Abstract
Oxidative stress (OS) is considered as one of the etiologic factors involved in several signals and symptoms of inflammatory bowel diseases (IBD) that include diarrhea, toxic megacolon and abdominal pain. This systematic review discusses approaches, challenges and perspectives into the use of nontraditional antioxidant therapy on IBD, including natural and synthetic compounds in both human and animal models. One hundred and thirty four papers were identified, of which only four were evaluated in humans. Some of the challenges identified in this review can shed light on this fact: lack of standardization of OS biomarkers, absence of safety data and clinical trials for the chemicals and biological molecules, as well as the fact that most of the compounds were not repeatedly tested in several situations, including acute and chronic colitis. This review hopes to stimulate researchers to become more involved in this fruitful area, to warrant investigation of novel, alternative and efficacious antioxidant-based therapies.
Keywords: Inflammatory bowel diseases, Oxidative stress, Nutraceuticals, Antioxidant therapy, Biomarkers, Complementary and alternative medicine, Ulcerative colitis, Crohn's disease
Graphical abstract
Legend: 8-oxodG=8-oxo-2′-deoxyguanosine; AGE=advanced glycation end-products; COX2=cyclooxygenase 2; GR=glutathione reductase; GSH=reduced glutathione; iNOS=inducible nitric oxide synthase; GPx=glutathione peroxidase; GST=glutathione S-transferase; H2O2=hydrogen peroxide; LPO=lipoxygenase; MDA=malonaldheyde; NF-kB=nuclear factor Kappa-light-chain enhancer of activated B cells; Nrf2=nuclear erythroid 2 factor; NOX=NADPH oxidase; HO•=hydroxyl radical; O2•–=anion radical superoxide; NO•=nitric oxide; RONS=reactive oxygen and nitrogen species; SOD=superoxide dismutase; TBARS=thiobarbituric acid reactive substances; ˧ decrease; (–) inhibition; (+) stimulus.
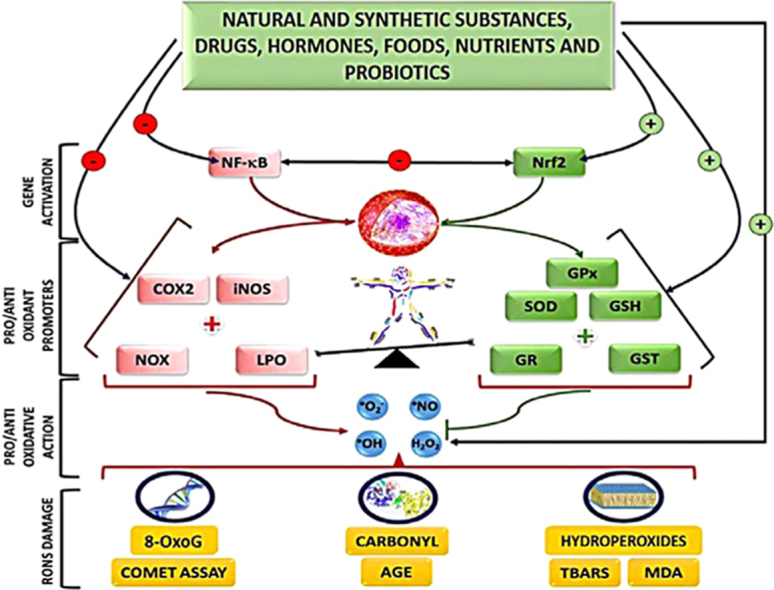
Highlights
-
•
Major biomarkers used for evaluation of antioxidant therapy were MPO, TBARS/MDA and glutathione levels.
-
•
Challenges were identified for the yet poor use of antioxidant therapy in IBD.
-
•
This review stimulates the investigation of alternative and efficacious antioxidant therapies.
1. Introduction
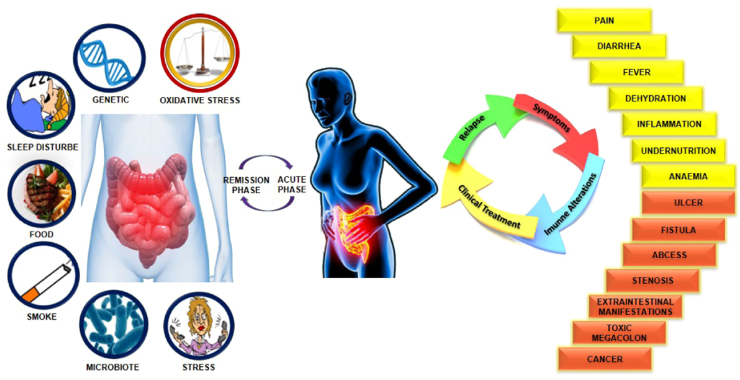
Inflammatory bowel diseases (IBD) are most commonly represented by Crohn's disease (CD), which involves any segment of the gastrointestinal tract, and ulcerative colitis (UC), that occurs in the inner lining of the colon (large intestine) or rectum. IBD is characterized by chronic or relapsing immune activation and inflammation within the gastrointestinal tract [1]. The etiology of IBD remains unclear but environmental factors, as well as infectious, immunological, and psychological ones, together with genetic susceptibility could be the major causes for the onset of UC [2] (Fig. 1). Although the prevalence and incidence of IBD is increasing (150–250/100,000 population), especially in developed countries, it is rarely fatal. It can however, greatly diminish the quality of life because of the pain, vomiting, diarrhea and other socially unacceptable symptoms it causes. The increased risk of colorectal cancer (from 0.5% up to 20% per year) is a serious complication of IBD, particularly in the case of UC [3].
Fig. 1.
Important risk factors associated with inflammatory bowel diseases and immunological changes.
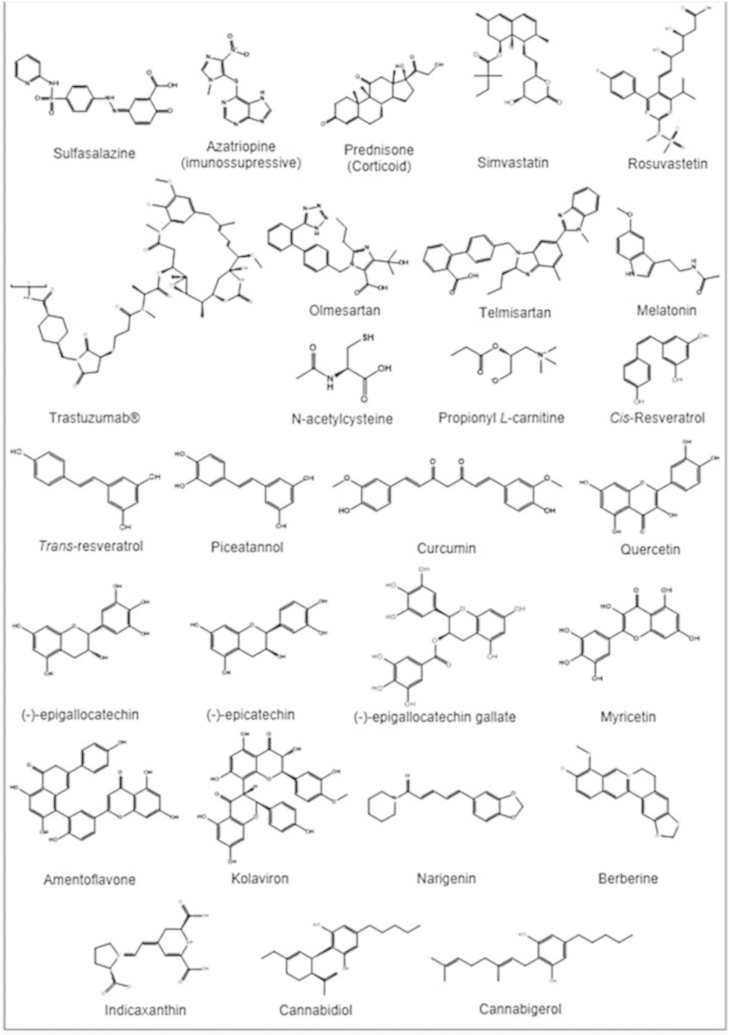
The current therapy for IBD relies on the use of sulfasalazine, corticosteroids, immunosuppressive agents, such as azatriopine, and biological therapy (Fig. 2) represented by the anti-TNFα (tumor necrosis factor alpha) antibody as the mainstream treatment for down-regulating aberrant immune responses and inflammatory cascades [4]. However, the adverse effects associated with these drugs over prolonged treatment periods and the high relapse rate limit their use [5]. Sulfasalazine, for instance, can exacerbate colitis, resulting in diarrhea, abdominal cramps and discomfort [6]. Antibiotics, one of the commonly used therapies, could adversely change the environmental conditions of microbiota and trigger resistance. Moreover, immunosuppressant and anti-inflammatory drugs (such as corticosteroids) have many undesirable side effects [7] and the combined therapy, using corticosteroids plus infliximab does not appear to provide any additional benefit over infliximab monotherapy [8]. Furthermore, these drugs display limited beneficial actions. In the long term, ≤25–33% of patients with UC will require surgery if pharmacological treatments are not successful, or in the case of complications such as fistulae, stenosis, or abscesses (particularly in Crohn's patients) [9]. The potential of these drugs when used over a long period of time, to induce severe side effects, together with the high costs of the therapy for patients, warrant investigation of novel and alternative pharmacological approaches [10].
Fig. 2.
Main substances used/tested in inflammatory bowel disease therapy (articles published from 2009–2015/06).
In recent years, several studies had focused on reactive oxygen species (ROS) and reactive nitrogen species (RNS) as the etiologic factors for IBD [11], [12], [13], [14]. The gastrointestinal tract is a major site for generation of pro-oxidants, whose production is primarily due to the presence of a plethora of microbes, food ingredients and interactions between immune cells [15]. Furthermore, the antioxidant capacity of patients with IBD is reduced, even in the asymptomatic phase of the disease [16]. To scavenge RONS, intestinal cells have several enzymatic and non-enzymatic antioxidants, including superoxide dismutase (SOD), reduced glutathione (GSH) and catalase (CAT), but excessive generation of RONS enhances lipid peroxidation (LP) and could deplete antioxidant defenses [17].
It should be noted that OS is clearly involved in IBD, once immune activation such as inflammation [18] occurs and could be a major contributing factor to tissue injury and fibrosis that characterize CD [19]. In this regard, reduction of plasma antioxidants and total intestinal antioxidant capacity has been observed in CD [20]. Like in CD, several studies have shown oxidative stress in UC. Patients with UC often have antioxidant nutrient deficiencies at the time of diagnosis [21], [22], [23] and that could suggest an increase of OS.
In this context, recent studies have suggested that the administration of antioxidants, from different sources, with additional anti-inflammatory action may be beneficial in the treatment of IBD because inflammation is caused by OS and leads to the increase of OS that contributes to tissue damage [24], [25], [26].
Several reports on non-traditional therapy for IBD have been published. However, those publications put special emphasis on antioxidant and/or anti-inflammatory activity [25], [26], [27] or regulation of gut microbiota [28], [29]. Unlike other published approaches, this review is broader and aims to describe and analyze the effect of functional foods and isolated nutrients, probiotics, natural active compounds from vegetal sources, drugs, hormones and other synthetic substances, all of them reported as antioxidants, in IBD.
