Abstract
Autoreactive B lymphocytes that commonly arise in the developing repertoire can be salvaged by receptor editing, a central tolerance mechanism that alters BCR specificity through continued L chain rearrangement. It is unknown whether autoantigens with weak cross-linking potential, such as insulin, elicit receptor editing, or if this process is dysregulated in related autoimmunity. To resolve these issues, an editing-competent model was developed in which anti-insulin Vκ125 was targeted to the Igκ locus and paired with anti-insulin VH125Tg. Physiologic, circulating insulin increased RAG-2 expression and was associated with BCR replacement that eliminated autoantigen recognition in a proportion of developing anti-insulin B lymphocytes. The proportion of anti-insulin B cells that underwent receptor editing was reduced in the type 1 diabetes-prone NOD strain relative to a non-autoimmune strain. Resistance to editing was associated with increased surface IgM expression on immature (but not transitional or mature) anti-insulin B cells in the NOD strain. The actions of mAb123 on central tolerance were also investigated, as selective targeting of insulin-occupied BCR by mAb123 eliminates anti-insulin B lymphocytes and prevents type 1 diabetes. Autoantigen-targeting by mAb123 increased RAG-2 expression and dramatically enhanced BCR replacement in newly developed B lymphocytes. Administering F(ab’)2123 induced IgM downregulation and reduced the frequency of anti-insulin B lymphocytes within the polyclonal repertoire of VH125Tg/NOD mice, suggesting enhanced central tolerance by direct BCR interaction. These findings indicate that weak or faulty checkpoints for central tolerance can be overcome by autoantigen-specific immunomodulatory therapy.
Keywords: B cells, Autoimmunity, Diabetes, Tolerance/Suppression/Anergy, Transgenic/Knockout mice
Introduction
Discrimination between foreign and self antigens by the immune system is critically important to maintaining protective immunity while limiting autoimmune disease. 55–75% of newly formed B cells in humans are estimated to be autoreactive, whereas the mature repertoire is markedly depleted of autoreactive specificities (1). Several immune tolerance mechanisms act in concert to limit this liability in developing B lymphocytes, including anergy, receptor editing, and deletion. Anergy compromises the functional status of autoreactive B cells, whereas deletion eliminates them from the repertoire (2–4). However, the major mechanism of central tolerance is receptor editing, by which the B cell receptor (BCR) specificity is changed through novel L chain rearrangement (5–8). Faulty central tolerance has been linked with autoimmune diseases such as type 1 diabetes, systemic lupus erythematosus, and rheumatoid arthritis (9–12). Understanding how central tolerance is maintained or lost for specific autoantigens will provide insight into ways to limit the participation of such B cells in autoimmune disease.
Immunization of non-autoimmune mice with heterologous insulin frequently induces insulin autoantibodies (13), as does therapeutic administration of insulin for the treatment of human diabetes (14–17). Insulin autoantibody detected in the prodrome of type 1 predicts diabetes in both patients and NOD mice (18–20), revealing that breaches in immune tolerance ignite disease. The hormone, insulin thus exemplifies an autoantigen that escapes tolerance in both normal and autoimmune states. The use of anti-insulin Ig transgenes has enabled the study of rare anti-insulin B lymphocytes that are inaccessible even in autoimmune NOD mice (21). These models include the H chain only VH125Tg model and the H chain plus L chain 125Tg model that produce B cell repertoires in which 1–2% or > 95% of all B cells bind insulin, respectively (22,23). Studies in these models show that anti-insulin B cells first encounter insulin in the bone marrow (24), and naïve immature B cells mobilize calcium in response to insulin stimulation of the BCR (25).
Immature B cells undergo receptor editing in response to recognition of multivalent antigens such as membrane-bound hen egg lysozyme (26,27), MHC class I (6,28), DNA (7,29), type IV collagen (30), and myelin oligodendrocyte glycoprotein (31), and in response to soluble HEL exposure (26,27), for which the BCR has a high affinity (5 × 1010 M−1) (32). Insulin differs from these antigens in many ways. Circulating insulin is a small protein present at very low concentrations (up to 1–5 ng/mL post-prandially), it is primarily monomeric in circulation and should not appreciably cross-link BCR, and the affinity of the 125Tg BCR interaction with rodent insulin is modest (8 × 106 M−1) (33–35). The 125Tg model is thus uniquely suited to study low intensity BCR interactions that are more representative of a developing B cell that has not yet undergone affinity maturation typically associated with higher affinity interactions. Whether insulin can elicit receptor editing, either in the context of health or autoimmunity, is not known. Accordingly, anti-insulin Vκ125 was targeted to the Igκ locus to generate mice in which Vκ125 is competent to undergo replacement. This model was crossed with VH125Tg mice to provide a novel model (VH125Tg/Vκ125SD) to study receptor editing-mediated L chain replacement that abrogates insulin recognition by the BCR.
In the NOD model, mice which lack insulin-binding B cells but retain a broad non-insulin reactive B cell repertoire are protected from type 1 diabetes, while disease develops in mice that harbor 1–2 % anti-insulin B cells, highlighting the importance of insulin recognition by B lymphocytes in promoting disease (22). Thus, altering the emergence of anti-insulin B cells into the repertoire has the potential to prevent adverse reactions to therapeutic insulins and to ameliorate the immunopathological process that causes T1D. Anti-insulin B cells whose BCR are occupied by circulating insulin can be bound by the anti-insulin Ab, mAb123, which recognizes a separate insulin epitope (21,23,24,35). Treatment with mAb123 eliminates anti-insulin B lymphocytes in vivo and prevents type 1 diabetes in NOD mice while preserving the broad, non-insulin-binding B cell repertoire (21). In addition to the potential for Fc recognition, mAb123 has the additional predicted functionality of altering BCR surface expression and signaling to reinforce central tolerance (21,23). We therefore hypothesized that mAb123 could act on the BCR to enhance receptor editing as a means to eliminate the anti-insulin B cell specificity from the repertoire.
In this study, we investigate the potential for insulin-reactive B cells to undergo central tolerance by receptor editing. These experiments demonstrate that BCR recognition of soluble insulin at physiologic levels is competent to induce editing of BCR with modest affinity. Physiologic insulin stimulates increased RAG-2 in anti-insulin B cells, a small proportion of which successfully edit the BCR to a non-insulin-binding specificity. Further, anti-insulin B lymphocytes are observed to undergo receptor editing less efficiently in NOD mice. The proportion of anti-insulin B cells that undergo receptor editing is increased following administration of mAb123 or F(ab’)2123 that recognize insulin-occupied BCR. Overall, these findings show that receptor editing less efficiently culls insulin autoreactivity in type 1 diabetes-prone NOD mice. This defect can be overcome by autoantigen-targeted therapy that reinforces this critical central tolerance checkpoint, thus reducing entry of a pernicious specificity into the mature repertoire.
Materials and Methods
Animals
VH125Tg/NOD (22) and VH125Tg/Vκ125SDNeo (36) mice were described previously. EIIA-Cre C57BL/6 (B6) mice (kindly provided by Dr. Richard Breyer, Vanderbilt University, Nashville, TN) were intercrossed with Vκ125SDNeo B6 mice to remove the loxP-flanked NeoR cassette to generate Vκ125SD mice (37). A probe provided by Dr. Roberta Pelanda, University of Colorado, Denver, CO (38) was used in Southern blot to detect the following alleles: endogenous (5.5 kb), Vκ125SDNeo (6.3 kb), and Vκ125SDNeo (5.1 kb) (Fig. 1). Vκ125SDNeo and Vκ125SD mice were also backcrossed onto the NOD background at least 8 generations. Spontaneous disease was routinely observed in the VH125Tg/Vκ125SD/NOD and VH125Tg/Vκ125SDNeo/NOD colonies (unpublished observations). Routine genotyping was performed using the primers FWD #444 5’-TATGATCGGAATTCCTCGAGTCTAGAGCGG-3’ and REV #88 5’-GCTCCAGCTTGGTCCCAGCA-3’).
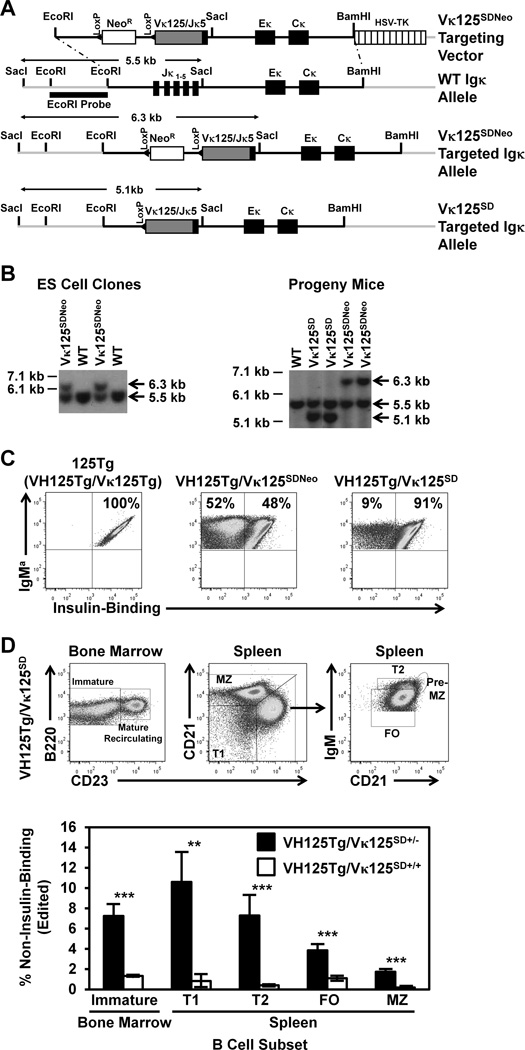
Figure 1. A proportion of anti-insulin B cells lose insulin-binding specificity in the presence of endogenous insulin in the Igκ transgenic model, VH125Tg/Vκ125SD.
(A) Targeting vector schematic, showing WT (non-targeted), targeted Vκ125SDNeo (NeoR gene retained), or targeted Vκ125SD (NeoR gene removed) alleles. (B) ES cell clones (Left) or progeny mouse tail DNA (Right) were digested with SacI and the following alleles were identified by probe hybridization: WT (5.5 kb), Vκ125SDNeo (6.3 kb), or Vκ125SD (5.1 kb, NeoR gene removed). (C) Splenocytes were freshly isolated from VH125Tg/Vκ125Tg (125Tg, Left), VH125Tg/Vκ125SDNeo (Middle), and VH125Tg/Vκ125SD (Right) C57BL/6 mice. Insulin-binding B cells were identified among B220+ IgMa+ live lymphocytes using flow cytometry, confirming expression of anti-insulin Vκ125. Data are representative of progeny from 6 independent founder lines. (D) B cell subsets were identified among B220+ live lymphocytes as follows: bone marrow: immature (IgM+ CD23−) or mature recirculating (IgM+ CD23+); spleen: T1 (CD21low CD23low IgMhigh), T2 (CD21mid CD23high IgMhigh), FO (CD21mid CD23high IgMmid), Pre-MZ (CD21high CD23high IgMhigh), and MZ (CD21high CD23mid IgMhigh), as shown by representative flow cytometry dot plots of VH125Tg/Vκ125SD mice (Top). Flow cytometry was used to assess the average percentage ± SD of non-insulin-binding (edited) B cells within the indicated B cell subset in n = 3 VH125Tg/Vκ125SD+/− (black) or n = 4 VH125Tg/Vκ125SD+/+ (white) B6 mice (Bottom). ** p < 0.001, *** p < 0.001, two-tailed t-test.
