time.
Though it is generally known that the aorta and large elastic arteries become wider, longer and more tortuous with age(10-14), systematic and comprehensive quantitative studies of geometric remodeling are rare. Existing data focus primarily on two aortic segments – the thoracic aorta and the abdominal aorta, and these studies rarely investigate the contributions of patient demographics and risk factors to the remodeling process. Correlating arterial geometric changes in different arterial beds with clinical risk factors and demographics could help produce better models for patient specific predictive imaging and also yield important insights on mechanisms underlying arterial remodeling in healthy and diseased arteries. Because of differences in arterial wall microstructure, we hypothesized that large elastic arteries remodel differently compared to smaller muscular arteries. The goal of our study was to test this hypothesis by precisely determining the diameters, lengths and tortuosity for the principal named arteries of the chest, abdomen, pelvis and upper thigh and then to examine how these morphological parameters correlate with age and comorbidities.
METHODS
Three-dimensional reconstructions
IRB approval was obtained to analyze our institution’s trauma database to identify suitable CTA scans with linked patient demographic and comorbidity information. A total of n=122 thin-section, contrast-enhanced chest-abdomen-pelvis CTAs were selected from the database to cover a variation in age (mean age 47±24 years, range 5-93 years) from each gender (64 Male / 58 Female) (Table 1). Since severely injured patients may present with significantly collapsed vessels due to hypovolemic hypotension(15), patients with systolic blood pressure <90 mmHg and patients with Injury Severity Score (ISS) ≥25 [severely injured] were excluded from the study. Average ISS in all patients was 6±5 (range 1-22). Scans of patients with aneurysms and injuries to the vessels of interest were excluded.
Table 1.
Subject demographics and risk factors. BMI = Body Mass Index (average value), HTN = Hypertension, DM = Diabetes Mellitus, CAD = Coronary Artery Disease.
| Age groups, years | 5-9 | 10-19 | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | >80 | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male/Female | 2 | 3 | 7 | 8 | 9 | 6 | 11 | 5 | 8 | 11 | 7 | 8 | 5 | 5 | 9 | 8 | 6 | 4 | 64 | 58 |
| BMI | 19 | 18 | 26 | 27 | 26 | 27 | 28 | 28 | 25 | 32 | 33 | 27 | 32 | 31 | 28 | 26 | 27 | 27 | 28 | 28 |
| HTN | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 0 | 2 | 6 | 3 | 6 | 2 | 3 | 8 | 4 | 2 | 3 | 22 | 22 |
| DM | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 4 | 0 | 1 | 2 | 1 | 1 | 1 | 7 | 7 |
| Dyslipidemia | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 4 | 2 | 3 | 1 | 2 | 7 | 3 | 1 | 2 | 16 | 14 |
| CAD | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 2 | 0 | 1 | 5 | 1 | 1 | 2 | 10 | 7 |
| Ever smoker | 0 | 0 | 1 | 2 | 6 | 2 | 4 | 1 | 3 | 7 | 4 | 3 | 1 | 1 | 7 | 1 | 2 | 0 | 28 | 17 |
| Total in group | 5 | 15 | 15 | 16 | 19 | 15 | 10 | 17 | 10 | 122 | ||||||||||
Scans were obtained with a GE LightSpeed VCT XT 64-channel CTA scanner (GE Healthcare, Waukesha, USA) with a slice thickness of 1.25 mm and a resolution of 512 × 512 pixels. Patients were injected with 75 mL intravenous contrast delivered at 3 mL/s. Three-dimensional reconstructions (Figure 1) of the entire aorta and the common carotid, subclavian, axillary, celiac, superior mesenteric, renal, iliac and common femoral arteries were performed using Mimics software (Materialize Co)(3,16) employing a combination of semi-automated thresholding and region growing techniques. As some arterial segments had densities similar to the surrounding tissue, all segmentations were verified by a vascular surgeon to ensure proper selection of vessel boundaries. Interobserver variability was assessed using a training dataset of five randomly selected scans. Each of these five three-dimensional reconstructions was overlaid onto the baseline image set and corresponding differences in arterial diameters were assessed at 10 random locations. Training was considered satisfactory when cumulative error was <5% in all locations.
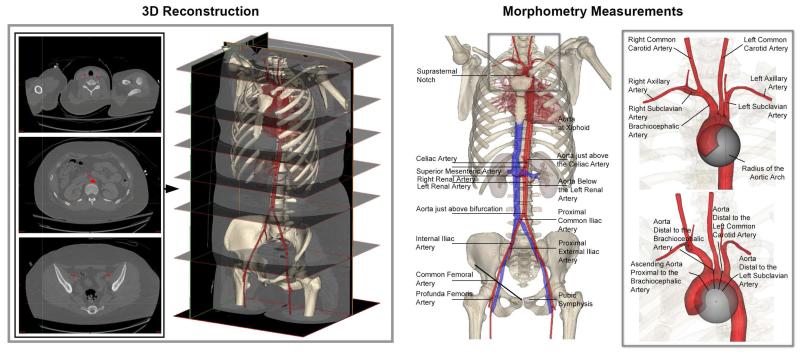
Figure 1.
Segmentation of the axial sections and three-dimensional reconstructions of the vascular tree (left panel) in a representative 21 year old female patient. Right panel demonstrates the measurement locations for the major arteries and the insert demonstrates a magnified view of the arch branches and measurement of the arch radius with an inscribed sphere.
