Abstract
Parkinson’s disease (PD) is a debilitating, progressive, neurodegenerative disorder characterized by progressive loss of dopaminergic neurons and motor deficits. Alpha-synuclein-containing aggregates represent a feature of a variety of neurodegenerative disorders, including PD; however, the mechanism that initiates and promotes intraneuronal alpha-synuclein aggregation remains unknown. We hypothesized protein radical formation as an initiating mechanism for alpha-synuclein aggregation. Therefore, we used the highly sensitive immuno-spin trapping technique to investigate protein radical formation as a possible mechanism of alpha-synuclein aggregation as well as to investigate the source of protein radical formation in the midbrains of Maneb and paraquat coexposed mice. Coexposure to Maneb and paraquat for 6 weeks resulted in active microgliosis, NADPH oxidase activation, and inducible nitric oxide synthase (iNOS) induction, which culminated in protein radical formation in the midbrains of mice. Results obtained with immuno-spin trapping and immunoprecipitation experiments confirmed formation of alpha-synuclein radicals in dopaminergic neurons of exposed mice. Free radical formation requires NADPH oxidase and iNOS, as indicated by decreased protein radical formation in knockout mice (P47phox−/− and iNOS−/−) and in mice treated with inhibitors such as FeTPPS (a peroxynitrite decomposition catalyst), 1400W (an iNOS inhibitor), or apocynin (a NADPH oxidase inhibitor). Concurrence of protein radical formation with dopaminergic neuronal death indicated a link between protein radicals and disease progression. Taken together, these results show for the first time the formation and detection of the alpha-synuclein radical and suggest that NADPH oxidase and iNOS play roles in peroxynitrite-mediated protein radical formation and subsequent neuronal death in the midbrains of Maneb and paraquat coexposed mice.
Keywords: Protein radicals, Peroxynitrite, Parkinson’s disease, Immuno-spin trapping, DMPO
Introduction
PD is a movement disorder characterized by slow and progressive loss of dopaminergic neurons in the substantia nigra and manifests mostly as a sporadic condition [1,2]. Although the cause of sporadic PD is not fully understood, various factors, including environmental toxins, heavy metals, and pesticides, have been implicated in disease pathogenesis [3,2,4]. An important aspect of human pesticide exposure is that it is usually to a mixture of pesticides rather than a single one, and it occurs over time [5]. Various chemicals, especially pesticides, and factors related to pesticide exposure (e.g., farming, well-water drinking and rural living) have been proposed as potential risk factors for PD [6-9].
The fungicide Maneb (Manganese ethylene-1,2-bisdithiocarbamate) and the herbicide paraquat (1,1-dimethyl-4,4-bipyridinium) are concurrently used in agriculture to treat a variety of crop pathologies [10,11]. Paraquat crosses the blood-brain barrier through neutral amino acid transporters, preferentially targets the nigrostriatal pathway, inhibits mitochondrial complex I, undergoes redox cycling and produces superoxide [12,13]. Maneb and paraquat together are well documented as a trigger that induces the PD phenotype in mice [14]. Epidemiological studies also show that combined exposure to Maneb and paraquat strongly increases the risk of PD in humans, especially with coexposure [10,11]. Considering the global use of pesticides and the presence of epidemiological evidence, the Maneb- and paraquat-induced model of PD seemed appropriate for studying the involvement of protein radicals in PD.
A common hallmark of several neurodegenerative disorders is the loss of specific populations of neuronal cells and the presence of abnormal protein aggregates containing specific modified proteins [15]. Because these modified proteins have a propensity to aggregate and interfere with cellular processes and signaling, either their production should be controlled or their removal must be quick enough to maintain cell survival. The ubiquitin-proteasome system is the primary pathway responsible for degradation of altered proteins [16,17]. Conversely, an impaired ubiquitin-proteasomal system, as often seen in various models of Parkinson’s disease, facilitates accumulation of modified proteins in the substantia nigra [18,19]. The pathological features of PD include an abnormal accumulation of the protein alpha-synuclein in particular throughout various brain regions in the remaining dopaminergic neurons in the nigrostriatal pathway [20,21].
Furthermore, inhibition of alpha-synuclein aggregation by Hsp90 renders it nontoxic and therefore affects neuronal survival [22]. Genetic ablation of alpha-synuclein has been shown to protect dopaminergic neurons from the environmental toxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), a precursor neurotoxin known to trigger the core neurological features of PD and specific dopaminergic neuronal death [23,22]. Certain mutations in SNCA (the gene which encodes alpha-synuclein), post-translational modifications, oxidative stress, and toxins interacting with oxidized dopamine are speculated to increase the propensity of alpha-synuclein to aggregate and accumulate [24]. Although protein aggregates and modified proteins such as alpha-synuclein [25,26] play crucial roles in neuronal regulation during PD pathogenesis, the underlying initiating mechanism of aggregation remains unknown [27,28]. It is generally accepted that protein radical formation might be a key mechanism that initiates aggregation or radical-driven protein modification. However, the formation, detection and consequences of protein radicals in vivo, especially in a neuronal context, have never been investigated.
Therefore, we aimed to investigate the underlying mechanism of the formation, detection and consequences of the alpha-synuclein radical in dopaminergic neurons of Maneb and paraquat coexposed mice. Through various lines of evidence, we report for the first time that NADPH oxidase and iNOS play crucial roles in peroxynitrite-mediated alpha-synuclein radical formation in dopaminergic neurons of Maneb and paraquat coexposed mice. Alpha-synuclein radical formation appeared in concurrence with the neuronal death that occurs in the Maneb- and paraquat-induced PD-phenotype. Possible biochemical consequences of protein radical formation may involve changes in activity, gain or loss of function, alteration in protein assembly, and formation of aggregates [29,30]. Understanding the protein radical-derived mechanisms in a pesticide-induced model of PD is important for defining protein radical-derived therapeutics that can specifically minimize or impair protein radical-mediated dopaminergic neurodegeneration.