NG bacterial artificial chromosome (BAC) RAG2-GFP reporter mice (39,40) were provided on a B6 background by Dr. Rachel Gerstein (University of Massachusetts Medical School, Worchester, MA) and were intercrossed with VH125Tg/Vκ125SD/B6 or VH281Tg/Vκ125SD/B6 to generate VH125Tg/Vκ125SD/RAG2-GFP/B6 or control VH281Tg/Vκ125SD/RAG2-GFP/B6. All mice were hemizygous for transgenes unless otherwise indicated. All NOD mice were confirmed to be non-diabetic (blood glucose < 200 mg/dL) at the time of the experiment. Both male and female mice were examined. All mice were housed under specific pathogen-free conditions, and all studies were approved by the institutional use and animal care committee of Vanderbilt University, fully accredited by the AAALAC.
Cell isolation, flow cytometry, and antibodies
Bone marrow was eluted from long bones with HBSS (Invitrogen Life Technologies) + 10% FBS (HyClone). Spleens or pancreatic draining lymph nodes were macerated with HBSS + 10% FBS. Bone marrow and splenic RBCs were lysed with Tris-NH4Cl. Cells were subsequently stained for flow cytometry analysis, including 7-aminoactinomycin D (7-AAD) and Ab reagents reactive with CD21 (7G6), CD23 (B3B4), CD43 (S7), B220 (6B2), Igκ (187.1), Igλ (R26-46), IgMa (DS-1), IgMb (AF6-78) (BD Biosciences or eBioscience), or IgM (µ chain specific, Invitrogen). Human insulin (Sigma-Aldrich) was biotinylated (36) and was used to detect insulin-binding specificity. Avidin-fluorochrome conjugates (BD Biosciences) were used to detect biotinylated reagents. A BD Biosciences LSR II flow cytometer was used for sample acquisition. Data were analyzed using FlowJo software (Tree Star, Inc.).
Full-length anti-insulin mAb123 IgG1 (HB-123, ATCC) or isotype control IgG1 (CRL-2395, ATCC) antibodies were purified from hybridoma cell lines by the Vanderbilt Antibody and Protein Resource. mAb123 F(ab’)2 fragments [F(ab’)2123] were generated using the Mouse IgG1 Fc and F(ab’)2 Preparation Kit (Thermo Fisher Scientific) per the manufacturer’s instructions and exchanged into sterile 1X PBS. Non-denaturing SDS-PAGE gel electrophoresis was used to confirm purity of the F(ab’)2 products and absence of full-length Ab. All full-length and F(ab’)2 antibody fragments were injected i.p. in sterile 1X PBS pH 7.
Vκ/Jκ usage identification
Freshly isolated splenocytes were prepared as above and B cells were negatively sorted by magnetic activate d cell sorting (MACS) using CD43 beads (Miltenyi). Total B lymphocyte populations (B220+ IgMa+ live non-doublet lymphocytes) were isolated using flow cytometry sorting. In some experiments, B cells were sorted into insulin-binding or non-insulin-binding populations based on staining with biotinylated insulin. RNA was purified from >1000 cells sorted into lysis buffer using the RNAqueous-Micro Total RNA Isolation Kit (Life Technologies). cDNA was prepared and Igκ were amplified using the following primers: Forward: 5’-ATTGTKMTSACMCARTCTCCA-3’ and Reverse: 5’-CAGATGTTAACTGCTCACTGGATG-3’. PCR product was ligated into PGEM-T Easy vector (Promega), plasmids were transformed into DH5α E. coli, and DNA isolated from positive colonies was sequenced, and identified using IgBLAST (http://www.ncbi.nlm.nih.gov/igblast/). All kits were used according to the manufacturer’s instructions.
Results
Generation of an Igκ-targeted model to study receptor editing in anti-insulin B cells
Receptor editing is known to mediate central tolerance for BCR that bind multivalent Ag and for BCR engineered from high affinity mAb (6,7,26–31,41); it is less clear how this form of tolerance applies to BCR that exhibit weaker interactions with cognate Ag. To assess whether insulin-binding B cells undergo receptor editing in response to physiologic insulin, an Igκ-targeted anti-insulin Vκ125 BCR transgenic mouse model was generated as outlined in Methods. As shown in Fig. 1A, Vκ125/Jκ5 (identical to the L chain coding region of 125Tg mice) was cloned into a targeting vector used to replace the Jκ1-5 segments (38). This strategy eliminates the potential for other Vκ to productively rearrange on the targeted allele, but rearrangements can occur on the other endogenous (non-targeted) Igκ allele or in the Igλ locus. Southern blot of embryonic stem cell clone DNA shows the expected endogenous (5.5 kb) and Vκ125SDNeo (6.3 kb) alleles (Fig. 1B, left). Vκ125SDNeo mice were intercrossed with EIIA-Cre transgenic mice that express Cre recombinase during very early fetal development to eliminate the NeoR gene (37). Southern blot of tail DNA from progeny mice detected the endogenous (5.5 kb), Vκ125SDNeo (6.3 kb), or NeoR-deleted Vκ125SD (5.1 kb) alleles in the indicated mice (Fig. 1B, right).
To assess functionality of the targeted alleles, Vκ125SDNeo or Vκ125SD mice were intercrossed with VH125Tg mice to generate VH125Tg/Vκ125SDNeo and VH125Tg/Vκ125SD mice on the non-autoimmune B6 background. Flow cytometry was used to assess expression of IgMa and insulin reactivity in freshly isolated spleen B cells. The average frequency of IgMa+ cells amongst B cells (B220+ CD19+ live lymphocytes) in the spleen of VH125Tg/Vκ125SD mice was 98 ± 0.3 %. Fig. 1C shows that nearly all B cells recognize insulin in 125Tg (VH125Tg + Vκ125Tg) B6 mice that harbor non-targeted Ig transgenes as expected. In contrast, ~ 50 % of B cells lose insulin-binding specificity in VH125Tg/Vκ125SDNeo/B6 mice. This frequency is reduced to < 10% following NeoR removal in VH125Tg/Vκ125SD/B6 mice. These data confirm expression of the targeted anti-insulin L chain and indicate that retention of the NeoR gene significantly enhances replacement of the insulin-reactive BCR. Accordingly, all subsequent studies were performed with VH125Tg/Vκ125SD. An overall average of 5.2 ± 1.0 % non-insulin-binding B cells was observed, suggesting that encounter with physiologic insulin may drive L chain replacement in a proportion of VH125Tg/Vκ125SD B cells.
To determine if the loss of insulin reactivity observed in VH125Tg/Vκ125SD B cells is suppressed by increasing the barrier to receptor editing, the frequency of non-insulin-binding B cells in VH125Tg/Vκ125SD+/− mice was compared to VH125Tg/Vκ125SD+/+ mice, in which replacement of homozygous Vκ125SD is restricted to novel L chain rearrangement at the Igλ locus. The frequency of non-insulin-binding B cells was reduced in all B cell subsets (identified as in Fig. 1D, top panel) in VH125Tg/Vκ125SD+/+/B6 mice (Fig. 1D, bottom panel). Of note, and consistent with observations in the original 125Tg model (23), B1a (IgM+ CD5+) and B1b (IgM+ CD5− CD11b+ CD43+) B cell differentiation is largely impaired in VH125Tg/Vκ125SD/B6 mice as compared to WT/B6 mice (Supplemental Fig. 1A and data not shown).
Correlates of receptor editing are observed in anti-insulin B cells that encounter physiologic insulin in the bone marrow
Insulin is an essential hormone, without which uncontrolled blood glucose levels lead to metabolic stress and death, thus complicating control experiments to study receptor editing in the absence of insulin. To circumvent this issue, control VH281Tg/Vκ125Tg B cells can be studied, which harbor a nearly identical H chain that lacks 2 aa in CDR2 necessary for insulin binding (42). When combined with Vκ125SD, these two H chain transgenes provide a means to track mechanisms related to receptor editing in insulin binding B cells, in B cells that have lost insulin binding, and in “control” B cells that do not bind insulin. Representative flow cytometry plots in Figure 2A confirm non-insulin-binding (left panel) and insulin-binding (middle panel) specificities provided by these H chains when paired with Vκ125SD. As expected based on previous studies (23,24), a second anti-insulin Ab, mAb123, confirms insulin occupied BCR on immature B cells in VH125Tg/Vκ125SD/B6 mice (Fig. 2A, Right).
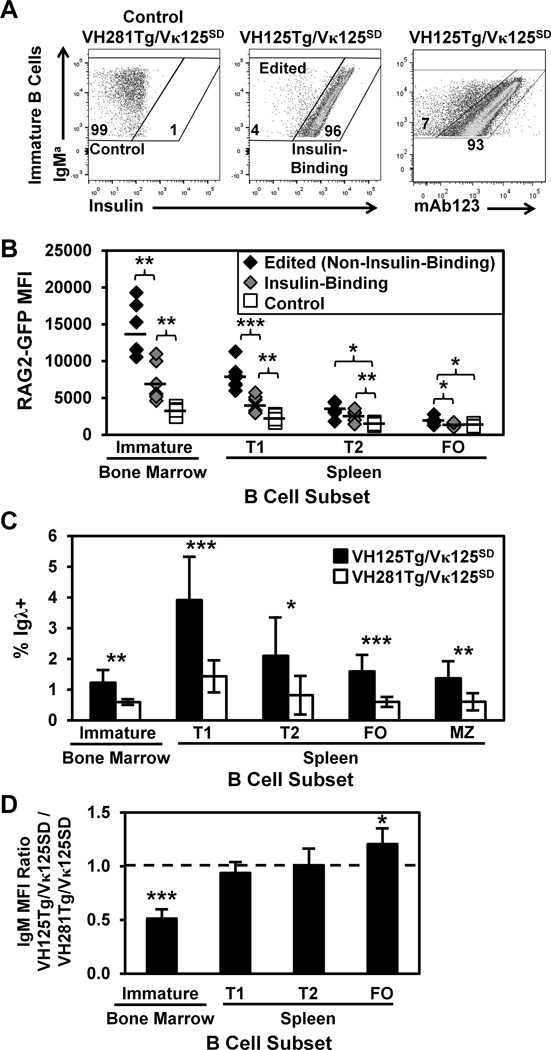
Figure 2. Anti-insulin specificity correlates with increased RAG2-GFP expression in developing B lymphocytes.