After reconstruction, centerlines were fit to the arterial volumes. Best-fit outer wall – to outer wall diameters were calculated perpendicular to the centerlines to avoid overestimation errors from oblique sectioning of vessels that were not perpendicular to the CTA scan axis. Arterial lengths were measured along the centerline as distances between the centerline intersections of major branches (Figure 1), and normalized to the length of the torso, defined as the distance between the pubic symphysis and suprasternal notch. Arterial tortuosity was measured as a fractional increase in length of the centerline of the tortuous vessel relative to a perfectly straight path(3,16). The angle of the aortoiliac bifurcation and the radius of the aortic arch were measured in three-dimensions. Arch radius was defined as the radius of an inscribed three-dimensional sphere (Figure 1) along the inner curve of the centerline through the aortic arch.
Demographics (age, gender, body mass index [BMI]) and cardiovascular risk factors (hypertension [HTN], diabetes mellitus [DM], dyslipidemia, coronary artery disease [CAD] and smoking history [never, former, current]) were extracted from the medical records linked with each CTA. BMI was calculated as weight(kg)/height(m)2.
Statistical Analysis
Multiple linear regression analysis was performed with SPSS v22 (IBM, Armonk, New York) using age, gender, BMI, HTN, DM, dyslipidemia, CAD, and smoking history parameters as independent variables. Stepwise linear regression was used to determine statistically significant predictors. A variable was entered into the model when the significance level of its F value was less than 0.05 and the variable was removed from the model when the significance level was above 0.10. Only statistically significant predictors are discussed below. Both unstandardized and standardized beta coefficients were determined for each model, and model quality was assessed with adjusted R2.
RESULTS
Diameters
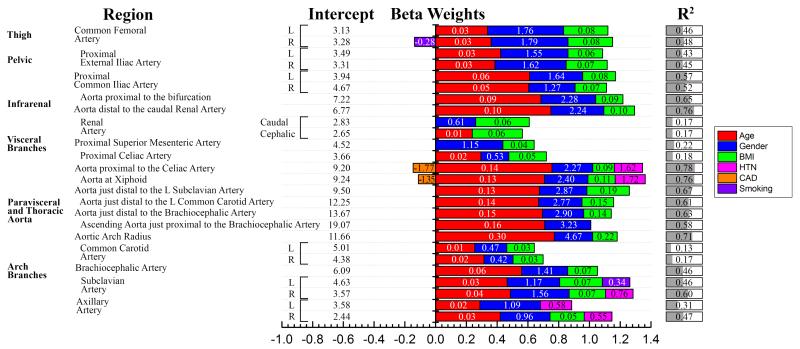
Best-fit arterial diameters at the locations specified in Figure 1 (right panel), and statistically significant beta values for patient demographics and risk factors associated with changes in these diameters, are summarized in Figure 2. The bar graphs represent standardized beta weights that demonstrate the influence of each parameter on the arterial diameter, while numeric values inside the bars are unstandardized beta weights used in the multiple linear regression models. When applicable, bilateral measurements of arterial diameters are provided and marked with a bracket. For clinical reference, average arterial diameters for each age group are summarized in Table 2.
Figure 2.
Best-fit arterial diameters (mm) can be predicted by patient demographics and risk factors. Unstandardized beta weights for the multiple linear regression models are provided with numeric values within the bars, while the length of the colored bars is made using standardized weights and represents the influence of each parameter on the diameter measurement. R2 demonstrates the quality of the model, i.e. how much variability in each diameter measurement is described by demographics and risk factors. BMI = Body Mass Index, HTN = Hypertension, CAD = Coronary Artery Disease. In addition: Gender (0=female, 1=male), Smoking (0=no, 1=former, 2=current). For example, the Aortic Arch Radius (mm) can be predicted as 11.66 + 0.30 Age [years] + 4.67 Gender [0=F, 1=M] + 0.22 BMI [kg/m2], and the combination of these demographics explain 71% of the variation in the predicted radius.
Table 2.
Group-averaged best-fit arterial diameters (mm) ± standard deviation (mm).