2. Methods
In 2009, Rahimi et al. [30] published a systematic review on the use of herbal medicines for the treatment of IBD [31]. These authors categorized herbal therapies used to date for UC and CD, and suggested their possible mechanisms of action. After that, several reviews about this topic were published [25], [26], [32], [33], [34], [35], [36], [37], [38], however, up to our knowledge, no one has so far discussed other chemical strategies, applying antioxidants like drugs, polyphenols and others in IBD treatment. In face of that, we reviewed the literature from 2009 up to 2015 (June) to in vitro and animal models of colitis induction and without limit of time to human studies, using the keywords inflammatory bowel disease or ulcerative colitis or Crohn disease associated to antioxidant in the databases of PubMed, ScienceDirect and Scopus. Articles reporting on substances studied in vitro or in vivo (enemas, oral supplementation or intraperitoneal application) were chosen. To minimize losses, the search was conducted by two researchers, independently.
Initially, the titles of the papers were read, with exclusion of duplicated articles. Thereafter, we identified if the reported work had dealt with antioxidant action (OS biomarkers), using the following phrases:
-
(1)
Scavenging of ROS/RNS ⇒ decrease of the levels of nitrite, nitric oxide (NO•), superoxide anion (O2•–), hydrogen peroxide (H2O2) and others;
-
(2)
Inhibition of RONS synthesis ⇒ decrease of the activity, protein expression or genic expression of inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX2), NADPH oxidase (NOX), lipoxygenase (LOX), myeloperoxidase (MPO), nuclear factor Kappa-light-chain enhancer of activated B cells (NF-κB) or Iκ-Bα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha);
-
(3)
Inhibition of RONS damage ⇒ biomarkers of LP (thiobarbituric acid reactive substances (TBARS), malondialdehyde (MDA), 4-hydroxynonenal (HNE) and others), protein damage (protein carbonylation, advanced glycation end-products (AGE) and others) and deoxyribonucleic acid (DNA) damage (8-oxo-2′-deoxyguanosine, 8-oxoguanine and others);
-
(4)
Increase of antioxidant defense (endogenous enzymatic and non enzymatic antioxidant defense and total antioxidant capacity).
Thereafter we categorized the therapies into topics, as following:
-
(a)
Drugs, hormones, synthetic antioxidants and chemicals derived from sources, other than vegetables;
-
(b)
Polyphenols and other natural active compounds from medicinal plants;
-
(c)
Functional foods, antioxidant nutrients and probiotics.
3. Results and discussion
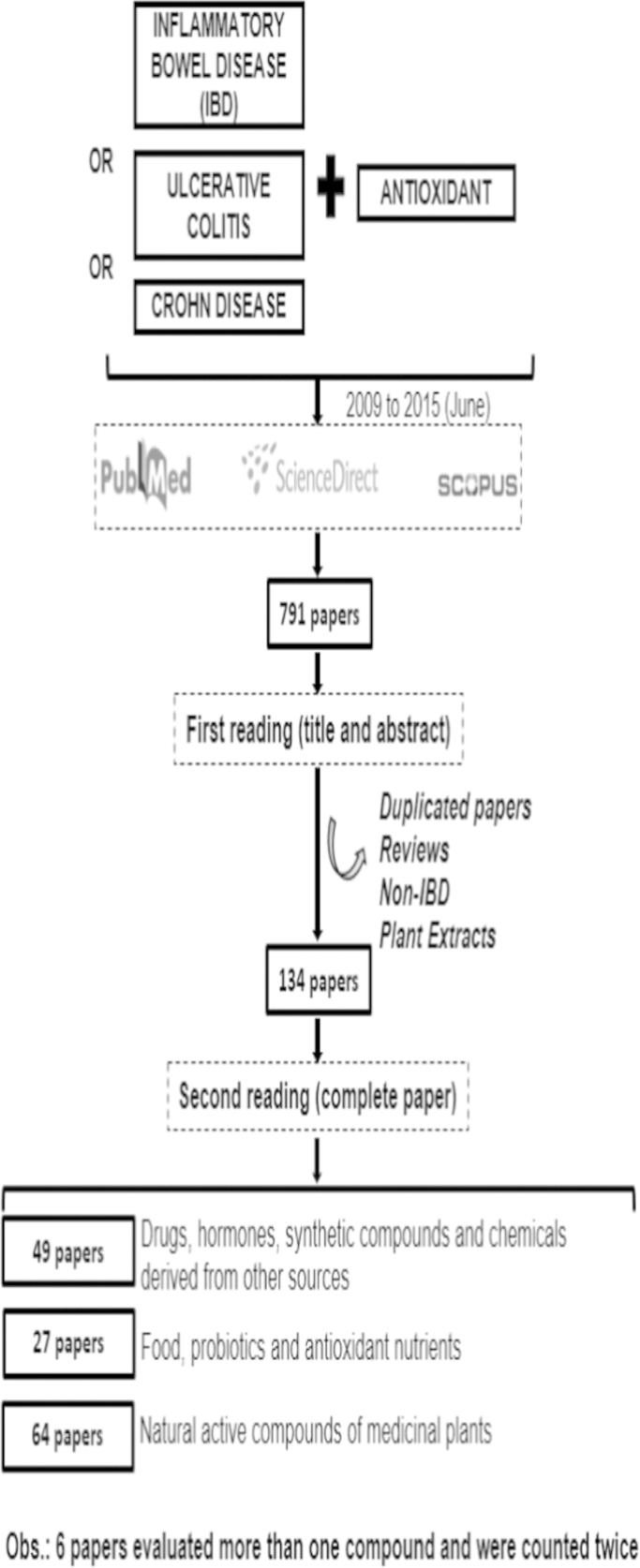
One-hundred and thirty four papers were found. A summary of the method and the total number of articles for each category are displayed in Fig. 3.
Fig. 3.
Main results from the database search.
In sequence, we will discuss the role of OS in IBD, the major biomarkers used in diagnosis, and the several classes of antioxidants available for IBD treatment.
3.1. Oxidative stress and IBD
OS is defined as an imbalance between oxidants (ROS, RNS) and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage [39]. ROS is generally taken to encompass the initial species generated by oxygen reduction as well as their secondary reactive products, and include free radicals such as the radical anion superoxide O2•−, hydroxyl radical (HO•), peroxyl (RO2•), alcoxyl (RO•) and hydroperoxyl (HO2•), and non-radical species such as singlet oxygen (1O2), H2O2 and hydrochlorous acid (HOCl). RNS include free radicals like nitric oxide (NO•), nitrogen dioxide (NO2•), anions like peroxynitrite (ONOO–), as well as nonradicals such as nitrous oxide (HNO2), nitryl chloride (NO2Cl) and alkyl peroxynitrites (ONOOR) [40].
Gastrointestinal (GI) tract is a key source of ROS production. Despite the protective barrier provided by the epithelial layer, ingested materials and pathogens can cause inflammation by activating the epithelium, polymorphonuclear neutrophils (PMNs), and macrophages to produce inflammatory cytokines and other mediators that contribute further to OS [41].
Dysfunction of the intestinal barrier accompanied by increased intestinal permeability is another characteristic symptom in the pathophysiology of IBD [42]. It is believed that the ability of commensal bacteria to adhere to the epithelial layer via oligosaccharides helps deter invasion by displacing pathogenic bacteria [43]. In this context, the increased permeability of gastrointestinal epithelial cells frequently results from the destruction of tight junctions (TJ), and release of different pro-inflammatory mediators, including ROS and RNS, which actively contribute to the pathogenic cascade that initiates and perpetuates the inflammatory response in the gut [44].
The main sites of RONS production in IBD are activated neutrophils and macrophages. During episodes of inflammation, these cells exhibit massive intestinal mucosa infiltration and release large amounts of these species [45]. The enhanced production of RONS is associated with chronic intestinal inflammation in the early stages of IBD, and their destructive effects on DNA, proteins and lipids may contribute to initiation and progression of CD [19] as well as UC [46], resulting in prominent inflammation, increased amounts of cytokines such as tumor necrosis factor (TNF)-α, interleukins IL-6 and IL-1β. Such biological compounds trigger the pathological responses and symptoms of IBD [47]. The increased OS associated with peripheral DNA damage may be one of the pathophysiological mechanisms in the development of UC associated colorectal cancer, together with actin carbonylation and nitration of actin and tubulin which disrupt the cytoarchitecture and cause tissue injury [48].
Another factor involved in the etiology of colitis is the enzyme Glutathione Peroxidase type 2 (GPx2), a gastrointestinal-specific form of GPx. According with a recent review, in contrast to other enzymes such MnSOD and CAT, which had been shown to be modestly reduced in inflamed issue, GPx2 had its expression increased during colitis [49]. An in vitro investigation with tissue of patients with colorectal cancer or UC, had identified that GPx2 is strategically located in endoplasmic reticulum where it can interfere with COX-2 activity by local removal of hydroperoxides and, thus, regulates the level of prostaglandin E 2 (PGE2), considered to be a key mediator of acute inflammatory responses [50]. The relevance of the hydroperoxide level to inflammatory processes in the gastrointestinal system is indeed evident from the spontaneous development of ileocolitis and intestinal cancer in GPx1/2 DKO mice. In this case, a synergism between both enzymes is suggested, but the role of GPx2 appears to be more important, since a single allele of GPx2 but not of GPx1 proved to be sufficient to prevent inflammation in this knockout mice [51]. Therefore, GPx2 activation may prevent undue responses to inflammatory stimuli and, in consequence, inflammation-driven initiation of carcinogenesis
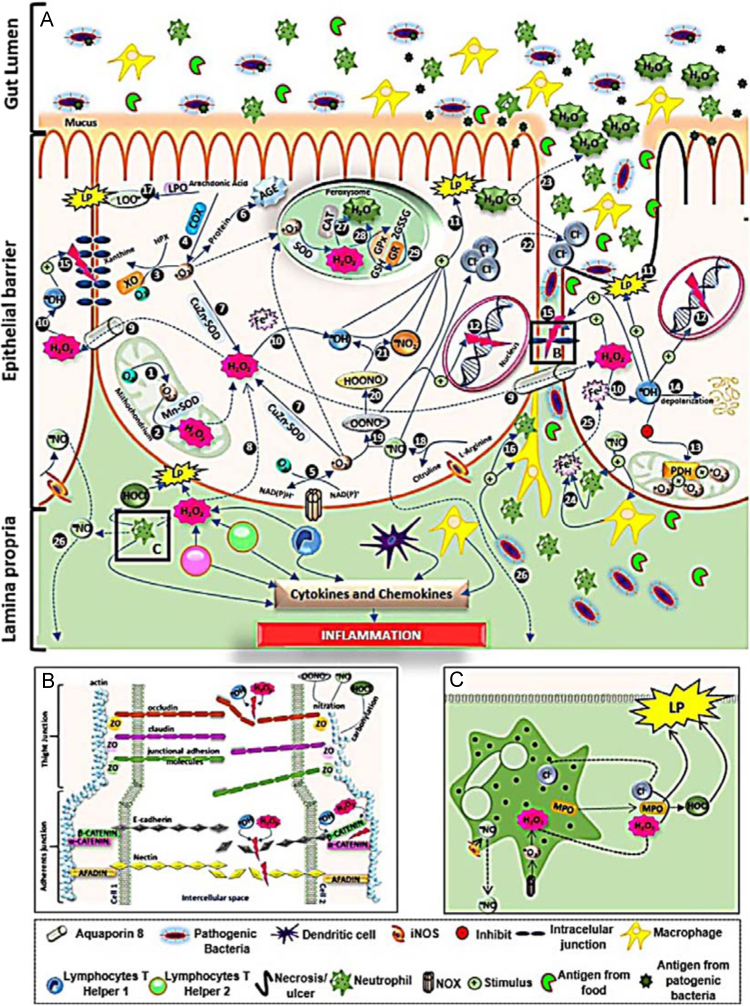
A summary of these alterations is displayed in Fig. 4. In brief, RONS cause intestinal tissue lipid peroxidation, necrosis/apoptosis and epithelial ulcers. These species are also responsible for the disruption of intercellular junctions, as well as leukocyte and neutrophil infiltration that produce ROS, RNS and cytokines that lead to the inflammatory process. Nitric oxide (NO•) acts on chloride anions and stimulates their release into the intercellular medium with a consequent loss of water leading to osmotic diarrhea. NO• also diffuses into the muscle layer and can cause toxic megacolon, a common complication of IBD, especially in the case of UC.