B cell subsets were identified in freshly isolated bone marrow and spleen as in Fig. 1D. (A) Representative flow cytometry plots of immature B cells depict Control (non-insulin-binding, Left), Edited (non-insulin-binding, Middle), or Insulin-Binding (Middle) B cells, based on reactivity with biotinylated insulin. Biotinylated mAb123 detects insulin-occupied BCR on immature VH125Tg/Vκ125SD B cells using flow cytometry (Right). (B) Immature, T1, T2, or FO B cell subsets were identified in n ≥ 6 VH125Tg/Vκ125SD/RAG2-GFP/B6 or control VH281Tg/Vκ125SD/RAG2-GFP/B6 5–12 wk old mice, in which GFP expression is driven by the RAG-2 promoter. RAG2-GFP MFI is shown for Edited/Non-Insulin-Binding (black diamonds), Insulin-Binding (grey diamonds), or control (white squares) populations identified as in Panel A that were GFP+. Averages are indicated by bars. (C) The average percentage ± SD of Igλ+ cells in the indicated B cell subset was compared between n ≥ 7 VH125Tg/Vκ125SD/B6 (black) and control VH281Tg/Vκ125SD/B6 (white) mice. (D) Flow cytometry was used to measure surface IgM expression on the indicated B cell subsets in n ≥ 7 mice per group. The IgM MFI of VH125Tg/Vκ125SD cells was divided by the IgM MFI of VH281Tg/Vκ125SD B cells within the developmental subset indicated. A value < 1 indicates lower IgM expression in VH125Tg/Vκ125SD B cells; the average ratio ± SD is shown. * p < 0.05, ** p < 0.001, *** p < 0.001, two-tailed t-test (B–C) or one sample, two-tailed t test (D).
Increased RAG expression is observed in B cells driven to undergo receptor editing (6,28,43,44). To determine if BCR occupancy by physiologic insulin enhances RAG expression in VH125Tg/Vκ125SD/B6 mice, the NG BAC RAG2-GFP reporter mouse model was employed, in which the RAG-2 promoter drives green fluorescent protein (GFP) expression from a BAC (39,40). Of note, GFP is not tightly regulated through degradation targeting as RAG is; as such, GFP persists longer than RAG-2 (39). However, as GFP expression indicates recent RAG-2 expression (39), this model was intercrossed to generate anti-insulin VH125Tg/Vκ125SD/RAG2-GFP/B6 mice or control, non-insulin-binding VH281Tg/Vκ125SD/RAG2-GFP/B6 mice. Figure 2B shows that RAG2-GFP MFI is elevated in both insulin-binding and edited (non-insulin-binding) RAG2-GFP+ immature, transitional 1 (T1), and transitional 2 (T2) B cells in VH125Tg/Vκ125SD/RAG2-GFP/B6 mice compared to the same subsets in control, non-insulin-binding VH281Tg/Vκ125SD/RAG2-GFP/B6 mice. Edited (non-insulin-binding) immature B cells show the highest levels of RAG2-GFP expression (4-fold increase versus control immature B cells). No difference in the percentage of RAG2-GFP+ B cells was observed between the groups examined within a given developmental subset (not shown). This is consistent with data in multiple other systems that show RAG expression in early development even when successful H and L chain rearrangements are present, suggesting that even non-autoreactive BCR transgenic B cells are likely to report RAG2-GFP (45,46). To further assess the occurrence of receptor editing in B cells with differential insulin-binding specificity, the frequency of Igλ+ B cells was assessed in the indicated subsets (Panel C). The percentage of Igλ+ B cells was significantly increased in all B cell subsets of VH125Tg/Vκ125SD mice compared to control VH281Tg/Vκ125SD mice (Fig. 2C). These data show that anti-insulin B cells exposed to physiologic insulin in vivo express increased RAG-2 and increase novel rearrangements at the Igλ locus.
Reduced surface expression of IgM results in loss of basal BCR signaling which subsequently drives receptor editing in immature B cells (44,47). To test whether insulin occupancy was associated with reduced IgM expression in immature B cells exposed to physiologic insulin, mAb123 was used to identify insulin-occupied BCR in freshly isolated bone marrow. Insulin recognition by immature bone marrow B cells was associated with 2-fold decreased surface IgM expression compared to surface IgM on control, non-insulin-binding B cells isolated from VH281Tg/Vκ125SD mice (Fig. 2D). In contrast, reduced IgM expression was not observed in transitional or mature anti-insulin B cells in the spleen (Fig. 2D). These data are consistent with previous in vitro studies that show insulin downregulates surface IgM expression on immature anti-insulin B cells (25) and indicate that insulin encounter by immature cognate B cells initiates a process associated with receptor editing.
Novel Vκ/Jκ rearrangement is enhanced by B cell recognition of circulating insulin and correlates with loss of anti-insulin specificity in VH125Tg/Vκ125SD B cells
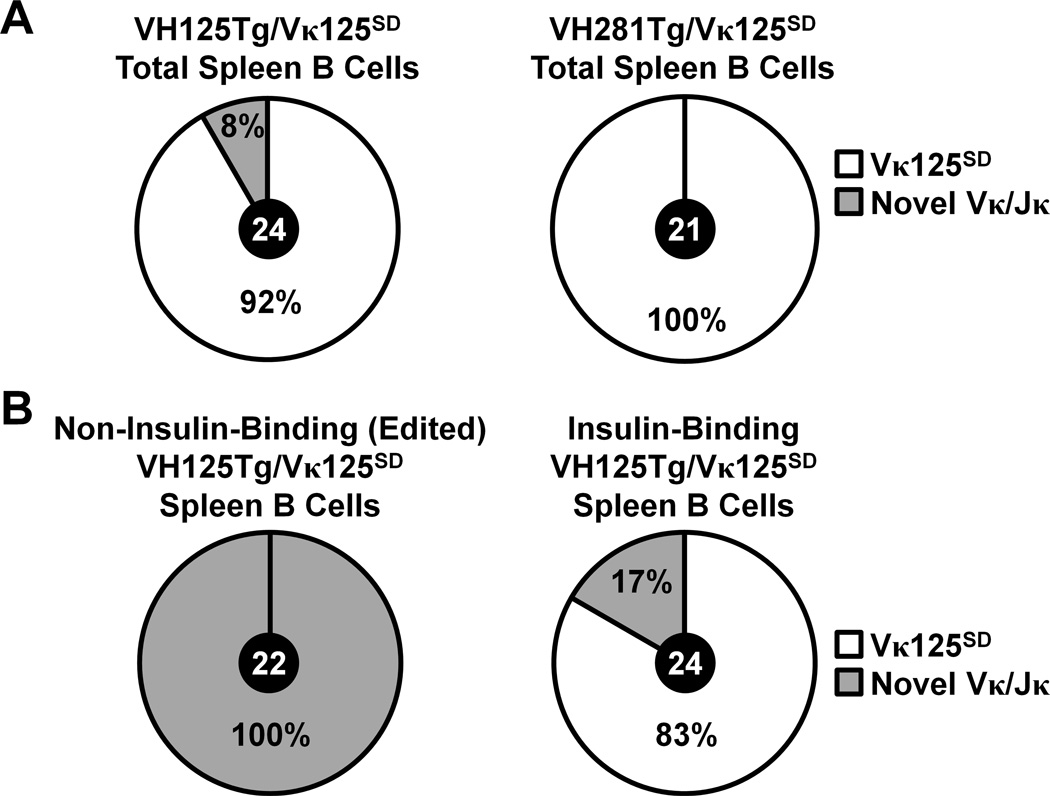
To directly test whether circulating insulin exposure leads to increased receptor editing in cognate B cells, Vκ/Jκ usage was compared between splenic B lymphocytes flow cytometry purified from anti-insulin VH125Tg/Vκ125SD or control VH281Tg/Vκ125SD mice as in Methods. Although a wide array of L chains has been observed to pair successfully with VH125Tg (48–50), only two (Vκ4-74 and Vκ4-57-1) have been confirmed to produce an insulin-specific BCR in B6 or NOD mice (24). As shown in Fig. 3A (left), 8 % (2/24) of isolates replaced the targeted allele in VH125Tg/Vκ125SD mice, comparable to the percentage of non-insulin-binding B cells observed by flow cytometry (Fig. 1C). In contrast, 100 % (21/21) of isolates retained the targeted allele (a Vκ4-74/Jκ5 distinguished by a nt change in CDR2, GenBank accession #M34530 http://www.ncbi.nlm.nih.gov/genbank) in control VH281Tg/Vκ125SD mice (Fig. 3A, right). To further confirm that the targeted Vκ125SD allele was replaced in non-insulin-binding B cells in VH125Tg/Vκ125SD mice, this population was flow cytometry purified from the spleen and Igκ usage was assessed. 100% (22/22) of isolates from non-insulin-binding B cells expressed novel Vκ/Jκ, consistent with Vκ125SD replacement (Fig. 3B, Left). In contrast, the large majority (83%, 20/24) of isolates from insulin-binding B cells expressed the targeted Vκ125SD (Vκ4-74/Jκ5), as expected (Fig. 3B, Right). These data demonstrate that non-insulin-binding specificity correlates with lost expression of the targeted allele, consistent with receptor editing in response to circulating insulin.
Figure 3. Insulin autoantigen recognition is associated with replacement of Vκ125SD in VH125Tg/Vκ125SD B lymphocytes that lose the insulin-binding specificity.
Flow cytometry sorting was used to purify B cell populations from freshly isolated spleens. Cells were sorted into RNA lysis buffer, RNA was purified, cDNA was generated, and PCR was used to amplify Igκ as in Methods. The number of clones (black circle, center) and percentage of isolates that expressed the targeted Vκ125SD allele (Vκ4-74 Jκ5, white) or Novel Vκ/Jκ (grey) is indicated for each sorted population. (A) Total B cells (B220+ IgMa+ live lymphocytes) were purified from n = 2 VH125Tg/Vκ125SD/B6 (Left, GenBank Accession #KT250658- KT250681) or n = 2 VH281Tg/Vκ125SD/B6 (Right, GenBank Accession # KT250637-KT250657) mice. (B) Non-insulin-binding (Left, GenBank Accession # KT250682-KT250703) or insulin-binding (Right, GenBank Accession # KT250704-KT250727) total B cells (gated as in Panel A) were purified from n = 3 VH125Tg/Vκ125SD/B6 mice.