Materials and Methods
Materials
Apocynin, bovine serum albumin, N-3-(aminomethyl)benzylacetamide ·2HCl (1400W), Maneb (Manganese ethylene-1,2-bisdithiocarbamate), DAB, NADPH, paraquat ( 1,1-dimethyl-4,4-bipyridinium), paraformaldehyde, Triton X-100 , and 5,10,15,20,-tetrakis(4-sulfonatophenyl) porphyrinato iron (III) chloride (FeTPPS) were from Sigma (St. Louis, MO). 5, 5-dimethyl-1-pyrroline N-oxide (DMPO) was obtained from Dojindo Laboratories (Rockville, MD) and used without further purification. Chicken and rabbit polyclonal anti-DMPO antibodies were developed in our laboratory and used in the immuno-spin trapping studies. Mouse monoclonal anti-integrin □M (ox-42), rabbit polyclonal anti-iNOS, and P67 phox antibodies were from Santa Cruz Biotechnology (Dallas, TX). Mouse monoclonal anti-alpha synuclein, and anti-β-actin antibodies were from Abcam (Cambridge, MA). Rabbit polyclonal anti-tyrosine hydroxylase antibody was from Millipore (Billerica, MA). Micro BCA Protein Assay Kit, Permount, RIPA buffer, Surfact-Amps X-100, and Pierce Classic Immunoprecipitation Kits were from Thermo Scientific (Rockford, IL). Nitrocellulose membranes, Prolong Gold anti-fade reagent with DAPI and AlexaFluor secondary antibodies were from Invitrogen (Grand Island, NY). The Vectastain Elite abc kit was from Vector laboratories (Peterborough, UK).
Methods
Animal treatment
Adult (8-10-week-old), pathogen-free male C57BL6/J mice (Jackson Laboratories, Bar Harbor, Maine) were housed one to a cage for 1 week before any experimental dosing. Mice that contained the disrupted iNOS (iNOS−/−, stock number-002609) and p47phox (p47phox−/−, stock number-004762) were treated identically. Age-matched mice of C57BL6/J origin that had normal iNOS and NADPH oxidase activity served as the control animals for knockout experiments. The animals were housed under standard conditions of temperature and humidity with a 12 h light/dark cycle and fed a standard pellet diet and water ad libitum. Animals were treated with normal saline (control), paraquat (10mg/kg, i.p.) and Maneb (30mg/kg, i.p.) with or without pretreatment with apocynin (10mg/kg, i.p.), 1400W (15 mg/kg, i.p.), or FeTPPS (10mg/kg, i.p.) twice a week for 6 weeks. A minimum of 3-5 animals were used per group for biochemical, Western blot/ELISA and immunohistochemical studies. All animals were treated in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals, and the animal study proposal was approved by the institutional review board.
HPLC and MS
DMPO (5,5'-dimethyl-1-pyrroline N-oxide) was administered to mice at a dose of 2 g/kg,i.p., followed by transcardial perfusion and sacrifice after 2 hours. Livers (used as a positive control) and brains were dissected to analyze DMPO content in order to evaluate its entry into the brain. In brief, the nigrostriatal tissue and liver were homogenized in PBS containing 0.1 N HClO4 followed by sonication and centrifugation at 21,000 × g for 30 min at 4°C [13]. The supernatant was neutralized with sodium hydroxide to a pH of 7.0, centrifuged (14,000 rpm for 5 min at room temperature) and passed through a 0.2 μm filter. Samples thus obtained were loaded onto a column (Waters Atlantis T3, l = 150 mm) pre-equilibrated with 0.1% aqueous formic acid (flow rate: 0.2 ml/min). The fraction eluted at the retention time of 15 min was collected and investigated with mass spectroscopy for DMPO. MS and MS/MS experiments were carried out using a Waters Micromass Q-tof Micro (Waters, Milford, MA). Capillary voltage was 3300 V, sample cone voltage 30 V, extraction cone energy 4-5 eV, and collision energy 4-5 eV for MS and 15-20 eV for MS/MS). Helium was used as the collision gas and the product ion mass was 114.09. For calibration, a solution of glu-fibrinopeptide B (500 fmol/μL) in water/acetonitrile 80:20 (v/v) with 0.1% formic acid and a mass of 785.8496 (2+) was used. Data analysis was accomplished using MassLynx software supplied by the manufacturer.
In vivo immuno-spin trapping studies
DMPO was administered at a dose of 2 g/kg, i.p., two hours after the final dose of Maneb and paraquat (MP) at the 6-week timepoint. Animals were sacrificed 2 hours after DMPO injection, and the brain was dissected to isolate the nigrostriatal region. Tissues were homogenized in phosphate buffer containing a protease inhibitor cocktail and centrifuged at 10,000 RPM at 4°C for 20 minutes. These samples were used for anti-DMPO ELISA [2].
Confocal microscopy of brain tissue
For confocal microscopy of brain tissue, mice were anesthetized and perfused transcardially. Perfused mice brains were coronally dissected, postfixed, and cryoprotected in sucrose. The frozen sections containing the substantia nigra region (20 micron) were cryocut using a frozen tissue processor (Leica Instruments, Bannockburn, IL, USA) at the immunohistochemistry core facility at NIEHS. Tissue slices were then permeabilised (0.2% Surfact-Amps X-100 in PBS) for 5 minutes and blocked overnight (2% BSA in PBS with 0.1% Surfact-Amps X-100). Afterwards, slides were incubated for 2 h with the primary anti-DMPO (1:2,000) and/or anti-alpha-synuclein (1:2,000) and anti-tyrosine hydroxylase antisera (1:4,000) at room temperature, followed by appropriate secondary Alexa Fluor antisera diluted 1:1,000 for an hour. Slides were washed four times, dried and mounted using Prolong Gold anti-fade reagent with DAPI. Confocal images were taken on a Zeiss LSM 510-UV meta microscope (Carl Zeiss Inc. Oberkochen, Germany) using a Plan-NeoFluar 40X/1.3 Oil DIC objective, in some cases with 3X zoom (Figure 4 A).
Figure 4.