Fig. 4.
Oxidative stress and its association with the physiopathological process of IBD.
Legend description: reactive oxygen species (ROS) are produced through several metabolic pathways. (1) In mitochondria, during the respiratory process, the electron transport chain normally transforms 2–3% of O2 to O2•– (complex I and III). Then, this reactive species undergoes dismutation by Mn-SOD and generates (2) H2O2. In the cytosol, O2•– can be generated by (3) xanthine oxidase (XO), (4) cyclooxygenase 2 (COX2) and (5) NADPH oxidase (NOX) and generates (6) advanced glycation end-products (AGE) that are involved with the inflammatory process, or can form (7) H2O2 by CuZn–SOD. Hydrogen peroxide can be also produced by (8) immune cells: monocytes, lymphocytes and (A) neutrophils (principally), coming from leukocyte infiltration that is characteristic of IBD. The reactive molecule H2O2, unlike the O2•–, is long lived and highly biomembrane permeable, facilitated by the (9) aquaporin-8 channel. In the cytosol, together with metals such as Fe2+, Cu2+, Co2+ and Cr2+, and by (10) the Fenton and Haber-Weiss reactions, H2O2 forms the hydroxyl radical (HO·) which is highly toxic and can directly cause (11) lipid peroxidation (LP). Other actions by HO• are: (12) DNA fragmentation, (13) inactivation of mitochondrial pyruvate dehydrogenase (PDH), (14) mucin depolarization and (15) destruction of (B) apical intercellular junctions. These damaging actions cause (11) tissue disruption and cellular death and then ulcerations in the mucosa, typical IBD damage; (12) mutagenesis and cancer; (13) increased mitochondrial O2•– generation and OS, which has a direct relationship to the severity of the disease; (14) decreased formation of the mucous layer and promotion of (15) bacterial translocation, which stimulates macrophages and neutrophil recruit, as well as increased intestinal permeability; and facilitates (16) leukocytes and neutrophil infiltration, causing inflammation. Another enzyme present in the cytosol, lipoxygenase (LPO) also causes LP via (17) lipoperoxide (LOOH) formation.
All reactive nitrogen species (RNS) are formed from nitric oxide (NO•), produced by (18) inducible nitric oxide synthase (iNOS) that converts l-arginine to l-citruline. The NO• reacts with O2•– to furnish (19) the peroxynitrite anion (ONOO–), which then becomes (20) peroxynitrous acid (ONOOH). The latter then generates the new species (21) of nitrogen dioxide (NO2•) that causes LP. ONOO– is highly reactive and can cause (12) DNA fragmentation and RNS/ROS stimulation (10) of LP. Nitric oxide has dangerous actions on enterocytes. This gas stimulates (22) chloride secretion to the mucosa with a consequent (23) water release into the intestinal lumen causing osmotic diarrhea, the most frequent symptom of IBD. RNS are chemotactic mediators of macrophages and neutrophils that (24) stimulate ferrous release from defense cells (25) into the intracellular environment. Nitric oxide, despite having a half-life of ≅ 3 s, can (26) diffuse to the muscle layer (smooth muscle) and causes no-cholinergic and no-adrenergic relaxing and consequently toxic megacolon, a common clinical complication of IBD.
Because of disrupted permeability, antigens derived from food digestion and from commensal or pathogenic bacteria can overcome the unprotected mucosal barrier and provoke a continuous intestinal immune response that then causes tissue damage. The inflammatory response often results in continued epithelial injury, which causes erosions and ulcerations leading to an increased exposure to intestinal microbiota and amplification of the inflammatory response.
Antioxidant cell defenses involve enzymes and non-enzymes present in peroxisomes. In this organelle, H2O2 can be transformed into water (H2O) by (27) catalase (CAT), principally in the inflammatory process, and (28) by glutathione peroxidase (GPx). For peroxisomes to obtain GPx there is a need to convert reduced glutathione (GSH) to its oxidized form (GSSH). For regeneration of GSH, the presence of (29) glutathione reductase (GR) – glutathione cycle is necessary.
Adapted from Refs. [46], [71], [132].
A – Disruption of intercellular junctions
The intercellular junctions of the intestinal epithelium are constituted of tight junctions (TJ), adherent junctions (AJ), and desmosomes. The epithelial tight junctions form a barrier to the entry of allergens, toxins and pathogens across the epithelium into the interstitial tissue. ROS and RNS can promote disruption of both, TJs and AJs. NO· and ONOOH cause nitration of actin present in the cell cytosol. Concomitant to RNS, H2O2• and HO• can cause dephosphorylation (serin/thyrosin) or phosphorylation (thyrosin) of occludin – connected to actin by ZO (Zonula occludens) (1, 2 or 3) proteins – and phosphorylation (tyrosine) of β-catenin – which connect E-cadherin with α-catenin and this to actin. Both, actin rearrangement (caused by nitration) and (de)phosphorylation of adherent/linker proteins, promote disruption of intercellular junctions, barrier dysfunction and consequent increase of intestinal permeability to neutrophil and bacterial/toxin infiltration and hence the inflammatory processes.
B – Neutrophils and OS
Neutrophils are the principal ROS and RNS sources during the inflammatory process. These cells produce O2•– from NOX and NO• from iNOS. H2O2 and Cl- are produced by the myeloperoxidase (MPO) enzyme, which leads to the production of hypochlorus acid (HOCl). Both MPO and HOCl cause LP and tissue damage.
To regulate the destructive effects of RONS, vital tissues are equipped with an intricate antioxidant defense system. These antioxidant control systems consist of enzymes such as SOD, CAT, GPx and peroxiredoxins [52], as well as antioxidant compounds, like α-tocopherol, β-carotene, vitamin C, GSH, bilirubin, urate, zinc, selenium and copper [53]. When this system fails, OS occurs [12]. Increased formation of RONS in the colonic mucosa in animal models of IBD has been known to be correlated not only with disease severity and progression, but also with extra-intestinal manifestations of UC, such as hepatobiliary disorder [54].
Inhibition of both inflammatory mediators and ROS production would provide an important protective and therapeutic treatment for IBD [47], [55], [56]. Therefore, identifying new antioxidants for IBD treatment has recently attracted much attention worldwide and will be discussed below.
For instance, some authors observed no difference [57], decreased [58] or increased levels [14], in plasma/erythrocyte, of antioxidant enzymes in patients with active intestinal inflammation. In a study [16], the authors showed significant modifications regards levels of OS markers in the serum of patients with active IBD, such as decreased SOD and GPx activity, and a low antioxidant profile and increased lipid peroxidation in patients in the remission phase. In tissue biopsies, an increase of malondialdehyde (MDA) was found, in both UC and CD patients [59] and reduced GPx and glutathione (GSH) activity/levels in inflamed CD mucosa [60]. A possible explanation given by the authors for low GPx and SOD in remission patients is the consumption of antioxidants present in the diet or supplementation during active phases. Another and more tempting hypothesis is that the patients suffering from IBD, even if they are in remission, have a low antioxidant defense, i.e. a pre-existing redox imbalance condition [18].
Despite the difficulty of comparing antioxidant capacity, due to the use of different methods, single versus chemical products in mixture, one table, listing chemicals, pure and in mixture, functional foods, and others, is herein included and the main biomarkers of OS analyzed (see Table 1). There are several controversies and contradictory results, which point out to the necessity of further experimental research.
Table 1.
Compounds and mixtures of compounds with antioxidant effects tested in inflammatory bowel disease: articles published from 2009–2015/06.
| Compounds | Molecular mechanism/ plant origin | Refs |
Antioxidant action |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
↓RONS |
↓ RONS synthesis |
↓ RONS damage |
Improved antioxidant defense |
||||||||||||||
| NO• | ROS | iNOS | COX2 LPO NOX | MPO | NF-κB /Iκ-Bα | LP | PTN | DNA | SOD | CAT | GPX | GR GSH GST | Nrf2 | AOC | |||
| Drugs | |||||||||||||||||
| Telmisartan | Angiotensin II receptor antagonist | [99] | X | X | X | X | X | X | X | X | X | ||||||
| [100] | X | X | |||||||||||||||
| Olmesartan | [251] | X | X | X | |||||||||||||
| Rivastigmine | Cholinergic agent | [252] | X | X | |||||||||||||
| Aminoguanidine | Selective iNOS inhibitor | [154] | X | X | X | ||||||||||||
| Sildenafil citrate (Viagra) | Therapy for erectile dysfunction terase | [253] | X | X | X | ||||||||||||
| [254] | X | X | X | ||||||||||||||
| Setarud (IMOD) | Immunomodulator | [167] | X | X | X | ||||||||||||
| Carvedilol | Nonselective β-adrenoceptor blocker | [255] | X | X | X | X | X | X | X | ||||||||
| Spironolactone | Aldosterone receptor antagonist | [256] | X | X | X | ||||||||||||
| Fluvoxamine | Selective serotonin reuptake inhibitors | [257] | X | ||||||||||||||
| Fc11a-2 (1-ethyl-5-methyl-2-phenyl-1H-benzo[d]imidazole) | – | [258] | X | ||||||||||||||
| Sinvastatin | Inhibitor of hydroxymethylglutaryl COA reductases | [101] | X | X | X | X | |||||||||||
| Rosuvastatin | [101] | X | X | X | X | ||||||||||||
| Amlodipine | Calcium channel blockers | [259] | X | X | X | ||||||||||||
| Lazaroid U-74389G | Nonglucocorticoid analogs of methylprednisolone | [260] | X | X | X | ||||||||||||
| Hormones | |||||||||||||||||
| Melatonin | Sleep metabolism | [104] | X | X | X | X | X | X | |||||||||
| [96] | X | X | X | X | |||||||||||||
| [234] | X | X | |||||||||||||||
| [109] | X | X | |||||||||||||||
| [108] | X | ||||||||||||||||
| Ghrelin | Stimulation of appetite and gastrointestinal motility | [261] | X | X | X | ||||||||||||
| Adrenomedullin | Gastrointestinal peptide | [262] | X | X | X | ||||||||||||
| Synthetic compounds | |||||||||||||||||
| N-acetylcysteine (NAC) | [120] | X | X | X | X | X | |||||||||||
| [235] | X | X | X | ||||||||||||||
| [236] | X | ||||||||||||||||
| [237] | X | X | X | X | |||||||||||||
| [238] | X | X | X | X | |||||||||||||
| Bis(1-hydroxy-2,2,6,6-tetramethyl-4-piperidinyl) decandioate | [239] | X | |||||||||||||||
| [240] | X | X | |||||||||||||||