Anti-insulin B cells undergo receptor editing less efficiently in the autoimmune NOD strain
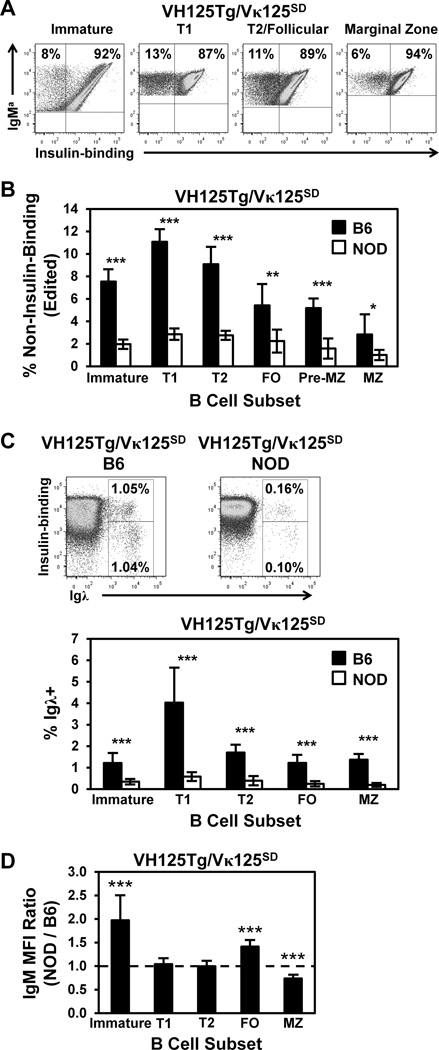
An increased frequency of anti-insulin B cells exit the bone marrow to populate mature B cell niches in VH125Tg/NOD mice relative to non-autoimmune VH125Tg/B6 mice (24). To directly test whether receptor editing is defective in the NOD strain for a relevant beta cell autoantigen, the frequency of non-insulin-binding B cells was compared in VH125Tg/Vκ125SD/B6 and VH125Tg/Vκ125SD/NOD mice. Representative flow cytometry plots in Fig. 4A show the frequency of non-insulin-binding B cells in subsets from B6 mice. A significantly reduced frequency of edited B cells was observed in all B cell subsets in VH125Tg/Vκ125SD/NOD mice compared to the B6 strain (Fig. 4B). The frequency of Igλ+ B cells was also examined in VH125Tg/Vκ125SD/NOD and B6 mice and was further used to detect allelic inclusion, another product of receptor editing (51,52). Representative flow cytometry plots (Fig. 4C, upper panels) show that Igλ+ B cells that lose or retain (allelic includer) insulin-binding specificity are significantly reduced in the NOD strain, a finding that is consistent across all subsets (Fig. 4C, bottom, and not shown). All NOD mice in these experiments were confirmed to have normal blood glucose levels (120 ± 19 mg/dL), thus indicating the findings are not due to insulin deficiency. Surface IgM expression was also compared in B6 versus NOD immature B cells as a possible explanation for the impaired receptor editing efficiency observed. As shown in Fig. 4D, immature VH125Tg/Vκ125SD B cells in NOD mice express 2-fold increased surface IgM (p < 0.001), a difference that is lost by transitional stages in the spleen. These data highlight a relative failure to downregulate surface IgM by immature, autoreactive B cells in NOD mice in response to circulating insulin encounter in the bone marrow.
Figure 4. Anti-insulin B cells undergo receptor editing less efficiently in the type 1 diabetes-prone NOD strain.
B cell subsets were identified using flow cytometry as in Fig. 1D in VH125Tg/Vκ125SD bone marrow or splenocytes freshly isolated from 8 –12 week old B6 (black) or NOD (white) mice. (A–B) The percentage of non-insulin-binding (edited) B cells was assessed within the indicated subset using flow cytometry Representative dot plots (A) and the average ± SD (B) is shown for n = 6 B6 or n = 7 NOD VH125Tg/Vκ125SD mice, n ≥ 2 experiments. (C) The percentage of Igλ+ B cells were further identified in total spleen as shown in representative plots (Top). The average total Igλ+ % ± SD in each subset is indicated for n ≥ 8 mice, n = 3 experiments (Bottom). (D) The IgM MFI of VH125Tg/Vκ125SD/NOD B cells was divided by the IgM MFI of VH125Tg/Vκ125SD/B6 B cells within the developmental subset indicated, n = 10 mice, n= 4 experiments. A value > 1 indicates higher IgM expression in NOD. * p < 0.05, ** p < 0.001, *** p < 0.001, two-tailed t-test (B–C), or one sample, two-tailed t test (D).
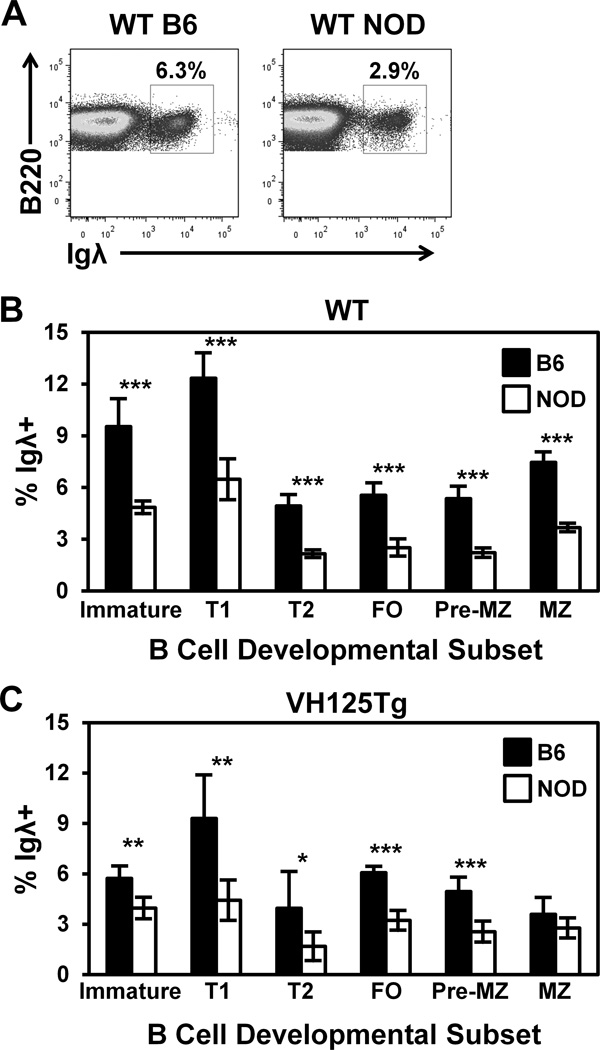
Igλ usage is reduced in NOD mice that express a polyclonal repertoire
Increased Igλ expression correlates with increased receptor editing (28,43,53). To test whether receptor editing is also impaired in NOD mice that rearrange H and L chains physiologically, the frequency of Igλ+ B cells was assessed during B cell development. The percentage of Igλ+ B cells was reduced in all subsets examined in WT/NOD mice compared to WT/B6 mice (Fig. 5A–B). NOD mice show biased VH gene usage (54), which may alter the extent of receptor editing by changing productive L chain pairing efficiency. To eliminate this bias, the VH125Tg model was used in which anti-insulin VH125Tg pairs with endogenous L chains. The frequency of Igλ+ B cells was reduced in both developing and mature B cell subsets in VH125Tg/NOD versus VH125Tg/B6 mice (Fig. 5C). Taken together, these data are consistent with receptor editing occurring less efficiently in NOD B cells that express polyclonal H and L chain repertoires, even when differences in H chain usage bias are eliminated.
Figure 5. Igλ usage is reduced in NOD mice, even in the absence of H chain usage bias.
B cell subsets were identified in freshly isolated bone marrow or spleen using flow cytometry as in Fig. 1D and the percentage of Igλ+ B cells was assessed in NOD or B6 mice, representative plots are shown in (A). The average Igλ % ± SD is shown for WT (B) or VH125Tg (C) B6 (black) or NOD (white) mice, n ≥ 6 8–12 week old mice per group, n ≥ 2 experiments. * p < 0.05, ** p < 0.001, *** p < 0.001, two-tailed t-test.
Therapeutic targeting of insulin-occupied BCR overrides defective central tolerance for anti-insulin B lymphocytes in type 1 diabetes-prone mice
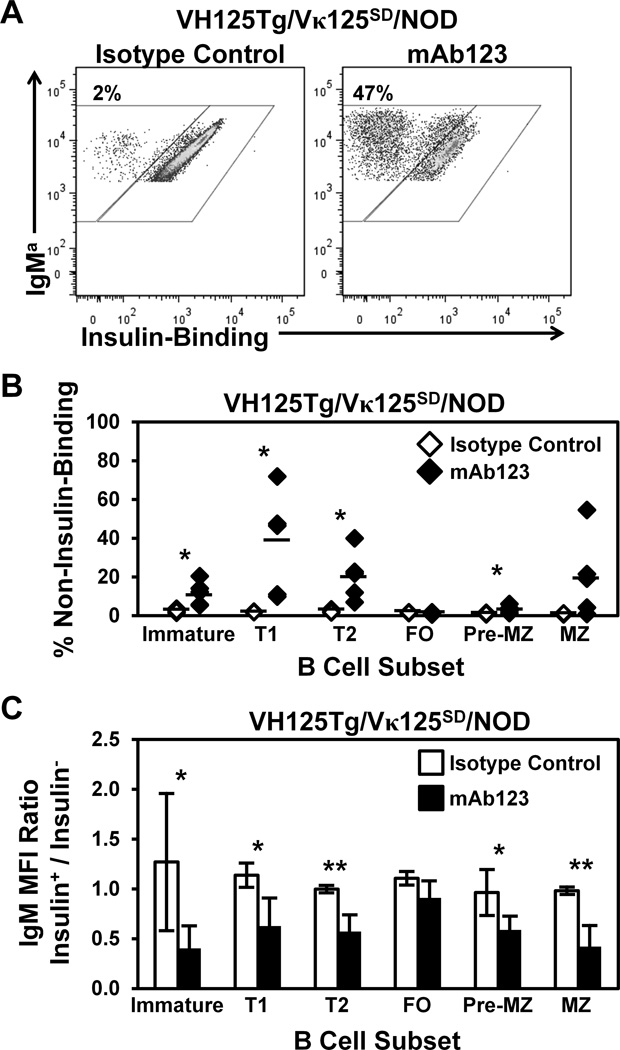
Anti-insulin mAb123 binds insulin-occupied BCR, selectively eliminates anti-insulin B cells, and prevents disease in NOD mice (21). We hypothesized that mAb123 treatment may alter BCR signaling in immature B cells to enhance central tolerance efficiency as one mechanism for the selective elimination of anti-insulin B cells. Receptor editing-driven changes in anti-insulin B cell frequency following therapy should be most rapidly apparent in the transitional B cell stages due to increased cell turnover in these compartments (55,56). VH125Tg/Vκ125SD/NOD mice were therefore administered a single dose of mAb123 or isotype control mAb and the frequency of non-insulin-binding B cells was compared in bone marrow and spleen B cell subsets after 2–7 d. Administration of isotype control mAb did not alter the basal frequency of non-insulin-binding B cells, as shown by representative dot plots (Fig. 6A, T1 B cells shown) and summarized in Fig. 6B. In contrast, mAb123 treatment leads to a dramatic increase in the proportion of non-insulin-binding B cells. The percentage of non-insulin-binding B cells was not substantially altered in follicular (FO) or pre-marginal zone (pre-MZ) subsets; although there was a trend towards an increase in non-insulin-binding B cells in the marginal zone (MZ), it was not statistically significant. A trend towards an increased number of non-insulin-binding B cells was observed in the following subsets: Immature (2-fold), T1 (5-fold), T2 (8-fold), pre-MZ (7-fold), and MZ (2-fold), and a concomitant decreased trend in the number of insulin-binding B cells was observed in the following subsets: Immature (2-fold), T1 (3-fold), and MZ (3-fold) (Supplemental Table I). These data show that a single dose of mAb123 increases the frequency of non-insulin-binding B cells in transitional B cell subsets, which may reflect enhanced receptor editing in immature, anti-insulin B cells.