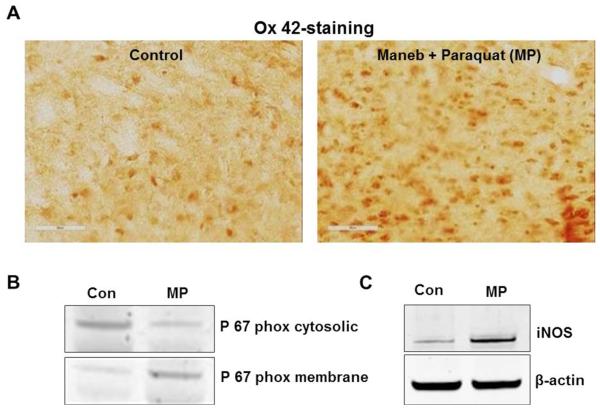
Maneb and paraquat (MP) induce active microgliosis in the midbrain of mice after 6 weeks of Maneb and paraquat coexposure. (A) Ox-42 staining in midbrain slices of sham, and Maneb and paraquat coexposed mice. (B) Western blot analysis of p67 phox in the cytosolic and membrane fraction of the nigrostriatal tissue of mouse brain following 6 weeks of Maneb and paraquat coexposure. (C) Western blot analysis of iNOS in the nigrostriatal tissue of mouse brain following 6 weeks of Maneb and paraquat coexposure. Data show mean values ± SEM from four independent experiments (n=4).
Immunoprecipitation of DMPO nitrone adduct and alpha-synuclein
Immunoprecipitation of DMPO nitrone adducts was carried out with the Pierce Classic Immunoprecipitation Kit with some modifications. Nigrostriatal homogenates were collected and protein concentrations measured with a Micro BCA Protein Assay Kit, and 1 mg of total protein was used for subsequent steps. The samples were precleared (1 h at room temperature) with 80 μl of control agarose slurry (50%). The homogenate was incubated overnight with 10 μg of normal mouse IgG (control) and 10 μg of rabbit polyclonal anti-alpha-synuclein antibody, and this antigen-antibody mixture was then incubated overnight to form the immune complex. This immune complex was incubated with protein A/G slurry for an hour with gentle end-over-end mixing. Samples were then eluted with sample-reducing buffer according to the manufacturer's instructions, followed by Western blotting with detection using anti-DMPO primary antibody.
Western blot analysis
Following separation by SDS-PAGE, proteins were transferred electrophoretically (15 volts for 40 minutes) to nitrocellulose membranes. Subsequently, membranes were blocked with 4% non-fat dry milk in 100 mM (bi)carbonate buffer, pH 9.6, for 2 hours. Membranes were washed once with washing buffer (0.05% Tween-20 in Tris-buffered saline, pH 7.4) and then incubated overnight with mouse monoclonal anti-DMPO antibody (1:250) / anti-alpha-synuclein antibody (1:3,000) / anti-iNOS antibody (1:2,000) / anti P-67 phox antibody (1:3,000) / anti-tyrosine hydroxylase antibody (1:4,000) / or anti-β –actin antibody (1:4,000). Then membranes were washed four times and incubated with appropriate fluorescent secondary antibody (1:10,000, diluted in washing buffer) labeled with infrared dyes for 2 h at room temperature in the dark. After incubation, traces of unbound antibody were eliminated by washing. An Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE) was used to acquire the images [2].
Enzyme-linked immunosorbent assay (ELISA)
Rabbit polyclonal anti-DMPO antibody was used to detect DMPO-protein-derived nitrone adducts using a standard ELISA as described previously [31]. In brief, brain homogenates (nigrostriatal region only) were immobilized by incubating reaction mixtures (2.5 mg/well) in 100 mM bicarbonate buffer, pH 9.6, for 90 min at 35°C. The plate was washed once and blocked (4% fish gelatin in PBS) for 90 min at 35°C. Following another wash, rabbit polyclonal anti-DMPO antibody (1:5,000) was added and incubated for 60 min. After incubation, plate wells were washed four times followed by incubation with anti-rabbit, IgG-alkaline phosphatase-conjugated secondary antibody (1:5,000) for 60 min at 35°C. After another four washes, CDP star (25 mM) was added and the chemiluminescent product was detected using a Tecan Spectra Fluor-Plus multifunction plate reader with Xfluor software (Tecan US, Research Triangle Park, NC, USA).
Immunohistochemistry
Integrin □M (ox-42) and TH-immunoreactivity were performed by the method described previously [13]. In brief, mice were anesthetized and the brain was perfused transcardially. Perfused mouse brains were coronally dissected, post-fixed, and cryo-protected in sucrose. Sections (20 μm thick) were cut serially using a cryostat, and the sections were then incubated with 0.5% H2O2 in methanol to block the endogenous peroxidase activity. The sections were incubated in blocking reagent (5% goat serum, 1% BSA, 0.1% Triton X-100 in PBS) for 2 h followed by incubation with rabbit polyclonal anti-TH antibody (1:4,000) and washed with PBS. The sections were then incubated with biotinylated anti-rabbit secondary antibody (1:300) for 2 h, washed and incubated with avidin-biotin complex for 90 min, and developed using DAB. The sections were mounted permanently with Permount, and images were captured with a brightfield microscope at 10× magnification. Coded slides were used to ensure unbiased counting of TH-positive neurons in every fourth serial section. The number of TH-positive neurons was counted bilaterally using a Metamorph image analysis tool. A minimum of four animals per group was used for counting TH-positive neurons. The results are expressed as percent of the control. Counting of TH-positive neurons was also performed in the VTA region of the controls and the experimental groups that received Maneb and paraquat and other pretreatments. The same method was used for integrin □M (ox-42) immuno-staining using monoclonal anti-integrin □M antibody (1:2,000).
Statistical analyses
All in vivo experiments were repeated three times with a minimum of four mice per group (n = 4; data from each group of four mice was pooled). One-way analysis of variance (ANOVA) was used for statistical analysis. The Newman–Keuls Post-Test was used for multiple comparisons. The results are expressed as mean ± SEM. The differences were considered statistically significant when p values were less than 0.05.