| Region | Age groups, years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-9 | 10-19 | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | >80 | |||
| Thigh | Common Femoral Artery | L | 4.9±0.5 | 7.1±1.3 | 6.5±1.2 | 7.3±1.3 | 7.2±1.1 | 7.9±1.7 | 8.3±2.0 | 8.0±2.7 | 8.4±1.5 |
| R | 4.8±0.5 | 7.1±1.4 | 6.5±1.1 | 7.4±1.3 | 7.3±1.2 | 8.0±1.7 | 8.4±1.9 | 8.2±2.5 | 8.6±1.6 | ||
| Pelvic | Proximal External Iliac Artery |
L | 4.6±0.5 | 7.0±1.3 | 6.6±1.2 | 7.3±1.4 | 7.3±1.4 | 7.8±1.2 | 8.5±1.9 | 8.3±2.6 | 8.8±1.6 |
| R | 4.6±0.6 | 7.0±1.3 | 6.6±1.1 | 7.3±1.3 | 7.3±1.3 | 7.9±1.6 | 8.3±1.6 | 8.3±2.4 | 8.4±1.7 | ||
| Proximal Common Iliac Artery |
L | 6.1±0.8 | 8.6±1.2 | 8.1±1.4 | 9.0±1.1 | 9.5±1.3 | 10.6±1.8 | 10.5±2.4 | 11.4±2.7 | 12.8±2.8 | |
| R | 6.1±0.8 | 8.7±1.2 | 8.0±1.4 | 9.2±1.2 | 9.4±1.1 | 10.6±1.6 | 10.4±1.6 | 10.9±2.3 | 12.2±2.6 | ||
| Infrarenal | Aorta proximal to the bifurcation | 9±1.4 | 13.1±1.5 | 12.8±1.7 | 14.2±1.3 | 15.0±2.0 | 16.4±1.6 | 16.1±2.6 | 17.8±3.4 | 18.8±3.7 | |
| Aorta distal to the caudal renal artery | 8.7±1.6 | 12.8±1.2 | 13.0±1.9 | 14.7±1.6 | 15.4±2.0 | 16.8±2.0 | 17.6±2.3 | 18.6±2.7 | 18.9±2.7 | ||
| Renal Artery | Caudal | 2.5±0.5 | 4.7±1.1 | 4.7±1.0 | 5.2±0.9 | 5.0±0.8 | 5.7±1.4 | 5.2±1.2 | 4.6±1.1 | 4.7±1.0 | |
| Cephalic | 2.4±0.3 | 4.7±0.9 | 4.0±0.9 | 4.9±0.8 | 5.1±1.0 | 5.4±1.2 | 4.7±1.1 | 5.0±1.1 | 4.6±0.8 | ||
|
Visceral
Branches |
Proximal Superior Mesenteric Artery | 4.6±0.6 | 6.5±1.0 | 6.0±1.2 | 6.2±0.9 | 6.3±1.2 | 7.1±1.6 | 6.0±1.3 | 6.2±1.5 | 6.0±1.4 | |
| Proximal Celiac Artery | 3.8±0.2 | 5.9±0.6 | 5.5±1.2 | 6.9±1.3 | 6.3±1.4 | 6.9±1.5 | 7.2±1.3 | 6.7±1.7 | 6.4±1.5 | ||
| Aorta proximal to the Celiac Artery | 10.7±1.6 | 15.5±1.9 | 15.7±2.1 | 18.9±1.5 | 19.4±2.3 | 21.9±2.3 | 23.1±3.3 | 23.5±3.3 | 23.7±2.4 | ||
|
Paravisceral
and Thoracic Aorta |
Aorta at Xiphoid | 10.5±1.9 | 16.2±2.2 | 16.6±2.1 | 19.2±2.1 | 19.8±2.4 | 22.3±2.5 | 23.5±3.1 | 23.7±3.2 | 23.8±2.4 | |
| Aorta just distal to the L Subclavian Artery |
12.1±1.7 | 18.6±2.5 | 19.4±2.7 | 22.7±2.1 | 21.9±2.4 | 25.0±3.7 | 26.1±3.3 | 26.2±4.5 | 26.0±3.4 | ||
| Aorta just distal to the L Common Carotid Artery |
13.3±1.8 | 20.5±3.0 | 21.1±3.0 | 24.3±2.4 | 23.9±2.4 | 26.9±2.9 | 27.9±3.4 | 27.8±5.1 | 28.3±3.6 | ||
| Aorta just distal to the Brachiocephalic Artery |
15.2±2.8 | 21.4±2.8 | 23.3±3.4 | 26.2±2.9 | 25.6±3.4 | 28.9±3.7 | 30.7±3.6 | 30.2±5.1 | 30.9±3.4 | ||
| Ascending Aorta just proximal to the Brachiocephalic Artery |
17.0±2.4 | 23.3±3.5 | 24.9±3.1 | 28.3±3.2 | 28.0±3.8 | 31.0±3.2 | 32.1±3.7 | 31.4±4.8 | 33.9±3.8 | ||
| Aortic Arch Radius | 17.3±2.0 | 26.0±4.7 | 27.5±2.8 | 31.3±3.1 | 33.5±4.0 | 39.3±6.3 | 38.5±8.1 | 44.5±8.1 | 45.2±7.0 | ||
|
Arch
Branches |
Common Carotid Artery | L | 5.5±0.4 | 6.7±1.2 | 6.1±0.9 | 6.8±0.7 | 6.3±0.7 | 7.1±1.5 | 6.9±1.4 | 7.1±1.5 | 6.9±0.6 |
| R | 5.4±0.5 | 6.2±0.9 | 5.5±0.9 | 6.1±1.0 | 5.8±0.9 | 6.5±1.1 | 6.8±1.0 | 6.7±1.3 | 6.6±1.1 | ||
| Brachiocephalic Artery | 7.0±1.2 | 9.8±1.5 | 10.6±2.1 | 11.8±1.0 | 10.7±1.3 | 11.6±2.0 | 12.2±1.8 | 13.1±3.1 | 13.5±1.5 | ||
| Subclavian Artery | L | 5.6±0.7 | 7.8±1.0 | 8.9±1.3 | 9.2±1.0 | 8.7±1.5 | 9.8±1.4 | 10.1±1.6 | 10.1±2.0 | 9.5±1.5 | |
| R | 5.5±0.6 | 7.3±1.5 | 7.3±1.2 | 8.5±1.7 | 8.4±1.3 | 9.5±1.9 | 9.2±1.7 | 10.1±2.3 | 10.5±1.7 | ||
| Axillary Artery | L | 2.4±0.8 | 4.8±0.9 | 4.5±1.2 | 5.4±1.0 | 5.0±1.1 | 5.9±1.2 | 5.6±1.3 | 5.6±1.7 | 5.7±1.5 | |
| R | 3.1±0.8 | 4.9±0.9 | 4.6±1.5 | 6.0±0.9 | 5.7±1.1 | 6.7±1.4 | 6.9±0.9 | 6.4±1.5 | 6.4±1.4 | ||
Most arteries increased their diameter with age, and the largest increase was observed for proximal elastic arteries. Figure 3A demonstrates that more proximal arteries, typically associated with higher elastin contents, increase their diameter faster than more distal arteries with less elastin. Demographics and risk factors explained more than 70% of variation in the diameter of the abdominal aorta, paravisceral aorta, and the aortic arch, but the same set of variables explained only 20% of the variance in the diameters of the less elastic and more muscular superior mesenteric and celiac arteries.