Figure 6. Faulty central tolerance in NOD mice is ameliorated by treatment with insulin autoantigen-targeted mAb123.
6–13 week old VH125Tg/Vκ125SD/NOD mice were injected i.p. once with 100 µg anti-insulin mAb123 (black, n = 5) or isotype control mAb (white, n = 4). Bone marrow and spleens were harvested 2–7 d later and B cell subsets were identified as in Fig. 1D, further gated as non-insulin-binding or insulin-binding B cells. (A) Representative flow cytometry plots for isotype control mAb (Left) or mAb123 (Right). (B) Individual mice are plotted, bars indicate the average. (C) The IgM MFI of insulin-binding (Insulin+) B cells was divided by the IgM MFI of non-insulin-binding (Insulin−) B cells in the same mouse. A ratio < 1 indicates reduced surface IgM expression in the insulin-binding population (relative to edited B cells). Bars indicate the average Insulin+ / Insulin− IgM ratio ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001, two tailed t test.
Decreased IgM expression is associated with receptor editing (44,47). To identify whether mAb123 may act by a similar mechanism, the ratio of IgM expression on insulin-binding vs. non-insulin-binding B cells following treatment with mAb123 was compared. IgM expression was decreased on insulin-binding B cells in the immature, T1, and T2 developing B cell compartments following mAb123 treatment (Fig. 6C). IgM reduction was also observed on insulin-binding B cells in pre-MZ, MZ, but not FO B cell subsets. Previous studies suggest that receptor editing is not induced in mature B cells (39); thus the reduction in surface IgM seen in the mature subsets following mAb123 reflects actions on B cells that cannot activate the receptor editing mechanism. Anergy is a peripheral tolerance mechanism that is also associated with decreased surface IgM (2,23,57), and may thus account for the reduced IgM expression observed in mature subsets. IgM downregulation in immature and transitional B cells in conjunction with the increased frequency of non-insulin-binding B cells suggests that central tolerance is reinforced by mAb123 treatment.
RAG-2 expression and the frequency of non-insulin-binding B cells are enhanced in developing anti-insulin B cells by mAb123 treatment
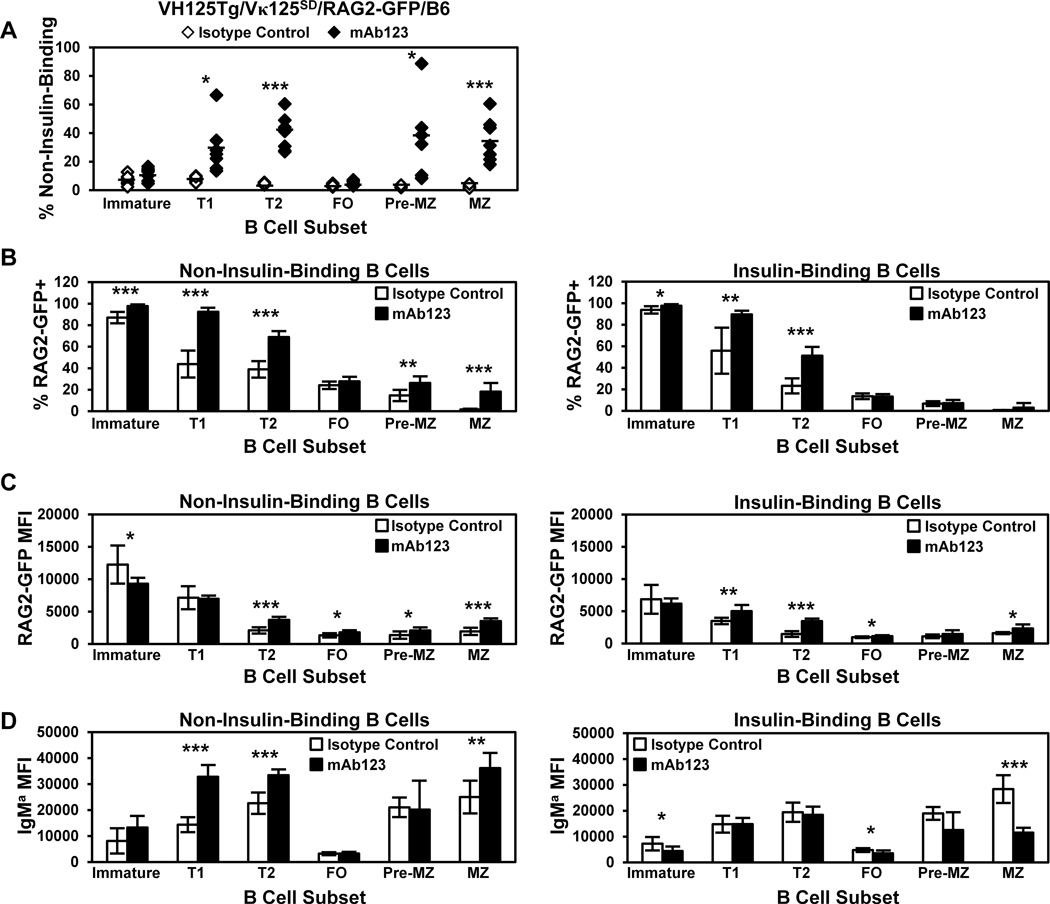
To further implicate receptor editing enhancement by mAb123 treatment, RAG-2 expression was assessed in anti-insulin B cells using RAG-2 GFP reporter mice (39,40), as anergy, deletion, or antibody-dependent cell-mediated cytotoxicity (ADCC) should not cause RAG-2 upregulation. VH125Tg/Vκ125SD/RAG2-GFP/B6 mice were administered mAb123 or isotype control mAb, and the frequency of edited (non-insulin-binding) B cells was assessed in B cell developmental subsets. As shown in Fig. 7A, a significantly increased percentage of non-insulin-binding B cells is observed in mAb123 vs. isotype control-treated mice in the T1 and T2 subsets. The percentage of non-insulin-binding B cells was also increased in the pre-MZ and MZ, but not FO subsets. A significant increase in the number of non-insulin-binding B cells was observed in Immature 17-fold) and T1 (6-fold) compartments, with an increased trend observed in the T2 subset (2-fold), and no increase was observed in mature subsets, as expected given the short duration of treatment (Supplemental Table II). A significant decrease in the number of insulin-binding B cells was observed in the T2 (6-fold), pre-MZ (5-fold), and MZ 19-fold) subsets, with a non-significant 2-fold decrease in the number of insulin-binding FO B cells. The data demonstrate the preferential targeting of short-term mAb123 therapy on the transitional rather than the follicular B cell compartment and indicates reduced exit of anti-insulin B cells from the bone marrow, rather than peripheral elimination as the explanation for the observed loss of anti-insulin B cells. The MZ B cell findings are consistent with the previous observation that this subset shows enhanced sensitivity to mAb123 depletion in VH125Tg/NOD mice (21).
Figure 7. Targeting insulin-occupied BCR increases RAG2-GFP expression and the frequency of non-insulin-binding B cells in developing subsets.
VH125Tg/Vκ125SD/RAG2-GFP/B6 7–17 week old mice were injected i.p. with 100 µg anti-insulin mAb123 (black, n = 7) or isotype control mAb (white, n = 6) i.p. every 2 days for 1 week. Bone marrow and spleens were freshly isolated 1 d after the final injection and B cell subsets were identified as in Fig. 1D. (A) The frequency of non-insulin-binding B cells in individual mice is plotted, bars indicate the average. (B–D) The average percentage of RAG2-GFP+ cells (B), the average RAG2-GFP MFI of RAG2-GFP+ cells (C), or the IgM MFI (D) of non-insulin-binding B cells (Left) or insulin-binding (Right) in each subset is shown, error bars indicate SD. * p < 0.05, ** p < 0.01, *** p < 0.001, two tailed t test.
The impact of mAb123 treatment on the frequency of RAG2-GFP+ B cells and the RAG2-GFP MFI were also investigated. The percentage of RAG2-GFP+ cells is increased in the T1 and T2 B cell stages in both non-insulin-binding B cells and in those that retain the insulin-binding specificity (Fig. 7B). RAG2-GFP expression was increased following mAb123 treatment in T1 and T2 insulin-binding B cells, as well as in T2 non-insulin-binding B cells (Fig. 7C). Surprisingly, the percentages of non-insulin-binding B cells in the pre-MZ and MZ subset that are RAG2-GFP+ are also increased. As shown in Fig. 7D, mAb123 treatment increased IgM expression in T1, T2, and MZ non-insulin-binding B cells. B cells that retained the insulin-binding specificity show decreased IgM expression in immature, FO, and MZ subsets following mAb123 treatment. These data show that mAb123 treatment increases the frequency of non-insulin-binding B cells, coincident with an increased percentage of RAG2-GFP+ cells and increased RAG2-GFP expression. Treatment with mAb123 downregulates IgM expression in insulin-specific B cells, particularly mature B cells. In contrast, IgM expression is increased in T1 and T2 B cells that have undergone receptor editing. The observed increases in RAG-GFP expression and the frequency of non-insulin-binding B cells in transitional subsets are consistent with mAb123 reinforcement of central tolerance through receptor editing.