Results
DMPO detected in brain after intraperitoneal injections
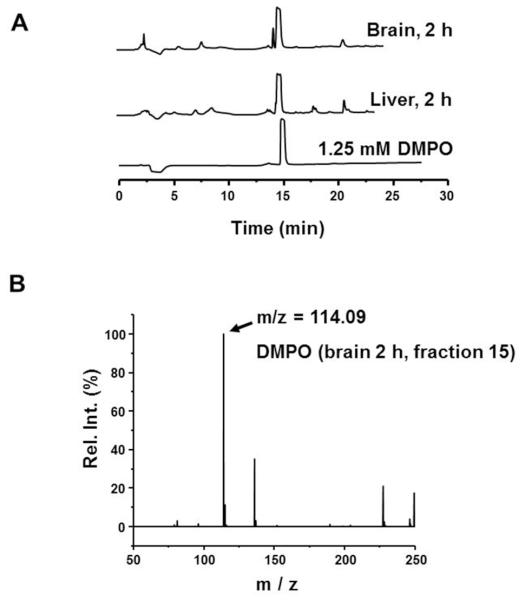
In order to trap protein radicals and facilitate their detection by immuno-spin trapping using the anti-DMPO antibody, DMPO must be shown to be present at the site of radical formation as a prerequisite. Typically, DMPO reacts with radicals on macromolecules such as proteins or DNA and forms a stable nitrone adduct, which can be detected by the anti-DMPO antibody [32]. In order to verify that DMPO can enter the brain after intraperitoneal injection, DMPO was injected intraperitoneally and the nigrostriatal tissue of the brain was analyzed for DMPO content using HPLC. Peaks resembling DMPO showed that DMPO reaches the brain after intraperitoneal injections (Figure 1A) at a concentration almost equal to that in the liver (used as a positive control). To further confirm that the peaks were those of DMPO, the fractions eluted at the retention time of DMPO (15 min) were collected and investigated with mass spectroscopy. MS and MS/MS experiments showed a peak with m/z of 114.09, as expected for DMPO (Figure 1B). Once the presence of DMPO in nigrostriatal tissue after intraperitoneal injections was confirmed, we further investigated protein radical formation in nigrostriatal tissue of mice coexposed to Maneb and paraquat.
Figure 1.
DMPO enters the brain after intraperitoneal injections. (A) HPLC chromatogram showing a peak corresponding to DMPO in brain and liver homogenates after 2 hours of DMPO injections (2g/kg, i.p.). (B) MS spectra showing peaks with m/z of 114.09, indicating the presence of DMPO in brain homogenates after intraperitoneal injections.
Maneb and Paraquat coexposure induces protein radical formation in the midbrain of exposed mice
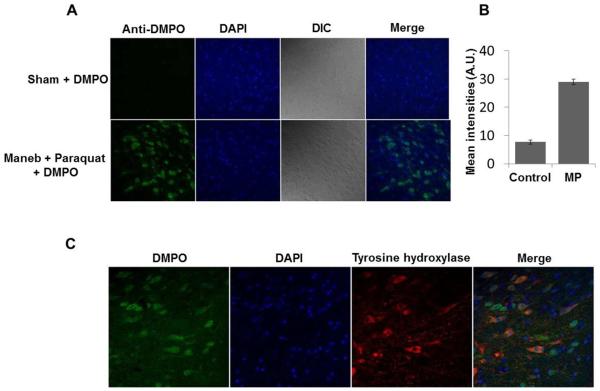
Immuno-spin trapping experiments with midbrain sections of Maneb and paraquat coexposed mice showed intense anti-DMPO staining. This result gives strong evidence of protein radical formation in the midbrain of MP coexposed mice (Figure 2A, B). Colocalization of tyrosine hydroxylase with anti-DMPO further indicated formation of protein radicals in dopaminergic neurons. Though the anti-DMPO signal was quite apparent in dopaminergic neurons, it was also present in adjacent cells, possibly microglia (Figure 2C). These results demonstrate the formation of protein radicals.
Figure 2.
Protein radical formation in the midbrain of mice after 6 weeks of Maneb (30mg/kg , i.p.) and paraquat (10mg/kg, i.p.) coexposure. (A) Confocal images showing the anti-DMPO staining in midbrain slices of Maneb and paraquat coexposed mice. (B) Fluorescence intensity quantification of anti-DMPO staining. (C) Confocal images showing the anti-DMPO staining in tyrosine hydroxylase-positive neurons and other adjacent cells.
Detection of alpha-synuclein protein-centered radicals in vivo by immuno-spin trapping
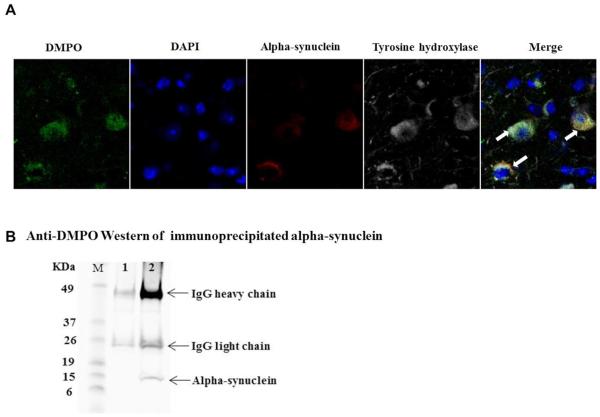
Once we confirmed the formation of radicals in the midbrain of Maneb and paraquat coexposed mice, we looked further for specific protein-centered radicals in dopaminergic neurons. Results with confocal microscopy (Figure 3A) showed colocalization of anti-DMPO, alpha-synuclein and tyrosine hydroxylase, which suggests radical formation on alpha-synuclein within dopaminergic neurons. To further confirm that the anti-DMPO signal was due to protein-centered radicals on alpha-synuclein, alpha-synuclein was immunoprecipitated and probed with anti-DMPO antibody. Proteins immunoprecipitated with normal mouse IgG (Lane 1, Figure 3B) did not show DMPO adducts. An anti-DMPO positive band on immunoprecipitated alpha-synuclein (Figure 3B, lane 2) further indicated that alpha-synuclein-centered protein radicals were formed in dopaminergic neurons of MP coexposed mice. Though Maneb and paraquat coexposure is known to trigger oxidative stress by complex 1 and/or complex 3 inhibition [5] and NADPH oxidase activation, a range of possibilities existed for how these protein radicals were formed. Because of a previous report that LPS-induced protein radical formation in BV2 microglial cells is peroxynitrite-mediated [2], subsequent experiments were designed to elucidate the underlying mechanism of peroxynitrite generation and protein radical formation in microglia and dopaminergic neurons of Maneb and paraquat coexposed mice.