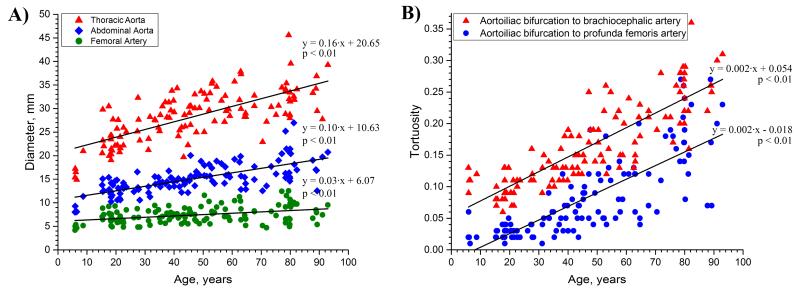
Figure 3.
A: Changes in the diameters of the aorta and femoral artery with age, and B: Changes in the tortuosity of the more proximal and the more distal segments of the aorta with age, demonstrating that elastic and muscular arteries undergo different circumferential but similar longitudinal remodeling.
Age had the strongest influence on arterial diameter, followed by gender, with male arteries being larger than female. BMI was the third most important contributor associated with arterial diameter. The contributions of gender and BMI suggest that larger arterial diameters were associated with larger body size; however normalization of diameters to BMI demonstrated that gender still had a statistically significant impact in most arterial locations. There were no correlations between gender and BMI.
The effects of risk factors on arterial diameters were small compared to the effects of demographics. Patients with HTN had wider descending thoracic aortas and axillary arteries, and patients with CAD had somewhat smaller descending thoracic aortas. Smoking appeared to have a significant, but minor effect on the femoral and subclavian arteries, and DM and dyslipidemia had no statistically significant effects on the diameter.
Lengths
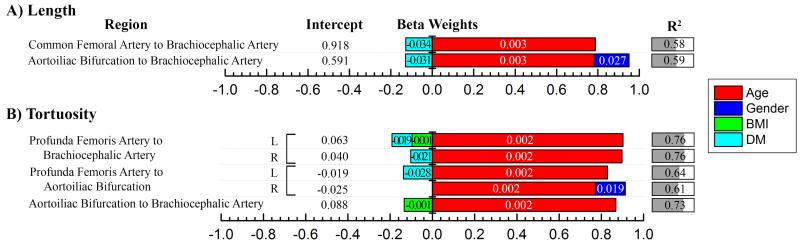
Lengths assessed as distances between major arterial branches, are presented in Figure 4A. All values were normalized to the length of the torso, i.e. the distance from the pubic symphysis to the suprasternal notch. For clinical reference, average normalized lengths for each age group are provided in Table 3 (upper panel). Figure 4A demonstrates that demographics and risk factors explained 58% of variability in the arterial length from the common femoral artery to the brachiocephalic artery, but this was mostly attributed to lengthening of the thoracic and visceral aorta, while length of the iliac arteries played only a minor role.
Figure 4.
A) Lengths of vascular tree normalized to torso length [suprasternal notch to pubic symphysis] and B) Tortuosity of the vascular tree can be predicted by demographics and risk factors. Unstandardized beta weights for the multiple linear regression models are provided with numeric values within the bars, while the length of the colored bars is made using standardized weights and represents the influence of each parameter on length (A) or tortuosity (B) measurement. R2 demonstrates the quality of the model, i.e. how much variability in each measurement is described by demographics and risk factors. BMI = Body Mass Index, DM = Diabetes Mellitus. Gender is 0 for female and 1 for male. For example, length of the vascular tree from the Common Femoral Artery to the Brachiocephalic Artery (normalized to torso length) can be predicted as 0.918 + 0.003 Age [years] – 0.034 DM [0=no, 1=yes], and the combination of these factors explain 58% of the variation in the predicted length.
Table 3.