mAb123 engagement of insulin-occupied BCR eliminates anti-insulin B lymphocytes independently of Fc recognition
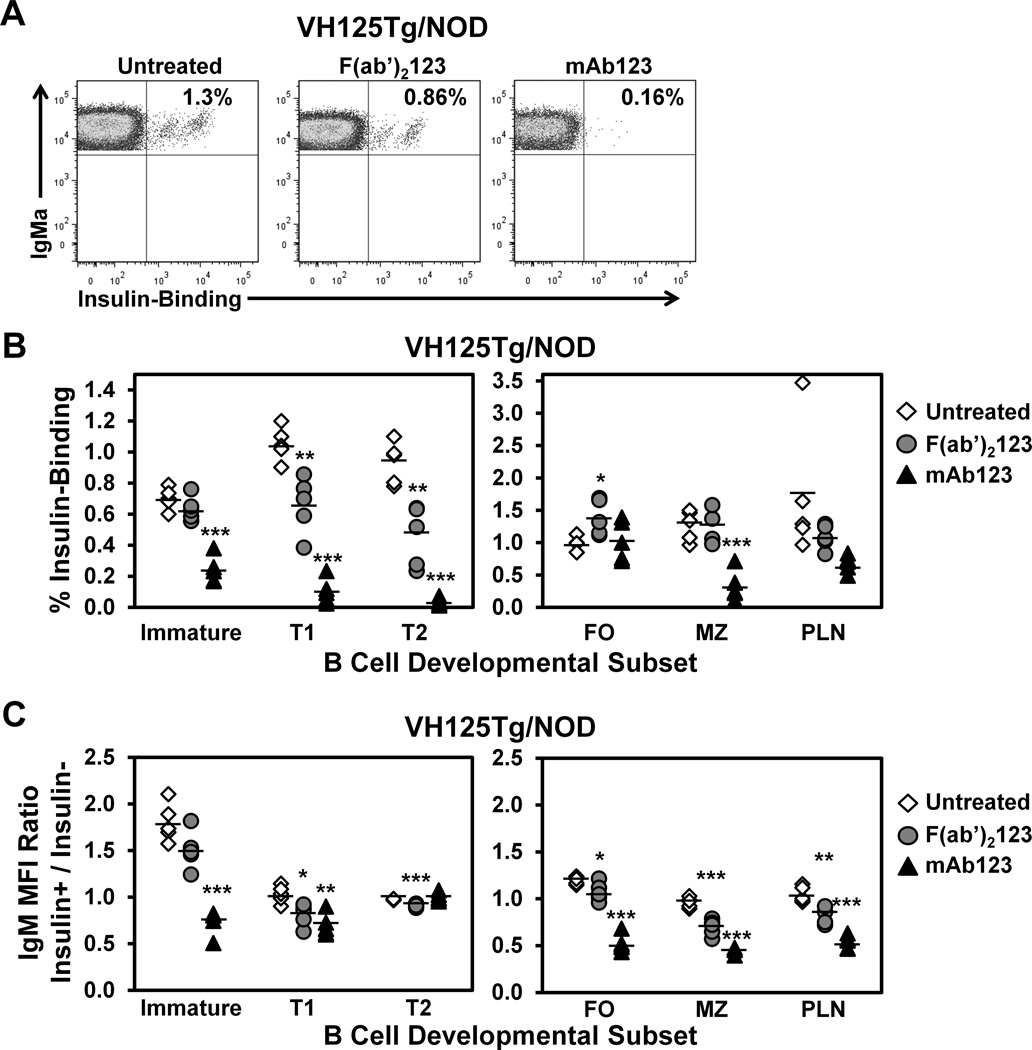
To dissect the contribution of BCR signaling vs. Fc recognition to anti-insulin B cell elimination by mAb123, VH125Tg/NOD mice were treated once with F(ab’)2123 fragments or full-length mAb123, and the percentage of insulin-binding B cells was assessed in B cell subsets the following day by flow cytometry. Representative dot plots in Fig. 8A show the percentage of anti-insulin B cells in the T1 B cell compartment in untreated mice, or following a single treatment with F(ab’)2123 or full-length mAb123. The results summarized in Fig. 8B indicate that F(ab)2123 treatment significantly decreases insulin-binding B cells in T1 and T2 subsets compared to untreated mice. In contrast, F(ab’)2123 treatment did not decrease the percentage of insulin-binding B cells in mature B cell subsets (FO, MZ, and pancreatic draining lymph node). As B cells turn over more rapidly in the transitional compartments, and given the short time frame post-treatment (1 d), these results are consistent with an immune tolerance mechanism that is elicited in immature B cells, such as receptor editing. A 2-fold reduction in the number of insulin-binding B cells was also observed in the T2 subset, though it was not statistically significant (Supplemental Table III). Full-length mAb123 elicits stronger anti-insulin B cell depletion in both developing and mature B cell subsets (Fig. 8B and Supplemental Table III), presumably due to the additional function of Fc recognition.
Figure 8. F(ab’)2123 depletes developing anti-insulin B cells.
VH125Tg/NOD mice were injected once i.p. with 75 µg F(ab’)2123 or 100 µg full-length mAb123 (to achieve ~ molar equivalence). After 1–2 d, mice were sacrificed and bone marrow, spleen, and pancreatic draining lymph nodes were freshly harvested. (A) Representative flow cytometry plots depict the frequency of insulin-binding B cells within the T1 B cell subset in untreated, F(ab’)2123-treated, or mAb123-treated mice. (B) The percentages of insulin-binding B cells in each subset (identified as in Fig. 1D) are plotted for n = 5 mice per group; untreated (white diamonds), F(ab’)2123- treated (grey circles), or mAb123-treated (black triangles), n = 3 experiments. (C) Flow cytometry was used to compare the IgM MFI of insulin-binding (Insulin+) vs. non-insulin-binding (Insulin−) B cells in subsets identified in (A). The insulin+ / insulin− ratio was calculated as in Fig. 6C. The average ratio ± SD is shown for untreated (white diamonds), F(ab’)2123-treated (grey circles), or mAb123-treated (black triangles) mice. Mice were 6–10 wk of age.
The relative surface expression of IgM was compared between insulin-binding B cells or non-insulin-binding B cells in the specified B cell subset in mice treated with F(ab’)2123 or mAb123 (Fig. 8C). F(ab’)2123 treatment reduces relative IgM expression in insulin-binding B cells in the T1 and T2 subsets, with a trend towards a reduction in the immature subset. Interestingly, IgM downregulation was also seen among FO, MZ, and pancreatic draining lymph node B cells following F(ab’)2123 treatment. These results support a role for both BCR-dependent and Fc-recognition dependent mechanisms in the elimination of autoreactive B cells by autoantigen-targeted therapy.
Discussion
Therapy that selectively eliminates autoreactive immune cells or disrupts their function without impacting the protective repertoire is a major goal for the treatment of autoimmune diseases. This is of particular importance for the treatment of diseases that affect children, such as type 1 diabetes. Autoantigen-specific tetramers show promise for targeting autoreactive T cells (58,59). Furthermore, autoantigen-specific elimination of insulin-reactive B cells prevents disease in NOD mice (21). Identifying immune tolerance mechanisms that fail to cull anti-insulin B lymphocytes could thus provide insight into how immune tolerance might be reinforced therapeutically to selectively remove anti-insulin B lymphocytes and prevent disease. In this study, we demonstrate the feasibility of targeting autoantigen-specific BCR as a means to reinforce central tolerance for insulin, a critical autoantigen in T1D. Using a new model in which receptor editing-mediated replacement of anti-insulin Vκ125 can be tracked, we find that physiologic levels of this small protein hormone elicit a proportion of anti-insulin B cells to undergo receptor editing in non-autoimmune mice. Investigation of NOD mice revealed that anti-insulin B cells undergo receptor editing less efficiently in the type 1 diabetes-prone strain. Using anti-insulin mAb123 that recognizes insulin-occupied BCR, we demonstrate that autoantigen-targeted therapy can increase RAG2-GFP expression and reduce the frequency of insulin-binding B cells within developing subsets. This approach can thus reduce or eliminate critical antigen presenting cells from the mature repertoire as a means to prevent expansion of cognate T cells.
Unlike many autoantigens that have been studied, insulin is present in circulation at low concentration, binds the 125Tg BCR with modest affinity, and is not predicted to induce a high degree of BCR crosslinking (33,35). Despite having limited predicted immunoreactivity, prior studies with the receptor editing-incompetent 125Tg model demonstrate that anti-insulin B cells are rendered anergic, and that this program likely initiates upon Ag encounter in the bone marrow (23,25,57). Data presented in these studies suggest that physiologic insulin encounter can provoke a second immune tolerance mechanism, receptor editing, in cognate immature B cells (Fig. 1–4). Immature B cells from anti-insulin VH125Tg/Vκ125SD mice show reduced surface IgM, increased RAG expression, and an increased frequency of Igλ+ B cells, as compared to control, non-insulin-binding VH281Tg/Vκ125SD mice (Fig. 2). All immature anti-insulin B cells upregulate RAG-2 in the VH125Tg/Vκ125SD model, yet only those which express the highest level of RAG2-GFP successfully replace the autoreactive BCR (Fig. 2). Taken together, these findings are consistent with a role for receptor editing in mediating B cell tolerance for insulin.
The current studies may underestimate the contribution of receptor editing to maintaining central tolerance for insulin in a natural polyclonal repertoire for several reasons. The VH125Tg/Vκ125SD model lacks all downstream Jκ which necessitates chromatin remodeling for rearrangement on the other allele, and should thus represent a relatively stringent barrier to receptor editing. This is suggested by the finding that nearly all anti-DNA B cells undergo receptor editing when downstream Jκ2-5 are present on the targeted allele, but virtually no anti-DNA B cells undergo receptor editing when downstream Jκ are absent (60). Competition for survival factors and follicular entry are also relaxed in the VH125Tg/Vκ125SD model, as the vast majority of developing B cells recognize insulin (Fig. 1C). As such, the proportion of mature, edited B cells would likely increase in a setting where anergic anti-insulin B cells are at a normal survival disadvantage. The presence of H and L chain transgenes accelerates B cell development in the bone marrow (61), thus limiting the window of opportunity for receptor editing.
Previous studies showed that receptor editing occurred normally in NOD mice harboring 3–83 transgenic B cells that engaged the membrane-bound protein, MHC class I compared to non-autoimmune mice (62). In contrast, recombining sequence (RS) rearrangement, a hallmark of B cells that have undergone receptor editing, is reduced in both NOD mice and type 1 diabetic patients (10). Furthermore, little if any negative selection occurs between immature and transitional B cell stages in NOD mice (63). The current studies show that a reduced proportion of anti-insulin B cells undergo receptor editing in NOD mice (Fig. 4), providing one explanation of how anti-insulin B cells escape negative selection to invade the pancreas (24). These findings are not attributable to insulin levels, as all mice studied were euglycemic, and studies by others demonstrate that insulin production is not impaired and may be increased in prediabetic NOD mice (64). Furthermore, the reduced frequency of Igλ+ B cells in WT/NOD mice suggests that this defect is not restricted to anti-insulin B cells, and data from the VH125Tg model suggests that receptor editing deficiency is not solely due to H chain usage bias (Fig. 5). Autoantigen recognition by immature B cells leads to BCR internalization and subsequent loss of tonic BCR signaling necessary for positive selection that allows maturation; it is this loss of tonic signaling that drives receptor editing (44,47). Interestingly, immature anti-insulin B cells express 2-fold increased surface BCR in NOD vs. B6 mice, despite an identical VH125Tg/Vκ125SD transgene, and this difference is lost as cells enter the T1 and T2 transitional subsets (Fig. 4D). We hypothesize that this increase in surface IgM is associated with increased tonic signaling necessary for positive selection at this stage, rendering NOD immature B cells refractory to receptor editing induction in response to insulin encounter.