Figure 3.
Detection of alpha-synuclein-centered radicals in dopaminergic neurons. (A) Confocal images of cryocut midbrain sections from sham + DMPO- and Maneb + Paraquat (MP) + DMPO-treated mice showing the colocalization of anti-DMPO staining with alpha-synuclein and tyrosine hydroxylase. (B) Immunoprecipitation of DMPO adducts. Proteins were immunoprecipitated from nigrostriatal tissue homogenates using either normal mouse IgG as a control (lane1) or with anti-alpha-synuclein antibody followed by Western blot analysis of immunoprecipitates using rabbit anti-DMPO antibody. Blots are representative of four different immunoblots from an equal number of experiments.
Protein radical formation and concurrent microglial activation
Coexposure to Maneb and paraquat induced active microgliosis, as evidenced by increased integrin □M (ox-42) immunoreactivity in the substantia nigra (Figure 4A). Maneb and paraquat coexposure triggered translocation of the p67 phox subunit of NADPH oxidase, which is considered to be the signature for its activation. An increase in p67 phox levels in the membrane fraction along with decreased cytosolic levels in Maneb and paraquat coexposed animals indicated that NADPH oxidase was activated in the substantia nigra of Maneb and paraquat coexposed animals (Figure 4B). Furthermore, Maneb and paraquat coexposure also induced iNOS (Figure 4C), which produces a significant amount of nitric oxide that can react with superoxide to form peroxynitrite. Being highly diffusible, peroxynitrite can easily diffuse into neurons in the immediate vicinity and may decompose to highly reactive radicals that can form protein-centered radicals.
Alpha-synuclein radical formed by a dual role of NADPH oxidase and inducible nitric oxide synthase in Maneb and paraquat coexposed mice is peroxynitrite-mediated
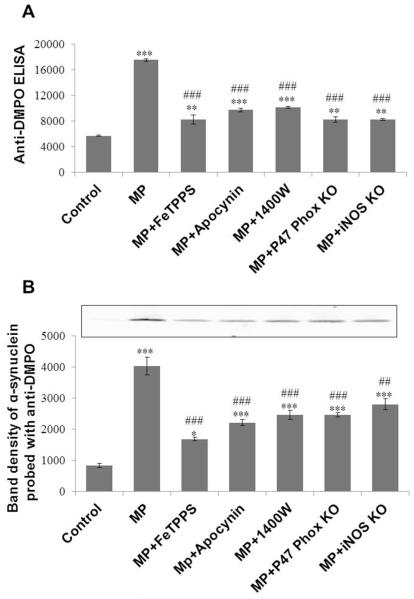
To investigate the possible mechanism of alpha-synuclein radical formation as well as protein radical formation in general, animals received several pretreatments in conjunction with Maneb and paraquat coexposure. Anti-DMPO ELISA showed increased protein radical formation in Maneb and paraquat coexposed animals, which was attenuated significantly in animals pretreated with a peroxynitrite decomposition catalyst (FeTPPS), an iNOS inhibitor (1400W), or an NADPH oxidase inhibitor (Apocynin) (Figure 5A). Furthermore, mice lacking p47-phox (NADPH oxidase) and iNOS were used to confirm their role in Maneb- and paraquat -induced protein radical formation in the nigrostriatal region of the brain. In Maneb and paraquat coexposed knockout mice (P47phox−/− and iNOS−/−), protein radical formation was significantly diminished compared to their wild-type littermates, as evident from anti-DMPO ELISA (Figure 5A). This result indicates the important roles of NADPH oxidase and iNOS in protein radical formation. Taken together, these results indicate the essential role of NADPH oxidase- and iNOS-mediated peroxynitrite in protein radical formation. To specifically quantify protein-centered radicals on alpha-synuclein, we immunoprecipitated alpha-synuclein from samples of all experimental groups. Band densitometry of individual bands obtained from samples of all experimental groups showed a consistent similarity with anti-DMPO ELISA (Figure 5B). This concurrence between the alpha-synuclein radical and total protein radical, which was significantly attenuated by iNOS/NADPH oxidase inhibition/ablation as well as by a peroxynitrite decomposition catalyst, clearly indicates that NADPH oxidase and iNOS play a crucial role in peroxynitrite-mediated protein radical formation in general.
Figure 5.
Effect of iNOS and NADPH oxidase inhibition/genetic ablation on Maneb- and paraquat-induced protein radical formation in the nigrostriatal tissue of mice after 6 weeks of Maneb and paraquat coexposure. (A) Anti-DMPO ELISA showing protein radical formation in Maneb and paraquat (MP) coexposed mice with or without pretreatment with FeTPPS (10mg/kg, i.p.), apocynin (10mg/kg, i.p.), or 1400W (15 mg/kg, i.p.), and in p47 phox or iNOS knockout mice. (B) Anti-DMPO Western blotting of immunoprecipitated alpha-synuclein from the nigrostriatal tissue of all experimental groups as in Figure A. Data show mean values ± SEM from four independent experiments (n=4). (*P<0.05, ** P<0.01, *** P<0.001, with respect to control, and ## P<0.01, ### P<0.001, with respect to the Maneb and paraquat coexposed group.)