Group-averaged arterial lengths normalized to torso length, and group-averaged arterial tortuosity ± standard deviation.
| Region | Age groups, years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-9 | 10-19 | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | >80 | |||
| Length | Common Femoral Artery to Brachiocephalic Artery |
1.00±0.02 | 0.98±0.03 | 0.97±0.02 | 1.00±0.04 | 1.03±0.04 | 1.07±0.05 | 1.07±0.04 | 1.15±0.08 | 1.16±0.11 | |
| Aortoiliac Bifurcation to Brachiocephalic Artery |
0.69±0.02 | 0.66±0.03 | 0.65±0.04 | 0.69±0.04 | 0.70±0.04 | 0.75±0.04 | 0.75±0.06 | 0.81±0.08 | 0.83±0.08 | ||
| Tortuosity | Profunda Femoris Artery to Brachiocephalic Artery |
L | 0.10±0.01 | 0.08±0.02 | 0.09±0.01 | 0.12±0.02 | 0.14±0.03 | 0.17±0.04 | 0.17±0.03 | 0.23±0.04 | 0.25±0.05 |
| R | 0.09±0.01 | 0.09±0.02 | 0.09±0.02 | 0.12±0.02 | 0.14±0.03 | 0.17±0.04 | 0.18±0.03 | 0.24±0.04 | 0.25±0.06 | ||
| Profunda Femoris Artery to Aortoiliac Bifurcation |
L | 0.03±0.02 | 0.03±0.01 | 0.03±0.01 | 0.05±0.03 | 0.07±0.04 | 0.09±0.04 | 0.10±0.05 | 0.17±0.05 | 0.16±0.07 | |
| R | 0.03±0.01 | 0.03±0.01 | 0.04±0.01 | 0.05±0.02 | 0.07±0.04 | 0.10±0.05 | 0.11±0.06 | 0.17±0.06 | 0.17±0.06 | ||
| Aortoiliac Bifurcation to Brachiocephalic Artery |
0.11±0.01 | 0.10±0.02 | 0.10±0.02 | 0.13±0.02 | 0.16±0.03 | 0.18±0.04 | 0.19±0.04 | 0.24±0.04 | 0.26±0.06 | ||
Similar to arterial diameters, age had the strongest influence on arterial lengths, with older vascular trees being longer. Elongation of the aorta with age resulted in inferior migration of the aortoiliac bifurcation in relation to the pubic symphysis landmark. Almost 50% of variation in this migration was attributed to age and gender, with the strongest effect demonstrated in males. Among all risk factors, only DM appeared to contribute negatively to the length of the vascular tree, while all other risk factors had no statistically significant effects of length.
Tortuosity and aortoiliac bifurcation angle
Measurements of arterial tortuosity are summarized in Figure 4B. For clinical reference, the average measurements for each age group are provided in Table 3 (lower panel). The vascular tree became more tortuous with age, and age was the strongest contributor to tortuosity, far surpassing the effects of gender and risk factors. The rate of tortuosity increase was the same for the more proximal and the more distal segments of the aorta (Figure 3B). The combination of age and DM explained more than 76% of variability in the total tortuosity from the profunda femoris to the brachiocephalic artery. Interestingly, presence of DM was associated with straighter arteries. The inferior migration of the aortoiliac bifurcation described above resulted in more tortuous iliac arteries. Though inferior migration of the aortoiliac bifurcation with aging might also be expected to cause widening of the aortoiliac bifurcation angle, this was not confirmed and no statistically significant correlation between age and bifurcation angle was observed. Nevertheless, the combination of age and HTN did explain 11% of variation in the aortoiliac bifurcation angle (βage = 0.406, βHTN = −0.236 where β is the standardized weight). BMI had minor negative contributions to the tortuosity of the vascular tree, and HTN, CAD, dyslipidemia and smoking were not associated with arterial tortuosity.
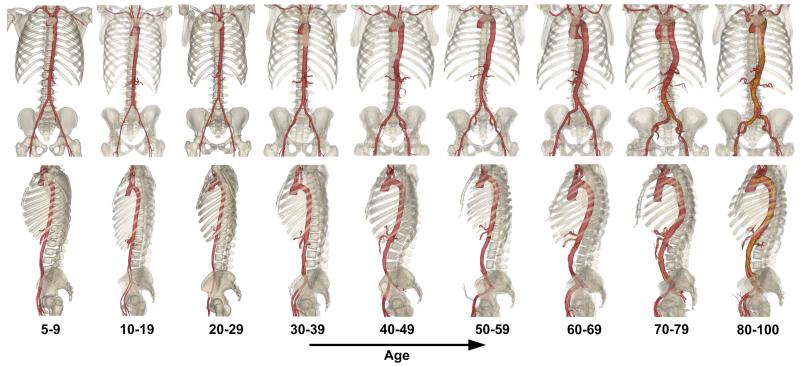
Representative three-dimensional reconstructions of vascular morphometry for patients in each age group are shown in Figure 5. The figure demonstrates increases in arterial diameters and tortuosity with age and inferior migration of the aortoiliac bifurcation.
Figure 5.
Representative reconstructions of vascular morphometry for patients from each age group demonstrating an increase in arterial diameter and tortuosity with age.
DISCUSSION
Advances in endovascular therapies over the past several years have led to the extensive use of minimally invasive techniques and devices to not only treat degenerative arterial diseases in the elderly, but also traumatic arterial injuries in much younger patients. A number of factors contribute to significantly lower short-term morbidity and mortality with endovascular aortic surgery compared to open surgery(4,5), and many of these factors have popularized the use of endovascular techniques for repair of more peripheral arteries in both civilian(6) and military trauma populations(7). Despite superior short-term results however, the potential for long-term complications in younger patients remains a significant concern(9). Arterial remodeling, resulting in diameter enlargement and elongation of arteries over time, can result in device migration, endoleaks, and reconstruction failure, predisposing to morbidity, mortality and expensive reinterventions(9). Though the effects of aging on certain parts of the vasculature have been reported previously(10,12), in this study we have comprehensively determined the dimensions and shapes of all major arteries in the chest, abdomen, pelvis and upper thigh, and included additional analysis on how these morphological parameters are influenced by age and comorbidities.