Numerous polymorphisms are observed in NOD Vκ (65). To investigate whether polymorphisms also exist in Igκ regulatory regions between NOD and C57BL/6 strains, the Wellcome Trust Sanger Institute’s Mouse Genomes Project, Query SNPs, indels, or SVs (https://www.sanger.ac.uk/sanger/Mouse_SnpViewer/rel-1410) was used. There are no differences in the Ei (intronic enhancer) or E3’ enhancer regions, however one deletion was observed in the Ed (downstream enhancer) upstream of a putative ETS binding site (not shown). It is unclear what whether ETS binding is altered, or what functional consequences this may have on Igκ transcription. These and other differences may contribute to altered central tolerance in NOD.
The proportion of B cells that have undergone receptor editing differs depending on the developmental compartment examined (Fig. 1E, 2C, 4, and 5). The immature B cell compartment is continually supplied with newly-formed anti-insulin B cells (24), whereas the T1 compartment reflects the full extent of receptor editing that occurred during the immature stage. This explains the relative increase in the frequency edited B cells between the immature to T1 transition (Fig. 1E and 4). It was however surprising to observe a reduced frequency of edited B cells in the FO B cell subset. This phenomenon was also observed in WT mice that lack BCR transgenes (Fig. 5), and in other studies (10,63,66,67). IgM expression is increased in edited T1 and T2 B cells driven to undergo receptor editing by mAb123 treatment, but not in B cells that retain the insulin-binding specificity (Fig. 7D). We speculate that recently edited B cells synthesize higher levels of H and L chain protein to rapidly replace the autoreactive BCR and maintain tonic signaling necessary for cell survival at this stage of development. We theorize that a fitness cost is associated with undergoing receptor editing, perhaps associated with the metabolic stress of synthesizing increased BCR protein.
While global B cell-depleting therapy has shown promise for the treatment of type 1 diabetes in humans and mice (68,69), this therapy brings substantial immunosuppressive risk, particularly for children (70). As an alternative to global B cell depletion, a strategy targeting insulin autoantigen was developed. The frequency of non-insulin-binding (edited) B cells is dramatically increased in the T1 and T2 B cell compartments following short-term mAb123 treatment in both NOD and B6 strains (Fig. 6–7). mAb123 does not bind insulin bound to the insulin receptor (71); thus, as expected, it did not alter blood glucose when chronically administered to NOD mice (21). RAG2-GFP expression is increased in non-insulin-binding vs. insulin-binding T1 and T2 B cells, and in insulin-binding T1 B cells following mAb123 treatment (Fig. 7). F(ab’)2123 elimination of developing anti-insulin B cells in VH125Tg/NOD mice (Fig. 8) further implicates receptor editing as a mechanism through which this therapy functions. Developing B cells transit through the T1 stage in ~2 days and through the T2 stage in 3–4 days (55,56). The reduced frequency of anti-insulin B cells in T1 and T2 B cell subsets following administration of a single dose of F(ab’)2123 is thus kinetically consistent with immature B cells triggered to undergo receptor editing (Fig. 8). We therefore interpret the loss of anti-insulin B cells, particularly in the T2 compartment, to reflect enhanced receptor editing of immature B cells that recently entered the spleen. This interpretation is strengthened by the finding that mAb123 treatment increases RAG-2 expression (Fig. 7). These findings suggest that autoantigen-targeted antibody therapy can reinforce central tolerance to mitigate insulin autoreactivity that continually arises in the developing repertoire.
The increased frequency of non-insulin-binding B cells in the macrophage-rich marginal zone following treatment with mAb123 likely involves Fc-mediated deletion of insulin-binding B cells (Fig. 6–8). Fig. 8 shows that short-term treatment with mAb123, but not F(ab’)2123 eliminates anti-insulin MZ B cells. We therefore conclude that Fc recognition, as anticipated, is contributing to anti-insulin B cell depletion by autoantigen-targeting mAb. F(ab’)2123 treatment leads to a selective decrease in IgM expression in mature anti-insulin B cells (Fig. 8), thus it is tempting to speculate that anergy may also be reinforced by this treatment. Additional studies are needed to explore if or how anergy programming is altered in anti-insulin B cells treated with autoantigen-targeting antibody. Immune tolerance outcomes observed in the 125Tg (VH125Tg/Vκ125Tg) and VH125Tg/Vκ125SD models suggest that they represent a balance point between fate decisions to undergo receptor editing or anergy, and offer an opportunity to better distinguish when and how these two programs diverge at the molecular level.
Receptor editing efficiency can be dramatically improved by insulin autoantigen-targeted antibody therapy, suggesting that such an approach might be used to selectively target autoreactive B cells in patients suffering with autoimmune diseases that arise from breaches in central tolerance. Increased emergence of autoreactive B cells is a recognized property of several autoimmune disorders. Some non-specific therapies used to treat autoimmune disease, such as IVIG, may also act through similar mechanisms since multiple specificities in such preparations may target autoantigens. A higher level of immune targeting selectivity is further augmented by the use of F(ab’)2, which limits any possible off target interactions with innate immune cells. Short-term treatment with F(ab’)2123 also implicates a role for Fc recognition in the elimination of mature B cells subsets, as they are less efficiently culled. Autoantigen-targeted mAb therapy thus holds dual functional capacity to eliminate specific B lymphocytes via reinforced immune tolerance through the antigen binding domain or by Fc recognition. Both of these functional activities should be considered in the design of clinical therapeutics depending on the nature of the autoantigen and the timing and nature of the B cell function that needs to be blocked.
Supplementary Material
Acknowledgements
We would like to thank Amita B. Rachakonda and Chrys Hulbert for technical contributions and Jonathan M. Williams for critical manuscript review (Vanderbilt University, Nashville, TN). We would also like to thank Dr. Roberta Pelanda (University of Colorado, Denver, CO) for providing the targeting construct and Southern blot probe used to generate Vκ125SD andVκ125SDNeo mice, and the Vanderbilt Transgenic Mouse/ESC Shared Resource for technical support in generating the transgenic mice. We would like to thank Dr. Richard Breyer (Vanderbilt University, Nashville, TN) and Dr. Rachel Gerstein (University of Massachusetts Medical School, Worchester, MA) for providing the EIIA-Cre/B6 and RAG-2/GFP/B6 mice, respectively. DNA Sequencing was performed by the Vanderbilt VANTAGE DNA Sequencing core. Flow cytometry experiments were performed in the Vanderbilt Medical Center Flow Cytometry Shared Resource. Monoclonal Ab were purified by the Vanderbilt Antibody and Protein Resource.
Footnotes
This work was supported by National Institutes of Health Grants T32 AR059039, T32 HL069765, T32 GM08554, and R01 AI051448 and Juvenile Diabetes Research Foundation Grants 3-2013-121 and 1-2005-167. This work was also supported through the following shared resources: the Vanderbilt Medical Center Flow Cytometry Shared Resource (supported by the Vanderbilt Ingram Cancer Center [P30 CA68485] and the Vanderbilt Digestive Disease Research Center [DK058404]), the Vanderbilt VANTAGE DNA Sequencing Facility (supported by the Vanderbilt Ingram Cancer Center [P30 CA68485], the Vanderbilt Vision Center [P30 EY08126], and the National Institutes of Health/National Center for Research Resources [G20 RR030956]), and the Vanderbilt Antibody and Protein Resource (supported by the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center [P30 CA68485]).
The Igκ sequences presented in this article have been submitted to GenBank (http://www.ncbi.nlm.nih.gov/genbank/) under accession numbers KT250637-KT250727.