| 3,4-Oxo-isopropylidene-shikimic acid (ISA) (derivative of shikimic acid (Illicium verum Hook)) | [241] | X | X | X | X | ||||||||||||
| [242] | X | X | X | X | X | X | X | ||||||||||
| [243] | X | X | X | X | X | X | |||||||||||
| 3-(3-Pyridylmethylidene)-2-indolinone | [93] | X | X | ||||||||||||||
| Chito-oligosaccharides | [263] | X | X | X | X | ||||||||||||
| Peptide P-317 | [264] | X | |||||||||||||||
| Chemicals derived from sources other than vegetals | |||||||||||||||||
| Superoxide dismutase recombinant Lactobacillus fermentum | [126] | X | X | X | X | ||||||||||||
| Lecithinized superoxide dismutase | [124] | X | X | ||||||||||||||
| Lipoic acid | [55] | X | X | X | X | X | X | ||||||||||
| Citicoline | [265] | X | X | X | |||||||||||||
| Propionyl-L-carnitine | [128] | X | X | ||||||||||||||
| Butyrate, Lactobacillus casei, and L-carnitine | [266] | X | X | X | |||||||||||||
| Methylsulfonylmethane (dietary supplement) | [267] | X | X | X | X | ||||||||||||
| Spirulina (dried biomass of Arthrospira platensisa-microalgae) | [248] | X | |||||||||||||||
| [247] | X | X | X | X | X | ||||||||||||
| Dunaliella salina (microalgae) | [247] | X | X | X | X | X | |||||||||||
| Aspergillus nidulans (fungus) | [268] | ||||||||||||||||
| Inonotus obliquus (fungus) | [269] | X | X | ||||||||||||||
| Ursodeoxycholic acid (secondary bile acid) | [270] | – | – | – | |||||||||||||
| Polyphenols | |||||||||||||||||
| Colon specific resveratrol | [137] | X | |||||||||||||||
| Trans Resveratrol | [138] | X | |||||||||||||||
| [244] | X | X | X | X | |||||||||||||
| [140] | X | ||||||||||||||||
| Resveratrol | [142] | X | X | ||||||||||||||
| [135]* | X | ||||||||||||||||
| [141] | X | X | X | X | |||||||||||||
| [143] | X | X | |||||||||||||||
| Piceatannol (hydroxylated analog of resveratrol) | [143] | X | X | ||||||||||||||
| Caffeic acid | [271] | X | |||||||||||||||
| 4-Vinyl-2,6-dimethoxyphenol (canolol) | [11] | X | X | X | X | ||||||||||||
| Verbascoside | [272] | X | X | X | |||||||||||||
| Ellagic acid | [273] | X | X | X | X | ||||||||||||
| [274] | X | X | X | ||||||||||||||
| β-Sitosterol, campesterol, stigmasterol and brassicasterol | [32] | X | |||||||||||||||
| Curcumin | [151] | X | X | X | X | ||||||||||||
| [152] | X | X | X | X | X | ||||||||||||
| [153] | X | X | |||||||||||||||
| [154] | X | X | X | ||||||||||||||
| Coumarin | [246] | X | X | X | X | ||||||||||||
| [76] | X | X | X | ||||||||||||||
| [245] | X | X | |||||||||||||||
| Apple polyphenols extract | [275] | X | |||||||||||||||
| Green tea polyphenols | [276] | X | |||||||||||||||
| Epi-gallocatechin-3-gallate plus 1-piperoylpiperidin (piperine) | [277] | X | X | ||||||||||||||
| Piperine | [278] | X | X | X | X | X | |||||||||||
| Peracetylated epi-gallocatechin-3-gallate | [163] | X | X | X | |||||||||||||
| Epi-gallocatechin-3-gallate | [279] | X | |||||||||||||||
| Proanthocyanidins from grape seeds | [165] | X | X | ||||||||||||||
| [166] | X | X | X | X | |||||||||||||
| [85] | X | X | X | X | |||||||||||||
| Quercetin | [155] | X | X | X | |||||||||||||
| [156] | X | X | X | ||||||||||||||
| [280] | X | X | X | X | |||||||||||||
| Diplacone | [281] | X | X | X | |||||||||||||
| Mimulone | [281] | X | |||||||||||||||
| Amentoflavone | [83] | X | X | X | X | X | |||||||||||
| Myricetin | [172] | X | X | X | X | X | |||||||||||
| Naringenin (4,5,7-trihydroxyflavonone) | [170] | X | X | X | X | ||||||||||||
| Kolaviron | [171] | X | X | X | X | X | X | ||||||||||
| 3′-Hydroxy-5,7,4′-trimethoxyflavone from Zeyheria montana | [282] | X | X | ||||||||||||||
| Other natural active compounds of medicinal plants | |||||||||||||||||
| Thymoquinone | Nigella sativa | [283] | X | X | X | ||||||||||||
| Sesamol (phenolic derivate) | Sesamum indicum | [284] | X | X | X | ||||||||||||
| Indicaxanthin (pigment) | Opuntia ficus-indica | [177] | X | X | X | X | X | ||||||||||
| Ginsenoside Rd | Panax notoginseng | [285] | X | X | X | X | X | ||||||||||
| Iridoid glycosides | Folium syringae | [286] | X | X | X | X | |||||||||||
| Embelin (quinone) | Embelia ribes | [287] | X | X | X | ||||||||||||
| [288] | X | X | X | ||||||||||||||
| Sanguinarine (alkaloid) | Sanguinaria canadenses and Argemone mexicana | [289] | X | X | |||||||||||||
| Taurohyodeoxy-cholic acid | Pulvis fellis suis | [290] | X | ||||||||||||||
| Berberine (alkaloid) | Coptidis japonica and Mahonia aquifolium | [178] | X | X | X | X | X | X | X | ||||||||
| Cannabigerol | Cannabis sativa | [179] | X | X | X | X | X | ||||||||||
| Cannabidiol | [180] | X | X | X | X | ||||||||||||
| 3,3′-Diindolylmethane | Brassica | [291] | |||||||||||||||
| Flavanolignan | Sylmarin (Silybum marianum)+Selenium nanoparticles | [292] | X | X | X | X | X | X | |||||||||
| Aloin (anthraquinone) Aloesin (chromone) | Aloe vera Aloe-gel | [293] | X | ||||||||||||||
| Oligonol | 17.6% of catechin-type monomers and 18.6% of proanthocyanidin dimers and trimers | [294] | X | X | X | X | X | X | |||||||||
| Cavdina | Corydalis impatiens | [295] | X | X | X | X | X | ||||||||||
| Fraxinellone (lactone) | Cortex dictamni | [296] | X | X | X | X | |||||||||||
| Asiatic acid (triterpenoid compound) | Centella asiatica | [297] | X | X | |||||||||||||
| Isatin (1H-indole-2,3-dione) | Heterocyclic compounds found in various medicinal plant species | [81] | X | X | X | X | X | ||||||||||
| Allicin | Garlic | [298] | X | X | X | X | X | X | X | ||||||||
| Functional food and nutrients | |||||||||||||||||
| Camel's milk | [31] | X | X | X | |||||||||||||
| Extra virgin olive oil diet enriched with hydroxytyrosol (polyphenol) | [199] | X | X | X | X | ||||||||||||
| [195] | X | X | |||||||||||||||
| [188] | X | X | |||||||||||||||
| [299] | X | X | X | ||||||||||||||
| [300] | X | X | X | X | |||||||||||||
| [301] | X | X | |||||||||||||||
| Vitamin E | [47] | X | X | X | X | ||||||||||||
| Selenium and vitamin E | [209] | X | X | X | |||||||||||||
| Vitamin C, vitamin E, and glutathione | [210] | – | – | – | X | ||||||||||||
| L-arginine and gallic acid | [302] | X | X | X | X | X | |||||||||||
| Anti TNFα+zinc acetate | [214] | X | |||||||||||||||
| Coenzyme Q10 | [259] | X | X | X | |||||||||||||
| Lacto-wolfberry (formulated product of wolfberries in skimmed milk) | [303] | X | X | X | X | X | |||||||||||
| Vitamins C and E | [215]* | X | |||||||||||||||
| Vitamin C, vitamin E, β-carotene, zinc, selenium and glutamine | [216]* | – | – | ||||||||||||||
| Probiotics | |||||||||||||||||
| Recombinant probiotic | [230] | X# | X# | ||||||||||||||
| Modified Lactobacillus casei BL23 strains | [220] | X | X | ||||||||||||||
| L. delbrueckii subsp. bulgaricus B3 strain; L. delbrueckii subsp. bulgaricus A13 strain | [227] | X | X | X | X | X | |||||||||||
| Lactococcus lactis subsp. cremoris FC | [304] | X | X | ||||||||||||||
| Ultrabiotique® (Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium lactis and Bifidobacterium breve). | [228] | X | |||||||||||||||
| Lactobacillus fermentum | [229] | X | |||||||||||||||
| TR5 (Streptococcus thermophilus, Lactobacillus reuteri, Bifidobacterium bifidium, Latobacillus acidophilus, and Latobacillus casei) | [305] | X | X | X | X | X | X | X | |||||||||
| Lactobacillus pentosus var. plantarum C29 | [306] | X | X | X | X | X | X | ||||||||||
| Lactococcus lactis | [307] | X | X | ||||||||||||||
| Ultrabiotique® (L. acidophilus, L. plantarum, B. lactis and B.breve) | [308] | X | X | X | |||||||||||||
| Lactobacillus plantarum 21 | [309] | X | X | X | |||||||||||||
| Butyricicoccus pullicaecorum | [310] | X | |||||||||||||||
AOC=antioxidant capacity; CAT=catalase; COX2=cyclooxigenase type 2; DNA=deoxyribonucleic acid; GPx=glutathione peroxidase; GR=glutathione reductase; GSH=glutathione; GST=glutathione S-transferase; iNOS=inducible nitric oxide synthase; Iκ-Bα=nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; LP=lipid peroxidation; LPO=lipoxygenase; MPO=myeloperoxidase; NF-κB=Nuclear Factor Kappa-light-chain-enhancer of activated B Cells; NO•=nitric oxide; NOX=nicotinamide adenine dinucleotide phosphate-oxidase; Nrf2=nuclear factor erythroid 2; PTN=protein; Refs=references; RONS=reactive oxygen and nitrogen species; ROS=reactive oxygen species; SOD=superoxide dismutase. –= did not alter; * human study; #clinical studies, in human beings
The relationship between colitis and cancer is also associated to activation of common tumor-related signaling pathways, including mutations of the p53 gene, the most frequently reported somatic gene alterations in human cancer, leading to accumulation of p53 gene products in tumor cells that can initiate an immune response with generation of circulating anti-p53 antibodies (p53Abs) [61]. Several studies have demonstrated that p53 overexpression is found in IBD patients and this fact is closely associated with neoplasia, especially in those with mucosa dysplasia [62]. The p53 activation is intrinsically associated with OS, by inducing the expression of p85, which may function as a signaling molecule during ROS-mediated p53-dependent apoptosis. p85 is a known regulator of phosphatidyl inositol-3 kinase (PI3K); however, its function during ROS-induced apoptosis is independent of PI3K [63]. p53 also induces NO•– cellular stress, and chronic inflammation, as well as elevated levels of the inducible nitric oxide synthase (iNOS) and increased TP53 mutation [64]. Staib et al. [65] identified 14 common genes among NO•, H2O2, DNA replication arrest, and hypoxia, and interestingly 8 of them are known as p53 target genes [65].
3.2. Oxidative stress biomarkers
As previously mentioned, OS is a crucial pathophysiological factor for human IBD. It is therefore important to identify the best biomarkers for the diseases, in order to verify whether the applied therapy is effective or not, in controlling the symptoms of IBD. Antioxidant enzymes such as SOD, GPx and CAT, and the non-enzymatic sulfhydryl groups play a major role in the organism defense against excess RONS generation, but changes in this profile seem to be the most difficult to quantify when looking at the various studies done on IBD. In fact, the identification of OS biomarkers is an important challenge to the scientific community as well as the analysis of the sensitivity of these biomarkers in the several biological matrixes (urine, blood, and tissue etc.) and how analytical procedures can compare, appropriately, each of these biomarkers [66].