Our data suggest that most arteries continuously increase their diameter, length and tortuosity over one’s lifespan. More proximal elastic arteries increase in diameter faster than muscular arteries, and all arteries demonstrate tortuosity increases. These findings might be explained by differences in the microstructure of elastic and muscular arteries(1-3). Proximal elastic arteries, such as the thoracic aorta, demonstrate a dense network of both circumferentially and longitudinally oriented elastin fibers uniformly distributed throughout the medial layer, while muscular arteries, such as the common femoral artery, contain mostly longitudinal elastin located primarily within the external elastic lamina (1-3,17,18). Since elastin matures early in life(19), elastic fibers stretch as vessels grow, resulting in considerable tension in maturity. Due to the location and organization of elastin in the arterial wall, this residual tension is both longitudinal and circumferential in elastic arteries, but mostly longitudinal in muscular arteries.
The elastin-generated tension likely serves two purposes. In the circumferential direction it smoothens stress distribution across the arterial wall thickness(20-22), while in the longitudinal direction, this tension ensures energy-efficient function by decoupling blood pressure and longitudinal force(2,17,23). In muscular arteries, longitudinal tension may help to accommodate end-organ motion, such as flexion of the femoropopliteal artery with movement of the legs or elongation of the renal artery with respiration-induced kidney motion(1,2,17,18). The effects of both circumferential and longitudinal tensions are demonstrated by the artery springing open when radially cut and by longitudinal foreshortening of healthy young arteries when transected(22).
Aging, vascular disease and certain risk factors are known to be associated with degradation and fragmentation of elastin(24,25), which in turn releases arterial wall tension and results in an increase of arterial diameter, length and tortuosity. In large elastic arteries having both circumferential and longitudinal elastin, the remodeling is bidirectional, and elastic arteries become wider in diameter, longer and more tortuous(3). In muscular arteries, having mostly a thin layer of longitudinal elastin, remodeling is predominantly longitudinal, and these arteries become more tortuous rather than increasing in diameter(2,17). Both of these speculations were confirmed by our analysis.
Similar results regarding widening and elongation of large elastic arteries with age have been reported previously by Hartley et al(10) and Sugawara et al(12) who studied the thoracic aorta. Hartley et al(10) found substantial widening of the aorta at the isthmus with age, but found no statistically significant differences between males and females, or patients with and without comorbidities. Our data demonstrate that when patient demographics and risk factors are used as independent variables, not only do gender and BMI have statistically significant contributions to the aortic diameter at the isthmus, but along with age they explain more than 67% of variation in this diameter – a value that is more than three-fold higher than when only age is considered(10).
Sugawara et al(12) studied elongation of the thoracic aorta, and although the influence of demographics and risk factors were not examined, they found substantial ascending aortic lengthening with age. Contrary to our findings however, they found no correlation between age and the length of the descending aorta which was measured from the top of the arch to the aortoiliac bifurcation. Our data demonstrate that this segment does lengthen with age, and that this lengthening is positively influenced by male gender and negatively by the presence of DM. Our results also indicate that the length of the entire aortic tree increases with age. Elongation of the proximal thoracic aorta is manifested by widening of the aortic arch, while in the descending and abdominal aorta, this elongation is demonstrated by an increase in tortuosity and inferior migration of the aortoiliac bifurcation. These results are consistent with the findings of Wolf et al(13) in reference to the infrarenal aorta and are of particular clinical interest, as these segments of aorta are frequent targets for endovascular repair with stent-grafts. Greater tortuosity not only increases the complexity of endovascular repairs in these regions, but may also predispose to stent-graft migration and endoleak(26,27).
Though geometric remodeling does not always imply disease, classic cardiovascular risk factors are frequently thought to significantly influence vascular remodeling. Surprisingly in this study, many of these risk factors did not appear to significantly influence geometric remodeling. Although patients with DM did have somewhat straighter and shorter arteries, these effects were dwarfed by age. The presence of HTN did contribute to larger thoracic aortic and axillary artery diameters, but did not affect arterial length or tortuosity. In a recent study of carotid arteries, we described the differences between normal and pathologic geometric remodeling, and demonstrated that when viewed in the context of aging, straighter rather than more tortuous arteries, may be associated with vascular disease(3). More robust risk factor definitions and assessment, including duration and severity of comorbidities such as DM, HTN and tobacco use, would be desirable to further confirm these results; however this would require substantially larger patient volumes with significantly more detailed analysis of clinical and laboratory variables.