TH immunoreactivity
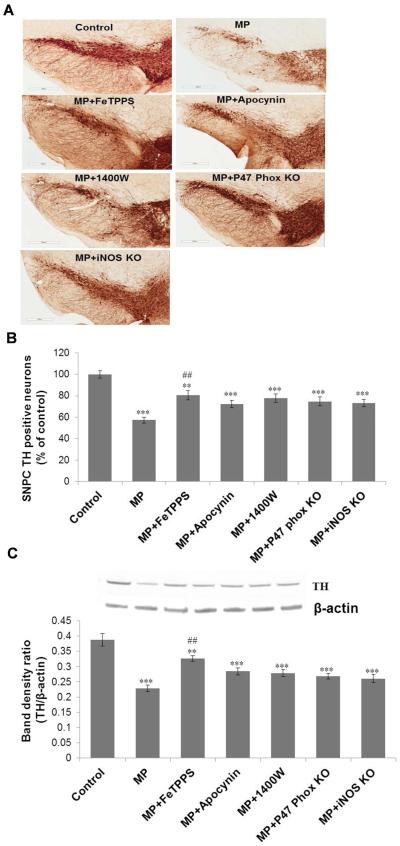
To investigate the possible link between protein radicals and dopaminergic neuronal death, tyrosine hydroxylase immunoreactivity was investigated. The significant reductions in tyrosine hydroxylase immuno-reactivity and the number of TH-positive neurons (Figure 6A-C) were observed in the substantia nigra region of the brains of exposed mice after 6 weeks of exposure. Pretreatment with apocynin, 1400W or FeTPPS conferred significant neuroprotection to Maneb and paraquat coexposed mice. As observed with protein radical formation, genetic ablation of p47 phox or iNOS conferred neuroprotection with diminished neuronal death induced by Maneb and paraquat coexposure (Figure 6). This concurrence between neuronal death and protein radical formation indicates a possible role of protein radicals in the neuronal death process.
Figure 6.
Tyrosine hydroxylase (TH) immunoreactivity in the substantia nigra of mouse brains following 6 weeks of Maneb and paraquat (MP) coexposure. (A) Upper panel shows TH immunoreactivity in frozen brain sections of control and treated animals with or without pretreatment with FeTPPS (10mg/kg, i.p.), apocynin (10mg/kg, i.p.), or 1400W (15 mg/kg, i.p.), and in p47 phox or iNOS knockout mice. (B) Lower panel shows the number of TH positive neurons in the substantia nigra pars compacta (SNPC) region of all experimental groups as in Figure A. (C) Western blot analysis of TH protein expression in the nigrostriatal tissue of mouse brains following 6 weeks of Maneb and paraquat coexposure in the presence and absence of pretreatments as indicated in the graph. The upper panel depicts a representative blot of TH protein; the lower panel depicts a densitometric analysis of the same with β-actin as the reference. The data are expressed as mean ± SEM (n=3–5). (** p<0.01, ***p<0.001 as compared with control and ## P<0.01, as compared with the Maneb and paraquat coexposed group.)
Discussion
There has been considerable progress in understanding the disease process of PD, which has led to effective symptomatic treatment of its motor symptoms, initially with levodopa (L-DOPA) and then later with dopamine receptor agonists and deep brain stimulation. Yet the efficacy of symptomatic therapy wanes after a few years, and patients develop worsened motor impairments and postural anomalies [33]. Most attention is currently focused on developing disease-modifying treatments. But slowing or halting the underlying neurodegenerative process required an even better understanding of the mechanism of disease pathophysiology, tested in environmentally relevant animal models such as the Maneb and Paraquat-induced PD phenotype in coexposed mice.
Although various models of Parkinson's disease as well as autopsy reports show alpha-synuclein aggregates forming in the substantia nigra, the role that protein free radicals play in their formation in vivo is unknown, as is the more general question of the role of protein radicals in the context of Parkinson's disease [34,24,35,2]. Knowing the central role of alpha-synuclein in PD pathogenesis, we have shown for the first time that alpha-synuclein radical is formed in the midbrain of Maneb and paraquat coexposed mice, which might be the key initial step that dictates its aggregation. This study also elucidates the roles of NADPH oxidase and iNOS, which play crucial roles in peroxynitrite-mediated protein radical formation in the dopaminergic neurons of Maneb and paraquat coexposed mice. The detection of protein radicals in dopaminergic neurons can be used to explore the possible implications of protein radicals, especially as the initiating mechanism for protein aggregation, which has implications in neurodegenerative diseases.
Aside from our report of protein radical formation in LPS-challenged BV2 cells [2], the only report showing involvement of protein radicals in a neuronal context shows mitochondrial protein radical adducts in an experimental model of amyotrophic lateral sclerosis [29]. With these two reports as proof of concept, the original goal of this study was to determine whether we could detect protein-radical formation in an animal model of PD to establish its involvement in the disease process. Since the presence of DMPO, a nitrone spin trap, is a prerequisite to detecting nitrone adducts using an anti-DMPO antibody, its entry into the brain after intraperitoneal injections was assessed with HPLC. Using known distributions of DMPO in different organs such as the liver, heart and blood after intraperitoneal injections [36] and its partition coefficient, which indicates its low lipophilicity [37], we measured its presence in nigrostriatal tissue after intraperitoneal injections. Despite its low lipophilicity, a detectible amount of DMPO enters the brain (Figure 1).
DMPO can permeate throughout the plasma membrane, enter into dopaminergic neurons and adjacent cells, bind to protein radicals and enable their detection by immuno-spin trapping as evidenced by an intense anti-DMPO signal in midbrain slices of Maneb and paraquat coexposed mice (Figure 2). The results obtained through this study not only confirm protein radical formation, but also identify for the first time a particular protein radical involved in PD pathogenesis (Figure 3A). A combinatorial approach using immuno-spin trapping in conjunction with immunoprecipitation allowed us to ascertain that alpha-synuclein was adducted to DMPO (Figure 3A) within the dopaminergic neurons of Maneb and paraquat coexposed mice. Parallel immunoprecipitation experiments using anti-alpha synuclein and anti-DMPO in conjunction with Western blotting showed a protein band that both immunoreacted to alpha-synuclein and co-immunoprecipitated with DMPO (Figure 3B).