As it has been said, UC and CD subjects exhibit increased LP [59], and for that, MDA or thiobarbituric acid reactive substances (TBARS), an indirect measure of MDA, is extensively used as a biomarker for oxidative damage. However, because other biological materials may react with thiobarbituric acid, resulting in high TBARS levels, this marker is under scrutiny. Other molecules derived from LP, such as hydroperoxides, isoprostanes, conjugated dienes and others have been used by the scientific community, but like MDA, have advantages and limitations [67]. Among these molecules, F2-isoprostane, measured by mass spectrometry, has been the best general indicator of non-enzymatic lipid peroxidation under normoxic conditions [68]
Myeloperoxidase (MPO), a heme protein secreted by phagocytes, especially neutrophils, is a marker for local leukocyte sequestration and inflammation. An increase in MPO activity and the generation of NO• has also been demonstrated in inflamed colon biopsies and both are related to the disease's progression [69]. Reports have demonstrated a significant correlation with other OS markers, such as MDA and H2O2, in patients with metabolic syndrome [70]. In bowel, MPO is present in neutrophils and macrophages derived from inflamed mucosa infiltration in the damaged area and rupture of the colonic barrier. They produce both inflammatory mediators (cytokines and chemokines) and OS through the generation of HOCl and oxidation of the unsaturated fatty acids present in the plasma membrane [71] (Fig. 4). Furthermore, MPO levels are found to be elevated in malignant tissues, suggesting that MPO-derived oxidants play a role in colon cancer [72].
For Hartmann, besides lipid peroxidation, the increase of iNOS expression and consequently NO• levels in rats, was associated with a significant decrease in anal sphincter pressure [73] and consequent increased diarrhea and other IBD symptoms. Furthermore, these compounds cause damage to cells of the mucosa and submucosa of the bowel [74]. iNOS is represented by three isoforms of NOS and is found in macrophages and smooth-muscle cells. It allows the production of NO• in large quantities as part of the mechanism of cell death in response to lipopolysaccharides, cytokines, and glucocorticoids. As such, this enzyme and its metabolites are involved in septic shock and inflammatory disorders [75].
Another common metabolite, GSH, has been used as an alternative indicator of both inflammation and OS, once its intracellular levels suffer modification due to its action in the extracellular pool, which is related with its function in detoxification and protection of cells from chemical- and oxidant-induced injuries [76]. In inflammatory diseases, for instance, the rate of consumption of GSH may increase dramatically [77]. For IBD subjects, contradictory results have been reported in terms of both GSH levels and GSH/GSSG ratio. In colon biopsies, either a decrease [78] or no changes in GSH levels [79], along with an increase in GSSG levels [78] had been detected. In fact, in animal colitis models, GSH levels are normally reduced [80], [81], [82].
According to Sakthivel et al. [83], SOD levels increase in UC induced by intrarectal acetic acid. The elevation of SOD and other antioxidants can be explained by the fact that in IBD, inflammation and OS are present and this SOD increase would protect the tissue against oxidative damage. However, in studies on UC induced by acetic acid [82], [84] and TNBS [81], [85], [86], SOD activity was reduced, which clearly indicates increased levels of OS, which may damage cells through lipid peroxidation of membranes and oxidation of cellular proteins [84]. Similar results were observed for GPx. According to Rabelo Socca et al [81] and [87], GPx activity in rats with UC induced by TNBS, increased, whereas for Mueller et al., in a DSS (dextran sulfate sodium) UC model, this enzyme activity was reduced [88].
The recognition of ROS/RNS and redox-mediated protein modifications as transducing signals has opened up a new field in cell regulation and provided a novel way of controlling disease processes. Such approach has been proven feasible for gene expression governed by the transcription factor NF-κB [89]. This mediator is activated when membrane cell receptors, such as interleukine 1 beta (IL-1β), TNF receptor 1 (TNFR1), nicotinamide adenine dinucleotide phosphate-oxidase (NOX) and toll-like receptors type 4 (TLR4) promote the phosporylation of its NF-κB inactive form (IκBα). After entry into the nucleus, NF-κB stimulates pro-inflammatory, pro-oxidant and pro-apoptotic genes, for instance, COX2 (cyclooxygenase 2), iNOS, caspases and others [90].
Still in the field of transcription factors, another anti-inflammatory mediator, nuclear erythroid 2 factor (Nrf2), is emerging as an important OS biomarker. When activated for antioxidant activity, in addition to inhibition of NF-κB, it causes attenuation of phosphorylated IκB and, in consequence, NF-κB degradation [91]. Nrf2 also stimulates activation of over two dozen genes involved in antioxidant and anti-inflammatory activities such as antioxidant response element (ARE), NAD(P)H quinone oxidoreductase-1 (NQO-1), heme oxygenase-1 (HO-1), peroxiredoxin 1, γ-glutamylcysteine ligase, GPx, and glutathione disulfide reductase [92]. Nrf2−/− mice had been used successfully in experimental model of colitis, confirming the closely role of this nuclear factor in bowel inflammation [93].
However, more recently, some results have brought doubts about the antioxidant and anti-inflammatory role of Nrf2. Kathiria et al. [94] identified an important effect of the prohibitin 1, a protein which regulates cell cycle progression, apoptosis, and transcription factor activity. It was shown to be decreased in mucosal biopsies from UC- and Crohn's disease-afflicted patients and in animal models of colitis. According to these authors, this protein maintained increased ARE activation and decreased intracellular ROS levels and increased colonic levels of the antioxidants HO-1 and NQO-1 via a mechanism independent of Nrf2. On the other side, [95] discovered a pro-oxidant action by Nrf2 via induction of transcription Kruppel-like factor 9 (Klf9) and further cell death.
Therefore, several agents, in special, dietary compounds, are used to modify or modulate molecular targets such as oxidative and or inflammatory markers, with the objective of prevention/amelioration of UC [37].
Thus, these results confirm the need for more investigations regarding redox profiles and markers in both states of health and disease. A summary of some biomarkers of OS found in this review is displayed in the Graphic Abstract.
3.3. Antioxidant therapy
When the antioxidant capacity of the damaged mucosa is compromised, the use of nontraditional therapy such as drugs, hormones, natural or synthetic substances and live organisms that eliminate RONS, inhibit cell damage (LP, protein and DNA modification) and improve the activity of antioxidant enzymes, can be beneficial, either associated or not with anti-inflammatory medicines [74]. Thus, agents that can target multiple molecular pathways, have fewer side effects, and are less expensive have enormous potential in the treatment of UC, with reduction of its severity, and in consequence, lead to a decrease in systemic damage [96].
3.3.1. Drugs, hormones, synthetic compounds and chemicals derived from sources other than vegetables
Pharmacological treatment of IBD attempts to minimize the symptoms associated with structural and functional abnormalities of the mucous membranes [23]. Current therapy includes corticosteroids and immunosuppressive agents (Fig. 2) and can cause serious adverse effects such as gastrointestinal problems (diarrhea, cramping, and abdominal pain), anemia, carcinogenesis, hepatotoxicity, nephrotoxicity and hypersensitivity reactions [97]. Another important observation refers to low or no response of some people to these medications [74].
3.3.1.1. Inhibitors of angiotensin II type 1
Among the studies evaluated in this review, the angiotensin II (Ang II) receptor antagonists (telmisartan and olmesartan – Fig. 2) have been shown to have beneficial outcomes (Table 1). These drugs act on angiotensin II type 1 (AT1) receptors, and as such, decrease angiotensin II action. This peptide promotes tissue inflammation through up-regulation/activation of NADPH oxidase with consequent generation of O2•– and causes activation of NF-kB, with sequential production of pro-inflammatory cytokines, chemokines, growth factors, and adhesion molecules, which cause inflammation and fibrosis [98]. Among several candidates as Ang II receptor blockers (ARBs) such as valsartan and olmesartan, telmisartan (TLM) (Fig. 2) was the only one with anti-inflammatory and antioxidant characteristics. It blocks Ang II AT1 receptors and is a partial agonist of peroxisome proliferator-activated receptor-gamma (PPAR-γ), a nuclear transcription factor that plays a role in the regulation of carbohydrate and lipid metabolism, and suppresses inflammatory gene expression, with this effect being independent of the blood pressure-lowering effects induced by AT1 receptor antagonism, thus showing a marked protective effect on colitis [99].
According to Arab et al. [99], oral treatment with telmisartan (TLM) in rats (10 mg/kg bw/d for 1 week before induced colitis+4 days after induced colitis) suppressed ROS generation by inhibition of NF-kB/p65, COX2 (cyclooxygenase type 2) and iNOS mRNA expression, as well as NF-kB/p65 protein colonic expression. Other antioxidant actions attributed to TLM were suppression of lipid peroxidation, scavenging of NO•, increasing of GSH levels, of total antioxidant capacity (TAC), and of SOD and GPx activity [99]. In a study by Guerra et al., they evaluated the use of TLM (1, 3, and 5 mg/kg bw/d) orally for 3 days before induction of UC in rats and also 2 and 24 h after induction, which showed that a dose of 5 mg/kg reduced colonic MPO activity and colonic levels of MDA [100]. These findings highlighted the beneficial effects of TLM for the treatment of IBD, which is mediated through modulation of colonic inflammation and OS (Table 1).
3.3.1.2. Inhibitors of hydroxymethylglutaryl COA reductases
The drug class called statins, in this review represented by simvastatin and rosuvastetin (Fig. 2), studied by Maheshwari et al. [101] presented an important antioxidant effect, represented by inhibition of MPO activity, decrease of lipid peroxidation and increase of SOD and GR activity in rats. The interest to test this class in UC is related to its anti-inflammatory and endothelial cell protective actions, independent of their antihyperlipidemic effects [102]. Besides attenuating OS, these drugs, especially simvastatin, also improve histologic parameters in TNBS-induced colitis in rats.
3.3.1.3. Hormones
Another nontraditional therapy, successfully tested on IBD, is hormone therapy, specifically melatonin (MEL) (Fig. 2). Chemically, MEL or N-acetyl-5-methoxytryptamine is a derivative of the essential amino acid tryptophan. In the gastrointestinal tract (GI), MEL comes from both pineal melatonin and de novo synthesis within the GI tract. It may have a direct effect on many GI tissues, serving as an endocrine, paracrine, or autocrine hormone, influencing the regeneration and function of epithelium, modulating the immune milieu in the gut, and reducing GI muscle tonus by targeting smooth muscle cells [103]. There is a large body of evidence depicting the protective effect of MEL in various experimental and clinical conditions [104]. It plays an important role as a regulator of inflammation, as well as in proper immune system and antioxidant system function for intestinal disorders [105]. In animal experiments, MEL administration was shown to have anti-inflammatory action through inhibition of IL-10, IFN-γ, TNF-α, IL-6, and NO•. As such, MEL also shows antioxidant properties such as free-radical scavenging; reduction of HO•, ONOO-, RO2• and singlet oxygen levels [106]; inhibition of COX2 expression and NF-kB activation [107] and iNOS expression [108]; and increase of Nrf2 (nuclear factor erythroid 2) expression [104], which is a nuclear mediator for anti-inflammatory and antioxidant genes. All these results suggest that MEL may exert benefits on UC by reducing or controlling inflammation and OS. According to Tahan et al. [109], 100 mg/kg.bw/d MEL i.p. for 3 days in mice, decreased ROS synthesis, by inhibition of colonic MPO activity, as well as RONS damage by decrease of MDA colonic levels. In this study, MEL also ameliorated antioxidant defenses by increasing colonic GSH levels and colonic SOD activity. In a study using mice conducted by Trivedi et al., a lower dose of MEL was tested: 1 mg/kg.bw/d MEL for 8 and 18 weeks, after colitis induction by dextran sulfate sodium (DSS). This treatment caused a significant decrease in the levels of inflammatory and oxidative colonic markers: MPO activity, COX2, signal transducer and activator of transcription 3 (STAT3) and NF-kB colonic expression, TBARS colonic levels and DNA damage [104].