The finding of continuous increases in diameter and length of large elastic arteries with increasing age raises concerns for potential device failure and the requirement for reinterventions in young trauma patients treated with endovascular interventions. Though additional confirmatory clinical studies investigating the effects of stent-grafts on the rate of arterial remodeling are needed, our data suggest that a hypothetical 20 year-old obese male with a 24mm thoracic aortic diameter treated with a 27mm stent-graft may overgrow his device by the age of 40. Unlike surgically implanted prosthetic grafts, because endovascular stent-grafts typically do not become incorporated into the aortic wall(28), this outward aortic remodeling may result in device collapse, migration or endoleak, potentially predisposing younger patients to additional morbidity, mortality and multiple expensive reinterventions over the course of a lifetime. A simple solution might appear to be to increase oversizing of the stent-graft at the time of implantation, but excessive oversizing may exacerbate arterial remodeling or result in short-term device collapse, “bird-beaking”, migration, endoleak, device fracture, artery wall necrosis, and stent-graft edge stenosis(29,30). Instead, these data demonstrate the need for improving stent-graft characteristics to properly balance the properties of the implant with the complex morphometry and mechanics of the recipient artery. These improvements may include the development of new “adaptive” devices that can expand along with a remodeling artery or grafts that heal into the artery wall within the seal zones. In the meantime, however, as suggested by Figure 2, maintaining a normal BMI in our hypothetical young male could possibly slow the remodeling process and delay artery wall overgrowth of stent-graft diameter by as much as a decade.
This study has several limitations, one of which is its descriptive nature. Though the associations between vascular anatomy, demographics and risk factors presented in this work are useful for estimating how arterial size and shape may change given vessel location, specific demographics and risk factors, they do not determine the cause and effect relations that can only be established with longitudinal studies. The second limitation is the relatively small sample size and our work continues to expand our population to gain an even better understanding of how the anatomy of the chest, abdomen and pelvic arteries change with aging and disease.
CONCLUSIONS
The vascular tree undergoes significant remodeling with aging, and this process is significantly influenced by patient demographics and some clinical risk factors. Elastic and muscular arteries remodel differently, possibly due to differences in their microstructure. The detailed description of arterial geometric remodeling in the context of demographics and risk factors described in this work can help physicians and biomedical engineers better understand the vascular system and optimize treatment methods for arteries in different anatomic locations.
ACKNOWLEDGEMENTS
Research reported in this publication was supported in part by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL125736. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also wish to acknowledge Dr. Paul J Schenarts and Diane Yetter for their help with procurement of trauma CTAs, Lucas Seiler for his help with the segmentation, Sheridan Nusz, Grant Bowen and Andreas Seas for their help with data analysis. Finally, we thank the Charles and Mary Heider Fund for Excellence in Vascular Surgery for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kamenskiy A, Dzenis Y, Kazmi SAJ, Pemberton M, Pipinos I, Phillips N, et al. Biaxial Mechanical Properties of the Human Thoracic and Abdominal Aorta, Common Carotid, Subclavian, Renal and Common Iliac Arteries. Biomech Model Mechanobiol. 2014;13(6):1341–59. doi: 10.1007/s10237-014-0576-6. [DOI] [PubMed] [Google Scholar]
- 2.Kamenskiy AV, Pipinos II, Dzenis Y a, Phillips NY, Desyatova AS, Kitson J, et al. Effects of age on the physiological and mechanical characteristics of human femoropopliteal arteries. Acta Biomater. Acta Materialia Inc. 2015 Oct 6;11:304–13. doi: 10.1016/j.actbio.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 3.Kamenskiy AV, Pipinos II, Carson JS, MacTaggart JN, Baxter BT. Age and disease-related geometric and structural remodeling of the carotid artery. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2014.10.041. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Cheng D, Martin J, Shennib H, Dunning J, Muneretto C, Schueler S, et al. Endovascular Aortic Repair Versus Open Surgical Repair for Descending Thoracic Aortic Disease A Systematic Review and Meta-Analysis of Comparative Studies. J Am Coll Cardiol. 2010;55(10):986–1001. doi: 10.1016/j.jacc.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 5.Xenos ES, Abedi NN, Davenport DL, Minion DJ, Hamdallah O, Sorial EE, et al. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. Elsevier. 2008 Nov;48(5):1343–51. doi: 10.1016/j.jvs.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 6.Reuben BC, Whitten MG, Sarfati M, Kraiss LW. Increasing use of endovascular therapy in acute arterial injuries: analysis of the National Trauma Data Bank. J Vasc Surg. 2007 Dec 1;46(6):1222–6. doi: 10.1016/j.jvs.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen TE, Clouse WD, Peck M a, Bowser AN, Eliason JL, Cox MW, et al. Development and implementation of endovascular capabilities in wartime. J Trauma. 2008 May;64(5):1169–76. doi: 10.1097/TA.0b013e31816b6564. discussion 1176. [DOI] [PubMed] [Google Scholar]