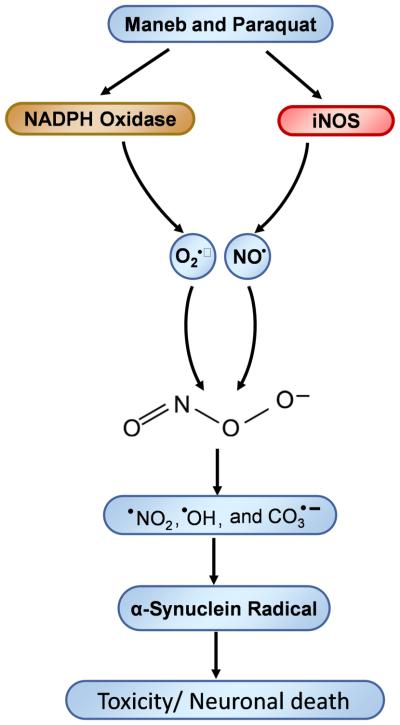
Whenever there is some disturbance in the brain’s microenvironment, microglia infiltrate that region and become activated, which is called microgliosis. Microgliosis involves NADPH oxidase activation and iNOS induction (Figure 4A-C), which leads to simultaneous production of superoxide anion and nitric oxide and, thereby, formation of peroxynitrite. Peroxynitrite, being a highly diffusible oxidant, diffuses through the plasma membrane and enters adjacent neurons [38]. Peroxynitrite can then decompose to form the reactive free radicals •OH, CO3•− and •NO2, which can induce protein radical formation within dopaminergic neurons as well as in adjacent cells (Scheme 1) [2]. Peroxynitrite can inhibit glutathione reductase and Cu, Zn and Mn superoxide dismutase enzymes [39,40], which may further favor peroxynitrite formation due to the sustained availability of superoxide in the ambient cellular environment. This sustained peroxynitrite production may lead to increased protein radical formation and accumulation of protein aggregates, which may initiate or accelerate the process of neurodegeneration.
Scheme 1.
Role of NADPH oxidase and iNOS in Maneb- and paraquat-induced peroxynitrite-mediated protein radical formation.
Inhibition or genetic ablation of NADPH oxidase/iNOS confers diminished total protein radical formation (Figure 5A) as well as the formation of the alpha-synuclein radical (Figure 5B). This decrease in alpha-synuclein radical formation when NADPH oxidase is inhibited or ablated concurs with reports that show that NADPH oxidase modulates alpha-synuclein aggregation in dopaminergic neurons [41]. Alpha-synuclein radical formation appears to be the initiating mechanism, which dictates its aggregation where NADPH oxidase and iNOS play crucial roles. These results, along with diminished protein radical formation in the presence of the peroxynitrite decomposition catalyst FeTPPS, demonstrate that protein radical formation is peroxynitrite-mediated and depends on both NADPH oxidase and iNOS. Experimental evidence from human and animal studies supports the theory that oxidative stress contributes to the pathogenesis of PD [42]. Tyrosine hydroxylase inactivation by peroxynitrite-mediated tyrosine nitration followed by impaired dopamine biosynthesis in PC12 cells indicates the role of post translational protein modification in PD [43]. This prompted us to investigate a possible link between peroxynitrite-mediated protein radical formation and neuronal death through toxic aggregation of proteins like alpha-synuclein.
The loss of TH positive neurons and overall decrease in TH protein levels, which actually indicates dopaminergic neuronal death in the Maneb and paraquat coexposed model of PD (Figure 6A-C), is in accordance with several reports so far [12,44]. Additionally, pretreatment of Maneb and paraquat coexposed animals with the peroxynitrite decomposition catalyst FeTPPS or the inhibition/ablation of NADPH oxidase/iNOS conferred neuroprotection, further supporting our interpretation that peroxynitrite-mediated protein radical formation, which triggers protein aggregation, plays a causal role in neuronal death. The crucial implications of peroxynitrite-mediated protein radical formation in this study are supported by a study of methamphetamine-induced toxicity in which dopaminergic neurotoxicity was prevented by targeting peroxynitrite using a peroxynitrite decomposition catalyst [45].
In summary, this study provides evidence that NADPH oxidase and iNOS play roles in peroxynitrite mediated alpha-synuclein radical formation, which might be the initiating mechanism for its aggregation. The detection of protein radicals and understanding the underlying mechanism of protein radical formation can be used to explore the possible implications of protein radicals specifically as the initiating mechanism for protein aggregation, which has implications for neurodegenerative diseases.
Acknowledgments
The authors gratefully acknowledge Dr. Ann Motten and Mary Mason for their valuable help in the preparation of the manuscript. We are very thankful to Dr. Thomas van‘t Erve and Dr. Birandra Sinha for reviewing the manuscript.
Grant Support: This work has been supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences.
Abbreviations
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- MP
Maneb and paraquat
- O2•−
superoxide
- TH
tyrosine hydroxylase
- NO•
nitric oxide
- ONOO−
peroxynitrite
Footnotes
Compliance with ethical standards: Authors declare compliance with ethical standards.
Conflict of interest - The authors declare no conflict of interest.
Animal uses - Experiments with animals were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals, and the animal study proposal was approved by the institutional review board.
Human samples - No human samples were used.