Abbreviations used: BAC: bacterial artificial chromosome, FO: follicular, GFP: green fluorescent protein, MZ: marginal zone, pre-MZ: pre-marginal zone, T1: transitional 1, T2: transitional 2
References
- 1.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 2.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, Trent RJ, Basten A. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 3.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 4.Nemazee D, Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radic MZ, Erikson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp. Med. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp. Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp. Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 9.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp. Med. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panigrahi AK, Goodman NG, Eisenberg RA, Rickels MR, Naji A, Luning Prak ET. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J Exp. Med. 2008;205:2985–2994. doi: 10.1084/jem.20082053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp. Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, Price C, Abraham C, Motaghedi R, Buckner JH, Gregersen PK, Meffre E. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin. Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewulonu UK, Nell LJ, Thomas JW. VH and VL gene usage by murine IgG antibodies that bind autologous insulin. J Immunol. 1990;144:3091–3098. [PubMed] [Google Scholar]
- 14.Diaz JL, Wilkin T. Differences in epitope restriction of autoantibodies to native human insulin (IAA) and antibodies to heterologous insulin (IA) Diabetes. 1987;36:66–72. doi: 10.2337/diab.36.1.66. [DOI] [PubMed] [Google Scholar]
- 15.Devendra D, Galloway TS, Horton SJ, Evenden A, Keller U, Wilkin TJ. The use of phage display to distinguish insulin autoantibody (IAA) from insulin antibody (IA) idiotypes. Diabetologia. 2003;46:802–809. doi: 10.1007/s00125-003-1107-7. [DOI] [PubMed] [Google Scholar]
- 16.Hall TR, Thomas JW, Padoa CJ, Torn C, Landin-Olsson M, Ortqvist E, Hampe CS. Longitudinal epitope analysis of insulin-binding antibodies in type 1 diabetes. Clin. Exp. Immunol. 2006;146:9–14. doi: 10.1111/j.1365-2249.2006.03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaeger C, Winter S, Eckhard M, Hardt P, Brendel MD, Bretzel RG. Binding characteristics and crossreactivity of insulin autoantibodies and insulin antibodies directed to three different insulin molecules. Acta Diabetol. 2008;45:191–194. doi: 10.1007/s00592-008-0041-z. [DOI] [PubMed] [Google Scholar]
- 18.Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 19.Steck AK, Johnson K, Barriga KJ, Miao D, Yu L, Hutton JC, Eisenbarth GS, Rewers MJ. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;34:1397–1399. doi: 10.2337/dc10-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry RA, Kendall PL, Thomas JW. Autoantigen-Specific B Cell Depletion Overcomes Failed Immune Tolerance in Type 1 Diabetes. Diabetes. 2012 doi: 10.2337/db11-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulbert C, Riseili B, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J Immunol. 2001;167:5535–5538. doi: 10.4049/jimmunol.167.10.5535. [DOI] [PubMed] [Google Scholar]
- 23.Rojas M, Hulbert C, Thomas JW. Anergy and not clonal ignorance determines the fate of B cells that recognize a physiological autoantigen. J Immunol. 2001;166:3194–3200. doi: 10.4049/jimmunol.166.5.3194. [DOI] [PubMed] [Google Scholar]
- 24.Henry-Bonami RA, Williams JM, Rachakonda AB, Karamali M, Kendall PL, Thomas JW. B lymphocyte"original sin" in the bone marrow enhances islet autoreactivity in type 1 diabetes-prone nonobese diabetic mice. J Immunol. 2013;190:5992–6003. doi: 10.4049/jimmunol.1201359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry RA, Acevedo-Suarez CA, Thomas JW. Functional silencing is initiated and maintained in immature anti-insulin B cells. J Immunol. 2009;182:3432–3439. doi: 10.4049/jimmunol.0803121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tze LE, Baness EA, Hippen KL, Behrens TW. Ig light chain receptor editing in anergic B cells. J Immunol. 2000;165:6796–6802. doi: 10.4049/jimmunol.165.12.6796. [DOI] [PubMed] [Google Scholar]
- 27.Hippen KL, Schram BR, Tze LE, Pape KA, Jenkins MK, Behrens TW. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. J Immunol. 2005;175:909–916. doi: 10.4049/jimmunol.175.2.909. [DOI] [PubMed] [Google Scholar]
- 28.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp. Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Prak EL, Weigert M. Editing disease-associated autoantibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Su SC, Hecox DB, Brady GF, Mackin KM, Clark AG, Foster MH. Central tolerance regulates B cells reactive with Goodpasture antigen alpha3(IV)NC1 collagen. J Immunol. 2008;181:6092–6100. doi: 10.4049/jimmunol.181.9.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litzenburger T, Bluthmann H, Morales P, Pham-Dinh D, Dautigny A, Wekerle H, Iglesias A. Development of myelin oligodendrocyte glycoprotein autoreactive transgenic B lymphocytes: receptor editing in vivo after encounter of a self-antigen distinct from myelin oligodendrocyte glycoprotein. J Immunol. 2000;165:5360–5366. doi: 10.4049/jimmunol.165.9.5360. [DOI] [PubMed] [Google Scholar]
- 32.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–759. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 33.Felig P. Physiologic action of insulin. In: Ellenberg M, Rifkin H, editors. In Diabetes Mellitus, Theory and Practice. New Hyde Park: Medical Examination Publishing Co., Inc.; 1983. pp. 77–88. [Google Scholar]
- 34.Blundell T, Dodson G, Hodgkin D, Mercola D. Insulin: The Structure in the Crystal and its Reflection in Chemistry and Biology. In: Anfinsen CB, Edsall JT, Richards FM, editors. Advances in Protein Chemistry. Academic Press; Ney York, NY: 1972. pp. 279–403. [Google Scholar]
- 35.Schroer JA, Bender T, Feldmann RJ, Kim KJ. Mapping epitopes on the insulin molecule using monoclonal antibodies. Eur. J. Immunol. 1983;13:693–700. doi: 10.1002/eji.1830130902. [DOI] [PubMed] [Google Scholar]
- 36.Bonami RH, Sullivan AM, Case JB, Steinberg HE, Hoek KL, Khan WN, Kendall PL. Bruton's Tyrosine Kinase Promotes Persistence of Mature Anti-Insulin B Cells. J Immunol. 2014 doi: 10.4049/jimmunol.1300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelanda R, Schwers S, Sonoda E, Torres RM, Nemazee D, Rajewsky K. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 39.Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, Melchers F, Meffre E, Nussenzweig MC. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 40.Yu W, Misulovin Z, Suh H, Hardy RR, Jankovic M, Yannoutsos N, Nussenzweig MC. Coordinate regulation of RAG1 and RAG2 by cell type-specific DNA elements 5' of RAG2. Science. 1999;285:1080–1084. doi: 10.1126/science.285.5430.1080. [DOI] [PubMed] [Google Scholar]
- 41.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 42.Thomas JW, Hulbert C. Somatically mutated B cell pool provides precursors for insulin antibodies. J Immunol. 1996;157:763–771. [PubMed] [Google Scholar]
- 43.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+IgD− bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 44.Schram BR, Tze LE, Ramsey LB, Liu J, Najera L, Vegoe AL, Hardy RR, Hippen KL, Farrar MA, Behrens TW. B cell receptor basal signaling regulates antigen-induced Ig light chain rearrangements. J Immunol. 2008;180:4728–4741. doi: 10.4049/jimmunol.180.7.4728. [DOI] [PubMed] [Google Scholar]
- 45.Verkoczy L, Duong B, Skog P, Ait-Azzouzene D, Puri K, Vela JL, Nemazee D. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J Immunol. 2007;178:6332–6341. doi: 10.4049/jimmunol.178.10.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan TG, Amesbury M, Gardam S, Crosbie J, Hasbold J, Hodgkin PD, Basten A, Brink R. B cell receptor-independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells. J Exp. Med. 2003;197:845–860. doi: 10.1084/jem.20022144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tze LE, Schram BR, Lam KP, Hogquist KA, Hippen KL, Liu J, Shinton SA, Otipoby KL, Rodine PR, Vegoe AL, Kraus M, Hardy RR, Schlissel MS, Rajewsky K, Behrens TW. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS. Biol. 2005;3:e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kendall PL, Yu G, Woodward EJ, Thomas JW. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. J Immunol. 2007;178:5643–5651. doi: 10.4049/jimmunol.178.9.5643. [DOI] [PubMed] [Google Scholar]
- 49.Woodward EJ, Thomas JW. Multiple germline kappa light chains generate anti-insulin B cells in nonobese diabetic mice. J Immunol. 2005;175:1073–1079. doi: 10.4049/jimmunol.175.2.1073. [DOI] [PubMed] [Google Scholar]
- 50.Henry RA, Kendall PL. CXCL13 Blockade Disrupts B Lymphocyte Organization in Tertiary Lymphoid Structures without Altering B Cell Receptor Bias or Preventing Diabetes in Nonobese Diabetic Mice. J Immunol. 2010 doi: 10.4049/jimmunol.0903710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J Exp. Med. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, Velez MG, Humann J, Rowland S, Conrad FJ, Halverson R, Torres RM, Pelanda R. Receptor editing can lead to allelic inclusion and development of B cells that retain antibodies reacting with high avidity autoantigens. J Immunol. 2005;175:5067–5076. doi: 10.4049/jimmunol.175.8.5067. [DOI] [PubMed] [Google Scholar]
- 53.Nadel B, Cazenave PA, Sanchez P. Murine lambda gene rearrangements: the stochastic model prevails over the ordered model. EMBO J. 1990;9:435–440. doi: 10.1002/j.1460-2075.1990.tb08128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leijon K, Freitas A, Holmberg D. Analysis of VH gene utilisation in the non-obese diabetic mouse. Autoimmunity. 1993;15:11–18. doi: 10.3109/08916939309004834. [DOI] [PubMed] [Google Scholar]
- 55.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp. Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 57.Acevedo-Suarez CA, Hulbert C, Woodward EJ, Thomas JW. Uncoupling of anergy from developmental arrest in anti-insulin B cells supports the development of autoimmune diabetes. J Immunol. 2005;174:827–833. doi: 10.4049/jimmunol.174.2.827. [DOI] [PubMed] [Google Scholar]
- 58.Hess PR, Barnes C, Woolard MD, Johnson MD, Cullen JM, Collins EJ, Frelinger JA. Selective deletion of antigen-specific CD8+ T cells by MHC class I tetramers coupled to the type I ribosome-inactivating protein saporin. Blood. 2007;109:3300–3307. doi: 10.1182/blood-2006-06-028001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vincent BG, Young EF, Buntzman AS, Stevens R, Kepler TB, Tisch RM, Frelinger JA, Hess PR. Toxin-coupled MHC class I tetramers can specifically ablate autoreactive CD8+ T cells and delay diabetes in nonobese diabetic mice. J Immunol. 2010;184:4196–4204. doi: 10.4049/jimmunol.0903931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yachimovich N, Mostoslavsky G, Yarkoni Y, Verbovetski I, Eilat D. The efficiency of B cell receptor (BCR) editing is dependent on BCR light chain rearrangement status. Eur. J. Immunol. 2002;32:1164–1174. doi: 10.1002/1521-4141(200204)32:4<1164::AID-IMMU1164>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 61.Melamed D, Kench JA, Grabstein K, Rolink A, Nemazee D. A functional B cell receptor transgene allows efficient IL-7-independent maturation of B cell precursors. J Immunol. 1997;159:1233–1239. [PubMed] [Google Scholar]
- 62.Silveira PA, Dombrowsky J, Johnson E, Chapman HD, Nemazee D, Serreze DV. B cell selection defects underlie the development of diabetogenic APCs in nonobese diabetic mice. J Immunol. 2004;172:5086–5094. doi: 10.4049/jimmunol.172.8.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quinn WJ, III, Noorchashm N, Crowley JE, Reed AJ, Noorchashm H, Naji A, Cancro MP. Cutting edge: impaired transitional B cell production and selection in the nonobese diabetic mouse. J Immunol. 2006;176:7159–7164. doi: 10.4049/jimmunol.176.12.7159. [DOI] [PubMed] [Google Scholar]
- 64.Amrani A, Durant S, Throsby M, Coulaud J, Dardenne M, Homo-Delarche F. Glucose homeostasis in the nonobese diabetic mouse at the prediabetic stage. Endocrinology. 1998;139:1115–1124. doi: 10.1210/endo.139.3.5823. [DOI] [PubMed] [Google Scholar]
- 65.Henry RA, Kendall PL, Woodward EJ, Hulbert C, Thomas JW. Vkappa polymorphisms in NOD mice are spread throughout the entire immunoglobulin kappa locus and are shared by other autoimmune strains. Immunogenetics. 2010 doi: 10.1007/s00251-010-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dingjan GM, Middendorp S, Dahlenborg K, Maas A, Grosveld F, Hendriks RW. Bruton's tyrosine kinase regulates the activation of gene rearrangements at the lambda light chain locus in precursor B cells in the mouse. J Exp. Med. 2001;193:1169–1178. doi: 10.1084/jem.193.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 68.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin. Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl. J. Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pescovitz MD, Torgerson TR, Ochs HD, Ocheltree E, McGee P, Krause-Steinrauf H, Lachin JM, Canniff J, Greenbaum C, Herold KC, Skyler JS, Weinberg A. Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin. Immunol. 2011;128:1295–1302. doi: 10.1016/j.jaci.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor SI, Schroer JA, Marcus-Samuels B, McElduff A, Bender TP. Binding of insulin to its receptor impairs recognition by monoclonal anti-insulin antibodies. Diabetes. 1984;33:778–784. doi: 10.2337/diab.33.8.778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.