- 8.Marty B, Maeder B, Gallino A, Mucciolo A, Karl von Segesser L. Does large oversizing of self-expandable endoprostheses compensate for aortic growth? J Vasc Surg. 2003 Dec;38(6):1368–75. doi: 10.1016/s0741-5214(03)00925-x. [DOI] [PubMed] [Google Scholar]
- 9.Miller LE. Potential long-term complications of endovascular stent grafting for blunt thoracic aortic injury. Sci World J. 2012. 2012 Jan;:897489. doi: 10.1100/2012/897489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartley MC, Langan EM, Cull DL, Taylor SM, Carsten CG, Blackhurst DW. Evaluation of the diameter of the proximal descending thoracic aorta with age: implications for thoracic aortic stent grafting. Ann Vasc Surg. Annals of Vascular Surgery Inc. 2009;23(5):639–44. doi: 10.1016/j.avsg.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Wenn CM, Newman DL. Arterial tortuosity. Australas Phys Eng Sci Med. 1990 Jun;13(2):67–70. [PubMed] [Google Scholar]
- 12.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. American College of Cardiology Foundation. 2008 Nov;1(6):739–48. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Wolf YG, Tillich M, Lee W a, Rubin GD, Fogarty TJ, Zarins CK. Impact of aortoiliac tortuosity on endovascular repair of abdominal aortic aneurysms: evaluation of 3D computer-based assessment. J Vasc Surg. 2001 Oct;34(4):594–9. doi: 10.1067/mva.2001.118586. [DOI] [PubMed] [Google Scholar]
- 14.Mao SS, Ahmadi N, Shah B, Beckmann D, Chen A, Ngo L, et al. Normal thoracic aorta diameter on cardiac computed tomography in healthy asymptomatic adults: impact of age and gender. Acad Radiol. 2008 Jul;15(7):827–34. doi: 10.1016/j.acra.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Prehn J, van Herwaarden J a, Muhs BE, Arnofsky A, Moll FL, Verhagen HJM. Difficulties with endograft sizing in a patient with traumatic rupture of the thoracic aorta: the possible influence of hypovolemic shock. J Vasc Surg. 2008 Jun;47(6):1333–6. doi: 10.1016/j.jvs.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Kamenskiy A, Pipinos I, Dzenis Y, Bikhchandani J, Gupta P, Phillips N, et al. Effects of Carotid Artery Stenting on Arterial Geometry. J Am Coll Surg. 2013;217(2):251–62. doi: 10.1016/j.jamcollsurg.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Kamenskiy AV, Pipinos II, Dzenis YA, Lomneth CS, Kazmi S a J, Phillips NY, et al. Passive biaxial mechanical properties and in vivo axial pre-stretch of the diseased human femoropopliteal and tibial arteries. Acta Biomater. Acta Materialia Inc. 2014 Dec 24;10(3):1301–13. doi: 10.1016/j.actbio.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacTaggart J, Phillips N, Lomneth C, Pipinos I, Bowen R, Baxter B, et al. Three-Dimensional Bending, Torsion and Axial Compression of the Femoropopliteal Artery During Limb Flexion. J Biomech. 2014;47:2249–56. doi: 10.1016/j.jbiomech.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 19.Mithieux SM, Weiss AS. Elastin. Adv Protein Chem. 2005 Jan;70(null):437–61. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- 20.Chuong CJ, Fung YC. On residual stresses in arteries. J Biomech Eng. 1986 May;108(2):189–92. doi: 10.1115/1.3138600. [DOI] [PubMed] [Google Scholar]
- 21.Holzapfel G a, Gasser TC. Computational stress-deformation analysis of arterial walls including high-pressure response. Int J Cardiol. 2007 Mar 2;116(1):78–85. doi: 10.1016/j.ijcard.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Humphrey JD. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. Springer. 2002 [Google Scholar]
- 23.Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech. 2009;42(1):1–8. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bizbiz L, Alpérovitch a, Robert L. Aging of the vascular wall: serum concentration of elastin peptides and elastase inhibitors in relation to cardiovascular risk factors. The EVA study. Atherosclerosis. 1997 May;131(1):73–8. doi: 10.1016/s0021-9150(97)06076-0. [DOI] [PubMed] [Google Scholar]
- 25.Wachi H. Role of Elastic Fibers on Cardiovascular Disease. J Heal Sci. 2011;57(6):449–57. [Google Scholar]
- 26.Figueroa CA, Taylor CA, Yeh V, Chiou AJ, Zarins CK. Effect of curvature on displacement forces acting on aortic endografts: a 3-dimensional computational analysis. J Endovasc Ther. 2009 Jun;16(3):284–94. doi: 10.1583/08-2667.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobo R, Kievit J, Leurs LJ, Buth J. Influence of severe infrarenal aortic neck angulation on complications at the proximal neck following endovascular AAA repair: a EUROSTAR study. J Endovasc Ther. 2007 Feb;14(1):1–11. doi: 10.1583/06-1914.1. [DOI] [PubMed] [Google Scholar]
- 28.Mallna M, Brunkwall J, Ivancev K, Jönsson J, Malina J, Lindblad B. Endovascular healing is inadequate for fixation of Dacron stent-grafts in human aortoiliac vessels. Eur J Vasc Endovasc Surg. 2000;19(1):5–11. doi: 10.1053/ejvs.1999.0867. [DOI] [PubMed] [Google Scholar]
- 29.Atkins MD, Marrocco CJ, Bohannon WT, Bush RL. Stent-graft repair for blunt traumatic aortic injury as the new standard of care: is there evidence? J Endovasc Ther. 2009 Feb;16(Suppl I):I53–62. doi: 10.1583/08-2669.1. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 30.Scheumann J, Heilmann C, Beyersdorf F, Siepe M, Brenner RM, Böckler D, et al. Early histological changes in the porcine aortic media after thoracic stent-graft implantation. J Endovasc Ther. International Society of Endovascular Specialists 1928 East Highland venue, #F104-605, Phoenix, AZ 85016 USA. 2012 Jun 12;19(3):363–9. doi: 10.1583/12-3845R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]