Changes in MEL metabolism was also observed in patients with UC. These changes are related to the severity of the disease, and according to [110], adjuvant MEL therapy may help in sustaining remission in patients with UC. Although none of these studies has evaluated OS, the potential benefit of MEL is clear. New trials to analyze the antioxidant effect of MEL are needed.
3.3.1.4. Synthetic substances
A synthetic substance (pro-drug) with various antioxidant effects on colitis is N-acetylcysteine or NAC (Fig. 2). It acts as a cysteine prodrug and a GSH precursor [111]. Being a thiol (R-SH), it can be oxidized by various radicals and also serve as a nucleophile [111]. NAC is an artificial antioxidant that has shown positive results in both IBD animal models [112] and clinical trials [113], in addition to being used with success in the treatment of several disorders, such as intoxication [111], cardiac ischemia-reperfusion [114], chronic obstructive pulmonary disease (COPD) [115], bronchitis [116], HIV/AIDS (Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome) [117], psychiatric disorders [118] and diabetes [119].
Antioxidant capacity reduction in a cellular environment is mainly due to a decrease of GSH and/or increased oxidized glutathione (GSSG) [116]. GSH depletion has been observed in both IBD patients and different models of colitis induction [120]. NAC increases antioxidant defense activity by providing cysteine, which is required for GSH synthesis. Furthermore, NAC decreases OS by RONS (HO•, NO2•) scavenging, along with carbonate radical (CO3•–) [111] and metal chelation (Cu+2, Fe+3, Cd2+, Hg2+ and Pb2+) which are metals involved in ROS generation and in the inflammatory response [121].
The anti-inflammatory and antioxidant activities of NAC have also been confirmed through observation of NF-kB inhibition [89]. This antioxidant activity may contribute to the therapeutic effects of NAC on IBD. According to [120], oral NAC (150 mg/kg bw/d) for 45 days along with 3 DSS cycles, generated a moderate impact on colonic oxidation of lipids and proteins, decreased colonic MPO and NO• serum levels, and improved colon antioxidant status by increasing GSH and CAT activities, suggesting that a long term NAC diet might be beneficial for IBD.
Within the category “synthetic antioxidants and chemicals derived from sources, other than vegetables”, we found substances naturally produced by human body or other non-probiotic alive organisms. Here, it stands the real therapeutic possibility of modified SOD and propionyl-l-carnitine (PLC) (Fig. 2), both already in clinical trial phase due to their high therapeutic power, however in this review, during the search period, there was no evaluation of OS markers (Table 1).
Due to its significant ROS scavenging ability, great interest has been focused on SOD, for therapeutic use. However, because of extremely rapid plasma clearance time, instability, and immunogenicity in vivo, the clinical application of SOD as a therapeutic agent has been very limited [122]. Several attempts to obtain active and stable SOD have been tested successfully in lung inflammation [123] as well as for IBD treatment. Inshihara et al. utilized a lecithinized human Cu/Zn–SOD (PC–SOD), in which four phosphatidylcholine-derived molecules were covalently bound to each SOD dimer. These authors observed, using inflammatory markers, that PC–SOD, administered intravenously (i.v.), decreased both MPO activity and phospho-NF-κB p65 [124]. In fact, administration of PC–SOD (40 mg/kg/d) for 14 days, had shown a safe and beneficial effect on UC-Disease Activity Index (DAI) [125].
Another modified SOD applied to IBD treatment was Lactobacillus fermentum, a recombinant superoxide dismutase (rec-SOD). This rec-SOD was able to improve redox imbalance by increasing oxidative defense (SOD colonic activity), decreasing ROS damage (LP) and as noted by [124] using PC–SOD, rec-SOD also reduced MPO, as well as NF-κB p65 expression in the colonic tissue [126], both in animal models.
Propionyl-L-carnitine (PLC), a natural ester derivative of L-carnitine that acts as an antioxidant molecule, is reduced in the serum of UC patients, suggesting that this molecule can serve as a source of L-carnitine, which plays an essential role in energy metabolism, since it transports activated long-chain fatty acids into the mitochondrial matrix, for β-oxidation [126]. In a phase II trial, PLC caused decreased symptoms (pain and rectal bleeding) in UC patients, compared to a placebo group [127]. Unfortunately, this multicentric trial did not investigate OS markers, and for that, they are not in our review. But it is possible to infer that, as identified by Scioli et al. [128], PLC can be an important scavenger and synthesis inhibitor of ROS, since classic IBD symptons have OS involved in the pathogenic process. These studies using experimental models and further studies in patients with UC contribute to a fundamental basis for its clinical use as a safe therapeutic alternative, but determination of safe doses and long-term studies are still necessary to ensure its correct prescription by health professionals.
3.3.2. Polyphenols and other natural active compounds of medicinal plants
Polyphenols or phenolic compounds refer to a broad group of molecules found mainly in plants that are characterized by the presence of one or more phenyl rings and one or more hydroxyl groups linked directly to the aromatic rings. They can be classified into five structural groups: phenolic acids, flavonoids, anthocyanins, stilbenes and lignans. A growing body of evidence indicates that polyphenols are health-promoting phytochemicals [129]. They possess antioxidant properties, acting directly as free-radical scavengers or indirectly by interfering with specific proteins in the redox signaling pathways that are involved in different biological functions [33].
Pure polyphenol standards can interfere with the induction of NF-kB and MAPKs signaling pathways in intestinal cells. Most of the studied polyphenols exhibited anti-inflammatory behavior by inhibiting the activation of the NF-kB cascade [130]. Furthermore, some polyphenols have been shown to have other functions, specifically in relation to the intestinal barrier (Table 1). In a study by Shigeshiro et al. [131], on experimental colitis, the administration of polyphenols: curcumin, quercetin, naringenin or hesperetin led, collectively, to restoring DSS-induced colitis, at least in part, through regulation of the colonic and tight junction (TJ) barriers. As described in the legend of Fig. 4, intercellular junctions are one of the mechanisms that provide intestinal integrity and RONS can promote disruption of these structures, by increasing intestinal permeability with neutrophil and bacterial/toxin infiltration, and by the inflammatory process [46], [132]. Although Shigeshiro et al. [131] did not demonstrate a direct relationship between the use of polyphenols and OS, it is understood that these components may reduce the harmful action of ROS on the intestinal mucosa.
3.3.2.1. Resveratrol
Among the polyphenols, one of the most extensive and commonly studied is the resveratrol-based class (Fig. 2). These compounds are widely distributed in all foods of plant origin such as red grapes, in certain berries, and in peanuts. A limiting factor for the use of resveratrol in the treatment of IBD is its low bioavailability, due to its rapid absorption and metabolism in the extensive upper gastrointestinal tract and liver. In order to improve its use for the treatment of this disease, a resveratrol-colon-specific drug delivery system was designed to overcome its poor solubility, limited stability, high metabolization in the upper gastrointestinal tract with increased efficacy, restoring enzymatic and nonenzymatic antioxidant defense, and decreased adverse effects [133], [134]. However, one of the 41 papers found is a pilot study [135], which observed important antioxidant and anti-inflammatory effects on the activity of plasmatic NF-κB, confirming that this polyphenol is able to attenuate UC activity and may act, also, as an anticancer agent [136].
Abdin investigated the use of a colon-specific delivery of resveratrol in rats with colitis and observed a decrease in colonic MPO activity [137). Similar results were observed for trans-resveratrol in studies by Larrosa et al. [138] and Yao et al. [139]. In addition, [140] reported additional antioxidant and anti-inflammatory effects of trans-resveratrol as observed by decreased COX1 and COX2 enzymes. Other markers for OS were improved after resveratrol use, such as decrease of colonic expression of iNOS and NF-kB in addition to MDA and colonic MPO levels [141], [142], [143].
Despite these promising results, the resveratrol dosage capable of maximizing health benefits without raising toxicity problems remains an area of controversial debate [144]. In fact, several studies have demonstrated that resveratrol can have a potent non-specific toxicity toward normal cells, particularly endothelial cells with a low oxidative condition. Resveratrol, in high concentration, had been recognized as a dangerous compound rather than as an antioxidant, by its metabolization through anti-xenobiotic enzymes, thus producing ROS [145]. As such, this evidence confirms the necessity for determining optimal doses of resveratrol to maintain the health of individuals.
3.3.2.2. Curcumin
Curcumin (Fig. 2), a hydrophobic polyphenol, is the major representative of curcuminoids, the main chemical constituents of the spice turmeric, and in curry powder. Turmeric is prepared from the root of the plant Curcuma longa, a member of the ginger family. It is native to India and Southeast Asia and has been used to treat a broad range of common ailments, especially inflammatory diseases, as an oral and topical medicine, in Indian Ayurvedic medicine for at least 4000 years, as well as in Chinese, Arabic and other traditional medicines [146]. Its beneficial effects on IBD were observed in animal models [147], through in vitro assays [148] and clinical trials [149], [150]. The main effects attributed to curcumin are related to anti-inflammatory and anticancer activities [136], however in analyzing Table 1, we can observe that curcumin, alone [151], [152], or in combination with other plants such as Gingko biloba [153] or compounds like aminoguanidine [154], show important antioxidant effects, like decrease of LP, and of RONS production, as well as increase of antioxidant enzymes. These results confirm that curcumin is a potential new treatment for IBD.
3.3.2.3. Quercetin
Quercetin (Fig. 2) was able to prevent oxidative injury and cell death by various mechanisms, for instance, as a ROS scavenger, an iron chelator [155] and an endogenous antioxidant (GSH) modulator [156]. It has been reported that quercetin plays an important role in cell cycle kinetics and proliferation and induction of apoptosis in cell culture [157]. Quercetin antioxidant capacity includes the most potent ROS, among them O2•-, HO•, RO2•, RO•, and reactive nitrogen species like NO• and ONOO–. According to its properties, [155] tested different orally administered doses (50 and 100 mg/kg.bw/d) for 10 days in TNBS (trinitrobenzene sulfonic acid)-colitis. In this study, both therapeutic doses significantly attenuated colonic MPO activity, MDA levels and serum NO• levels, while increasing the colonic levels of GSH. [156] evaluated the therapeutic effect and mechanisms of orally administered quercetin in microcapsules (100 mg/kg bw/d) on acetic acid-induced colitis. This microencapsulated form reduced MPO, increased GSH colon levels and presented an elevated antioxidant capacity evaluated through FRAP (Ferric Reducing Antioxidant Power).
In addition to their direct antioxidant action, some polyphenols, like quercetin, act in maintaining the intestinal barrier function by selective protein kinase C δ (PKCδ) inhibition, resulting in the promotion of occludin phosphorylation of TJ (tight junctions) assembly [158]. Although the precise mechanism underlying the quercetin mediated promotion of TJ protein assembly and expression remains unsolved, it is clear that this flavonoid induced the phosphorylation of occludin in human cell cultures concomitantly with the assembly of the TJs [159].
3.3.2.4. Catechin class
Another important polyphenols derived from the green tea, especially in the (–)-epi-gallocatechin-3-gallate (EGCG) fraction (Fig. 2), which represents up to 30% of the dry weight of green tea leaves [160]. Green tea is one of the most popular beverages consumed worldwide, being second, next to water. Although green tea consists of >2000 components, interest has focused on the polyphenols which include EGCG, (–)-epi-gallocatechin (EGC), (–)-epi-catechin gallate (ECG) and (–)-epi-catechin (EC) (Fig. 2), all of which have potent antioxidant properties [161].
In order to improve bioavailability, stability, and cancer preventive activities of EGCG, per-acetylated EGCG (AcEGCG) was synthesized by [162] and tested in colitis by [163]. According to these authors, dietary consumption of AcEGCG demonstrated to be more effective than EGCG in preventing DSS-induced colitis. The antioxidant action of EGCC can be explained by several mechanisms, including activation of Nrf2 signaling. Despite this, none of the studies evaluated the effect of EGCG on enzymatic expression or activity. EGCG, like quercetin, improved intestinal TJ barrier dysfunction. However, the molecular mechanisms underlying these EGCG-mediated effects remain unclear [131].