References
- 1.Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML. Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain. 2010;133:808–821. doi: 10.1093/brain/awp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, Chen SH, Kadiiska MB, Hong JS, Zielonka J, Kalyanaraman B, Mason RP. Inducible nitric oxide synthase is key to peroxynitrite-mediated, LPS-induced protein radical formation in murine microglial BV2 cells. Free Radic Biol Med. 2014;73:51–59. doi: 10.1016/j.freeradbiomed.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan SD, Dolatabadi N, Chan SF, Zhang X, Akhtar MW, Parker J, Soldner F, Sunico CR, Nagar S, Talantova M, Lee B, Lopez K, Nutter A, Shan B, Molokanova E, Zhang Y, Han X, Nakamura T, Masliah E, Yates JR, 3rd, Nakanishi N, Andreyev AY, Okamoto S, Jaenisch R, Ambasudhan R, Lipton SA. Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1alpha transcription. Cell. 2013;155:1351–1364. doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AK, Tiwari MN, Upadhyay G, Patel DK, Singh D, Prakash O, Singh MP. Long term exposure to cypermethrin induces nigrostriatal dopaminergic neurodegeneration in adult rats: postnatal exposure enhances the susceptibility during adulthood. Neurobiol Aging. 2012;33:404–415. doi: 10.1016/j.neurobiolaging.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 7.Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med. 1998;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 8.Kontakos N, Stokes J. Monograph series on aging-related diseases: XII. Parkinson's disease-recent developments and new directions. Chronic Dis Can. 1999;20:58–76. [PubMed] [Google Scholar]
- 9.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson's disease: a metaanalysis. Environ Res. 2001;86:122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 10.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson's disease risk from ambient exposure to pesticides. Eur J Epidemiol. 2011;26:547–555. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kachroo A, Irizarry MC, Schwarzschild MA. Caffeine protects against combined paraquat and maneb-induced dopaminergic neuron degeneration. Exp Neurol. 2010;223:657–661. doi: 10.1016/j.expneurol.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Singh BK, Ahmad I, Shukla S, Patel DK, Srivastava G, Kumar V, Pandey HP, Singh C. Involvement of NADPH oxidase and glutathione in zinc-induced dopaminergic neurodegeneration in rats: similarity with paraquat neurotoxicity. Brain Res. 2012;1438:48–64. doi: 10.1016/j.brainres.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson's disease: what have we learned from pesticide-induced animal models? Trends Pharmacol Sci. 2009;30:475–483. doi: 10.1016/j.tips.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 17.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 18.Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann Neurol. 1998;44(3 Suppl 1):S110–114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- 19.Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;70:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 20.Forno LS. Concentric hyalin intraneuronal inclusions of Lewy type in the brains of elderly persons (50 incidental cases): relationship to parkinsonism. J Am Geriatr Soc. 1969;17:557–575. doi: 10.1111/j.1532-5415.1969.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 21.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 22.Daturpalli S, Waudby CA, Meehan S, Jackson SE. Hsp90 inhibits alpha-synuclein aggregation by interacting with soluble oligomers. J Mol Biol. 2013;425:4614–4628. doi: 10.1016/j.jmb.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L. SIRT1 protects against alpha-synuclein aggregation by activating molecular chaperones. J Neurosci. 2012;32:124–132. doi: 10.1523/JNEUROSCI.3442-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med. 2013;5:344–352. doi: 10.1002/emmm.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gundersen V. Protein aggregation in Parkinson's disease. Acta Neurol Scand Suppl. 2010;190:82–87. doi: 10.1111/j.1600-0404.2010.01382.x. [DOI] [PubMed] [Google Scholar]
- 28.Franco MC, Ye Y, Refakis CA, Feldman JL, Stokes AL, Basso M, Melero Fernandez de Mera RM, Sparrow NA, Calingasan NY, Kiaei M, Rhoads TW, Ma TC, Grumet M, Barnes S, Beal MF, Beckman JS, Mehl R, Estevez AG. Nitration of Hsp90 induces cell death. Proc Natl Acad Sci U S A. 2013;110:E1102–1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radi R. Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res. 2013;46:550–559. doi: 10.1021/ar300234c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camilleri A, Vassallo N. The Centrality of Mitochondria in the Pathogenesis and Treatment of Parkinson's Disease. CNS Neurosci Ther. 2014;20:591–602. doi: 10.1111/cns.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Ganini D, Deterding LJ, Ehrenshaft M, Chatterjee S, Mason RP. Immuno-spin trapping of heme-induced protein radicals: Implications for heme oxygenase-1 induction and heme degradation. Free Radic Biol Med. 2013;61C:265–272. doi: 10.1016/j.freeradbiomed.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic Biol Med. 2004;36:1214–1223. doi: 10.1016/j.freeradbiomed.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 33.Beal MF. Experimental models of Parkinson's disease. Nat Rev Neurosci. 2001;2:325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- 34.Fu RH, Liu SP, Huang SJ, Chen HJ, Chen PR, Lin YH, Ho YC, Chang WL, Tsai CH, Shyu WC, Lin SZ. Aberrant alternative splicing events in Parkinson's disease. Cell Transplant. 2013;22:653–661. doi: 10.3727/096368912X655154. [DOI] [PubMed] [Google Scholar]
- 35.Oueslati A, Schneider BL, Aebischer P, Lashuel HA. Polo-like kinase 2 regulates selective autophagic alpha-synuclein clearance and suppresses its toxicity in vivo. Proc Natl Acad Sci U S A. 2013;110:E3945–3954. doi: 10.1073/pnas.1309991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu KJ, Jiang JJ, Ji LL, Shi XL, Schwartz HM. An HPLC and EPR investigation on the stability of DMPO and DMPO spin adducts in vivo. Res Chem Intermediat. 1996;22:499–509. [Google Scholar]
- 37.Turner MJ, 3rd, Rosen GM. Spin trapping of superoxide and hydroxyl radicals with substituted pyrroline 1-oxides. J Med Chem. 1986;29:2439–2444. doi: 10.1021/jm00162a004. [DOI] [PubMed] [Google Scholar]
- 38.Xie Z, Wei M, Morgan TE, Fabrizio P, Han D, Finch CE, Longo VD. Peroxynitrite mediates neurotoxicity of amyloid beta-peptide1-42- and lipopolysaccharide-activated microglia. J Neurosci. 2002;22:3484–3492. doi: 10.1523/JNEUROSCI.22-09-03484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez B, Demicheli V, Duran R, Trujillo M, Cervenansky C, Freeman BA, Radi R. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med. 2004;37:813–822. doi: 10.1016/j.freeradbiomed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Cristovao AC, Guhathakurta S, Bok E, Je G, Yoo SD, Choi DH, Kim YS. NADPH oxidase 1 mediates alpha-synucleinopathy in Parkinson's disease. J Neurosci. 2012;32:14465–14477. doi: 10.1523/JNEUROSCI.2246-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 43.Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc Natl Acad Sci U S A. 1998;95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singhal NK, Srivastava G, Patel DK, Jain SK, Singh MP. Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson's disease phenotype in the mouse. J Pineal Res. 2011;50:97–109. doi: 10.1111/j.1600-079X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- 45.Imam SZ, Islam F, Itzhak Y, Slikker W, Jr., Ali SF. Prevention of dopaminergic neurotoxicity by targeting nitric oxide and peroxynitrite: implications for the prevention of methamphetamine-induced neurotoxic damage. Ann N Y Acad Sci. 2000;914:157–171. doi: 10.1111/j.1749-6632.2000.tb05193.x. [DOI] [PubMed] [Google Scholar]