Three papers evaluated the effect of grape seed proanthocyanidines (GSPs) on induced UC. GSPs contain approximately 89% proanthocyanidins, with dimers (6.6%), trimers (5.0%), tetramers (2.9%) and oligomers (74.8%) [164]. The antiproliferative, antioxidant, and anticarcinogenic effects of GSPs can be explained, principally, by their action on decreasing expression of NF-κB targeted genes [85], [165], [166]. According to Ref. [165], it is possible to consider that GSPs suppress IκK (inhibitor of κB kinase) activation, and the inactivated IκK complex suppresses the phosphorylation-induced degradation of IκBα (IκB kinase α). GSPs were also able to increase colonic SOD activity [85], [166], confirming their antioxidant effect on improving enzymatic defense.
3.3.2.5. Others
Myricetin, narigenin, kolaviron and amentoflavone (Fig. 2) were also successfully, used for IBD (animal model). These substances were tested by oral [167], [168], [169] or i.p. [83] administration. Independently of routes, most flavonoids exhibited free radical scavenger ability, particularly toward NO• [155], [170], [171], [172], reduced lipid peroxidation [83], [155], [170], [171], [172], promoted an increase in antioxidant defense, principally through SOD activity [83], [155], [170], [171], [172] and GSH levels [83], [155], [156], [171]. In analyzing these results, the potential therapeutic use of flavonoids becomes evident. However, it should be pointed out that they concurrently have pro-oxidative properties, which have some associations with potential toxicity [173]. Furthermore, because this antioxidant power has only been tested in animal models, clinical trials are necessary to determine the flavonoid efficacy not only in the acute IBD phase, but also in the remission phase, in order to promote a better quality of life for IBD patients.
Other natural substances with antioxidant power had been identified in plants. A recent meta-analysis carried out on herbal therapy in humans concluded that herbal medicines may safely induce clinical response and remission in patients with IBD (histological index and adverse event) [174], however no parameter observed was related to OS. Furthermore, neither extract nor natural compound has been so far recommended by the International Society of Nutritional Therapy as adjuvant therapy for IBD [175], [176].
In this review, 21 active compounds were identified and tested in IBD therapy (Table 1), all of them in animal models. The pigment indicaxanthin (Fig. 2) [177], which antioxidant activity in vitro, involves reduction of the expression of COX-2 and iNOS associated to inhibition of NF-κB activation, and consequently inhibition of RONS synthesis and scavenging of NO• and ROS, stands out. The alkaloid berberine [178] obtained from the rhizome of Coptidis japonica and the stem bark of Mahonia aquifolium, which antioxidant action in TNBS-induced experimental colitis involves reduction of RONS synthesis (decreasing expression of COX2 and iNOS and MPO activity) plus decrease of MDA levels, stimulation of SOD and CAT activities and increase of GSH content is also noticeable. The active compounds of Cannabis sativa, cannabigerol [179] and cannabidiol [180] (Fig. 2), which present non-psychothropic actions and in face of their antioxidant activities such as scavenger of RONS and decrease of iNOS expression, may stimulate future studies.
Despite different alterations identified for antioxidant defense, the active compounds utilized in IBD treatment were able to improve redox imbalance, reducing the levels or activity of OS when the antioxidant was increased and vice versa.
Thus, identification and characterization of novel anti-inflammatory and antioxidant components of plants may aid in improving current nutritional formulas. In face of this evidence, it is feasible to assert that herbal therapy shows beneficial results for both inflammation and OS, in animal models.
3.3.3. Functional foods, antioxidant nutrients and probiotics
The impact of functional foods on the course of IBD has been examined for a small number of specific dietary factors (Table 1) [181].
3.3.3.1. Camel's milk
Camel's milk (CM) might represent a potential candidate for pharmacological efficacy on IBD with minimal adverse reactions [31]. It is different from other ruminant milk, due to its high content in minerals (calcium, iron, magnesium, copper and zinc), vitamins (A, B2, C and E) and insulin, and lower content in fat, cholesterol, protein and sugar [182]. It also contains a relatively large amount of polyunsaturated fatty and linoleic acids, which are essential for human nutrition [183]. With regard to the high risk of malnutrition in the course of IBD, nutritional support plays a crucial role in the management of these patients. CM has been consumed as an essential nutritional supplement with high energy and vitamin content to help immune-deficient patients [31]. Malabsorption syndrome has been identified as potential trigger for symptoms of IBD, such as abdominal pain, bloating and distension, altered bowel habits and malnutrition [184], [185].
Besides having antioxidant properties, CM is rich in lactoferrin, a protein with marked antioxidant and anti-inflammatory properties [186]. In view of these properties, Arab et al. evaluated the effects of CM (10 mL/kg.bw/d by gavage, one week before colitis induction (c.i.) and at the 4th day post c.i.) against TNBS-induced colitis in rats. Attenuation of colon injury was observed through suppression of OS via reduction of lipid peroxides (MDA), NO• and MPO activity along with boosting of the antioxidant defenses through restoration of colonic GSH and total antioxidant capacity (TAC) [31].
More studies are necessary to investigate if CM may be a valuable complementary approach for the management of IBD.
3.3.3.2. Extra virgin olive oil
Another functional food that may exhibit beneficial effects on IBD without undesirable effects that accompany the classical pharmacotherapy is extra virgin olive oil (EVOO) [187]. It is a typical ingredient in a Mediterranean diet. It is obtained from the fruit of the olive tree solely by mechanical or other physical means under conditions that do not lead to alteration of the oil [188]. EVOO could modulate responses against OS, for all antioxidant enzymes and consequently attenuate IBD [189]. EVOO consumption increases the activity of antioxidant enzymes such as CAT, SOD and GPX [190]. The protective role of EVOO is the result of its specific composition including high proportions of mono-unsaturated fatty acids (oleic acid), a balanced presence of polyunsaturated fatty acids and other minor components, such as α-tocopherol and phenolic compounds [191], such as simple phenols (hydroxytyrosol, tyrosol), aldehydic secoiridoids, flavonoids and lignans (acetoxypinoresinol, pinoresinol) [187].
Previous studies have shown that patients with IBD are among the highest risk groups for developing colon-rectal cancer (CRC) [192]. Experimental studies have indicated a role of dietary lipids on cancer, particularly in colon tumor development. For instance, it was demonstrated that high fat diets rich in ω-6 polyunsaturated fatty acids and saturated fatty acids promote carcinogenesis, while high fat diets rich in ω-3 fatty acids had a protective effect [193], [194]. Considering that chronic inflammation is the key predisposing factor to CRC in IBD, [195] evaluated an EVOO diet (5%) in DSS-induced colitis for 5 weeks. In these animals, suppression of COX2 and iNOS colonic expressions was observed. COX2 is expressed in response to growth factors (transforming growth factor α), pro-inflammatory cytokines (interleukin 1α) [196], while iNOS produces large amounts of NO• which is implicated in initiation, promotion and progression of tumors [197]. This study indicates that chronic feeding of a 5% olive oil diet for 5 weeks attenuates inflammation in DSS-induced colitis of rat colons, based on evaluations using COX2 and iNOS [195].
As already shown, phenolic compounds are recognized as holders of important antioxidant, anti-inflammatory, antimicrobial, antiproliferative, antiarrhythmic, platelet antiaggregant and vasodilatory effects, as well as able to modulate important cellular signaling pathways [188], [198]. EVOO has remarkable antioxidant potential derived from its high level of phenolic compounds [189]. Sánchez-Fidalgo et al. [199] investigated the effects of an EVOO diet enriched with its principal polyphenols-hydroxytyrosyl acetate (HTy-Ac) and 3,4-dihydroxyphenylglycol (DHPG) (Fig. 2) (both at 0.1% for 30 d before c.i.) on the severity of DSS-induced acute inflammation. Only the HTy-Ac supplemented group showed a reduction in COX2 and iNOS colonic protein expression. Furthermore, in DSS-treated mice, supplemental HTy-Ac blocked the activation of the NF-kB pathway and boosted antioxidant defenses through the decrease of MPO colonic activity, and reduction of the degree of polymorphonuclear neutrophils infiltration [199].
Given the few experimental studies performed, addressing the protective activity of olive oil on colon cancer and the anti-inflammatory and antioxidant properties of the compounds present in EVOO, further experimental and clinical studies are needed to investigate whether olive oil intake and its doses are effective to attenuate OS and inflammation in UC.
3.3.3.3. Micronutrients
Some micronutrients found in food, such as α-tocopherol or vitamin E and ascorbic acid (vitamin C), act as exogenous antioxidant defenses. For this reason, food rich in antioxidants or antioxidants administered as supplements are applied on a large scale in an attempt to alleviate ROS induced damage such as apoptosis, alteration in immune response modulation and innate immunity, and others [200].
Vitamin E is the blanket term that covers all biologically active tocopherols and tocotrienols and their derivatives. Numerous beneficial health effects have been proposed for vitamin E, which are attributed to its antioxidant activity [201]. Additionally, vitamin E is associated with a reduced risk of coronary heart disease [202] and colon cancer [203].
Chemically, vitamin E is a lipid-soluble vitamin, which is biologically active in tissues and plasma. Tocopherols, therefore, protect lipid membranes by quenching/scavenging singlet oxygen (and potentially also other ROS, such as HO•) [204]. Despite the beneficial action of vitamin C, which can contribute to vitamin E regeneration [205], in vivo studies have demonstrated contradictory results when this vitamin is combined with other antioxidants. According to [206], vitamin E, vitamin C and lipoic acid protect the arachidonic acid level in the brains of diabetic and non-diabetic rats. However, when a combination of high doses of vitamin E with oxidized sunflower oil is used, this vitamin presents pro-oxidant action [207].
The major impediment to vitamin E use in the treatment of acute inflammatory diseases such as UC, is its low solubility in water, preventing its oral ingestion or enema administration in patients. Therefore, the use of derivatives of vitamin E such as tocopheryl acetate and α-tocopheryl phosphate, which are more water soluble [208], have been preferred as dietary supplements.
α-Tocopherol was utilized alone [47] and in combination with other nutrients [209], [210]. Interestingly, the results had shown an absence of colonic antioxidant effect, when vitamin E was utilized with vitamin C and GSH [210]. Some meta-analyses have shown that antioxidant supplements do not result in the presumed health benefit, but paradoxically a high intake of antioxidants is associated with increased mortality [211], [212]. Those observations suggest a pro-oxidant effect of antioxidants and it seems that this deleterious effect is associated to Nrf2 mutations [213].
Other nutrients relevant to the maintenance of intestinal homeostasis are selenium (Se) and zinc (Zn), essential elements for the restructuring of antioxidant enzymes such as GPx and SOD. Selenium interacts with the active site of GPx, which is essential to combat ROS and to regenerate adipocytes by reacylation. On the other hand, zinc as a SOD cofactor is indirectly involved with oxidant defense. When this mineral was used in combination with vitamin E [209] or anti-TNF-α (a drug preconized for the treatment of CD) [214], an improved redox balance was observed in plasma and/or colonic tissue (Table 1).
Aghdassi et al., in 2003 [215], conduced the first randomized controlled trial on antioxidant (Vitamins C and E) on CD. These authors observed an important decreased of oxidative damage (reduction of LP in plasma) in patients, which received the supplementation for 4 weeks, versus placebo group. More recently, in 2008, Roggenbuck et al. [216] studied six patients with CD which received a nutritional formulation containing several antioxidants (Vitamin C, vitamin E, β-carotene, zinc, selenium and glutamine) for 4 weeks. According to those authors, this supplementation was not sufficient to improve oxidative stress and inflammatory biomarkers, but increased antioxidant status of plasma and inflamed mucosa. Thereby, they concluded that supplementation was inconclusive and stimulated to continuation this therapeutic approach in placebo-controlled trials.
From these results, it is fundamental before prescribing antioxidant nutrients, to understand that each antioxidant has its own unique biochemical profile and therefore antioxidants should not be treated as a group [201].
Recently, an interesting review conducted by Di Stasi and Costa [217] evaluated patent literature focused on chemical compounds, functional foods and biological therapy useful for the treatment of IBD. The paper selected 33 patented products during the period 2013–2014 and just one of them was included in this review, lipoic acid. However, the authors were critical about the quality of those patents, once most of them are not conclusive because they were based on data from unspecific methods that are not related to intestinal inflammation. Besides that, clinical studies have rarely been described in patients, strongly limiting their evaluation and medical applicability.
3.3.3.4. Probiotics
In recent years, a rising interest in the hypothesis that gut dysbiosis can result in immune impairment effects associated with IBD, resulted in several studies on probiotics and prebiotics. Probiotic bacteria may be defined as live microorganisms which, when administered in adequate amounts, confer a health benefit on the host [169].
The antioxidant action of probiotics can be due to ROS scavenging, metal ion chelation, enzyme inhibition, and the reduction and inhibition of the ascorbate autoxidation [218], [219], as well as the synthesis of antioxidant enzymes by bacteria used in the formulas [220]. Probiotic antioxidant power had been demonstrated in healthy subjects by increased Total Antioxidant Activity – TAA – and Total Antioxidant Status – TAS [221], in cardiovascular disease, by decreased ROS production [222], and in non-alcoholic fatty liver disease in children, as shown in a review by Yang et al. [223].
In a recent in vitro and in vivo study, using Lactobacillus and Bifidobacterium conducted by Amaretti et al., the authors identified that given the colon colonization by administered probiotics, it is conceivable that their overall protective effect could be related to activities taking place at the intestinal level, i.e., secretion of enzymes like SOD, metal-chelating activities, promotion of the production of antioxidant biomolecules such as exopolysaccharides that had shown in vitro antioxidant and free-radical scavenging activities [224].
Even it a specific pathogen has not yet been identified as an etiopathogenetic factor and a definite infectious cause cannot be considered alone, the involvement of gut microbiota in the pathogenesis of these diseases is generally recognized [225]. Several studies in UC patients have demonstrated anti-inflammatory effects and improvement of clinical markers as a result of probiotics use, in particular E. coli Nissle 1917, VSL#3, Lactobacilli and/or Bifidobacteria [169]. However, for CD, there have been no benefits that would recommend their use [226].
Although few studies (Table 1), and all in animal models, have investigated antioxidant action by probiotics, these microorganisms have been shown to increase SOD, CAT and GPx activity [220], [227], GSH levels [227], as well as to reduce MPO [228] and NO• [229] activity. However, a noticeable result was reported by Gardlik et al., who used recombinant probiotics. According to these authors, despite the lack of increase in MDA levels and advanced oxidation protein products in colitis induced by the DSS group, administration of all E. coli strains lowered these parameters, when compared to the control group [230].
This result suggests a potential antioxidant action caused by probiotics and the daily use of 200 mg of E. coli Nissle 1917 (Mutaflor) may be an option for UC patients who are unable or unwilling to take mesalazine, as recognized by the British Society of Gastroenterology [231]. However, one of the difficulties found in this review, regarding this nutraceutical, was the diversity of bacterial strains and therapeutic doses used.
Jonkers et al. conducted a systematic review about probiotics in management of IBD and concluded that future studies should focus on specific disease subtypes and disease location [232]. Besides, it is fundamental to investigate further all therapeutical possibilities of probiotics for IBD, including their antioxidant power.
4. Conclusions and future perspectives
Alternative therapies for IBD are a vast field of scientific interest. In this systematic review, 101 different substances/live organisms/functional foods with antioxidant capacity, principally for UC, were identified. However, few compounds were tested more than once, including: the angiotensin II receptor antagonist [99], [100], [233]; the inhibitors of hydroxymethylglutaryl COA reductases [101]; the hormone MEL [96], [103], [109], [234]; the synthetic substances N-acetylcysteine [120], [235], [236], [237], [238], bis(1-hydroxy-2,2,6,6-tetramethyl-4-piperidinyl)decandioate [239], [240] and 3,4-oxo-isopropylidene-shikimic acid [241], [242], [243]; resveratrol, some of its derivatives [137], [138], [140], [141], [142], [143], [244] and curcumin [151], [152], [153], [154]; the flavonoids proanthocyanidin from grape seeds [85], [165], [166]; quercetin [155], [156]; coumarin derivatives [76], [245], [246]; the microalga spirulina [247], [248]; active compounds such as cannabigerol [179] and cannabidiol [180] from Cannabis sativa [179], [180], which hampered the performance of a meta-analysis.
Several OS biomarkers were employed in all the papers, in particular: NO•, the pro-oxidant enzymes MPO and COX2, the transcriptor factor NF-kB; antioxidant enzymes SOD, CAT, GPx and the non-enzymatic antioxidant compound GSH or the ratio GSH/GSSG. This chemical diversity contributes to the understanding of the metabolic pathways. However, only few studies analyzed Nrf2 expression [11], [55], [96], [104] and, in view of its strong association with redox imbalance and inflammation, as discussed previously, the regulation of Nrf2-ARE signaling may be closely linked with pharmacological intervention. Thus, the knowledge of the mechanism and consequent control of these redox-dependent events where the tested substances are used can provide us with alternatives to attenuate inflammatory-mediated diseases at the molecular and metabolic level.
Besides the role of OS in IBD etiology, the consistent increase of RONS have an important action in the recurrence of the active phase (Fig. 1) and consequently the use of antioxidants may help in the maintenance of remission phase [36].
According to Piganelli & Delmastro (2011), optimal treatments may have to incorporate antioxidants together with anti-inflammatory agents, such as NFκB activation inhibitors, and must also take into consideration the limitations associated with the use of intact enzyme/protein therapies, such as bioavailability, immunogenicity-limited cellular accessibility, and the cost of production [249]. In this context, functional foods and natural active compounds of plants fit very well for the treatment of IBD
In vitro and in vivo preclinical models, like the ones cited in the review, are fundamental to confidently test several isolated compounds/extracts/nutraceuticals/hormones/foods, before human trials. This fact partially explains why only two papers, based on the criteria adopted (OS markers), reported the use of compounds in humans subjects: resveratrol [135], extract pycnogenol in children [250] and antioxidant nutrients [215], [216]. Others were performed and included in the present review, but could not enrich the statistics, once OS markers were not assessed.
It is clear that more research is necessary in order to implement the use of these compounds/drug/foods/synthetic substances, thus paving the way for new therapeutic possibilities to be brought to millions of IBD patients. Studies regarding pharmacokinetic parameters and metabolic profiles are essential and are normally the first approach in clinical trials [311].
It is essential that the same substance is tested several times in various conditions (acute, chronic, recidive, and CHC) and in different models before being tested in humans.
It is strongly desirable that research on IBD and their etiology or triggering factors in humans involves OS markers, because it is clear that this phenomenon is crucial to the onset of inflammation and that this, in turn, is directly related to the aggravation of the disease, by a concomitant formation of RONS. Until these issues are fully understood, in the fundamental and molecular levels, in terms of pharmacokinetics and metabolism, health professionals should assess individually the prescription of these substances/natural or synthetic compounds is valid for their patients with IBD. However, actually, in face of all results, the alternative antioxidant therapy to IBD seems promising and must be further and continuously investigated, especially, in clinical trials.
Acknowledgments
This review was supported by grants from the CNPq (4840044/2011-3, 407963/2013-8, and 458114/2014-6), PRONEX/FAPEAL/CNPq/2009-09-006 and PROCAD/CAPES/3004-2014.
Contributor Information
Fabiana Andréa Moura, Email: fabianamoura_al@hotmail.com.
Kívia Queiroz de Andrade, Email: kiviaqueiroz@hotmail.com.
Juliana Célia Farias dos Santos, Email: jcfs_nut@yahoo.com.br.
Orlando Roberto Pimentel Araújo, Email: orlanforpa@hotmail.com.br.
Marília Oliveira Fonseca Goulart, Email: mariliaofg@gmail.com.
References
- 1.Halpin S.J., Ford A.C. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am. J. Gastroenterol. 2012;107(10):1474–1482. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 2.Medhi B., Prakash A., Avti P.K., Saikia U.N., Pandhi P., Khanduja K.L. Effect of Manuka honey and sulfasalazine in combination to promote antioxidant defense system in experimentally induced ulcerative colitis model in rats. Indian J. Exp. Biol. 2008;46(August):583–590. [PubMed] [Google Scholar]
- 3.May D., Pan S., Crispin D.A. Investigating neoplastic progression of ulcerative colitis with label-free comparative proteomics. J. Proteome Res. 2010;10(1):200–209. doi: 10.1021/pr100574p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutgeerts P., Vermeire S., Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136(4):1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Barnes P.J., Adcock I.M. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1915–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 6.Tremblay L., de Chambrun G. Pineton, Vroey B. De, Lavogiez C., Delaporte E., Colombel J.-F., Cortot A. Stevens-Johnson syndrome with sulfasalazine treatment: report of two cases. J. Crohn's Colitis. 2011;5(5):457–460. doi: 10.1016/j.crohns.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Sartor R.B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126(6):1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Jw M., Wang Y., Dj T., Jk M., Bg F. Methotrexate for induction of remission in refractory Crohn's disease. Cochrane Database Syst. Rev. 2014;6(8) doi: 10.1002/14651858.CD003459.pub4. CD003459-CD003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H., Li Y.R. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp. Biol. Med. 2012;237:474–480. doi: 10.1258/ebm.2011.011358. [DOI] [PubMed] [Google Scholar]
- 10.Fok K.C., Ng W.W.S., Henderson C.J.A., Connor S.J. Cutaneous sarcoidosis in a patient with ulcerative colitis on infliximab. J. Crohn's Colitis. 2012;6(6):708–712. doi: 10.1016/j.crohns.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Fang J., Seki T., Tsukamoto T., Qin H., Yin H., Liao L., Nakamura H., Maeda H. Protection from inflammatory bowel disease and colitis-associated carcinogenesis with 4-vinyl-2,6-dimethoxyphenol (canolol) involves suppression of oxidative stress and inflammatory cytokines. Carcinogenesis. 2013;34(12):2833–2841. doi: 10.1093/carcin/bgt309. [DOI] [PubMed] [Google Scholar]
- 12.Rezaie A., Parker R.D., Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig. Dis. Sci. 2007;52(9):2015–2021. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- 13.Roessner A., Kuester D., Malfertheiner P., Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol. Res. Pract. 2008;204(7):511–524. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Tüzün A., Erdil A., Inal V., Aydin A., Bağci S., Yeşilova Z., Sayal A., Karaeren N., Dağalp K. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin. Biochem. 2002;35(7):569–572. doi: 10.1016/s0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]
- 15.Pawar P., Gilda S., Sharma S., Jagtap S., Paradkar A., Mahadik K., Ranjekar P., Harsulkar A. Rectal gel application of Withania somnifera root extract expounds anti-inflammatory and muco-restorative activity in TNBS-induced inflammatory bowel disease. BMC Complement. Altern. Med. 2011;11(1):34. doi: 10.1186/1472-6882-11-34. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achitei D., Ciobica A., Balan G., Gologan E., Stanciu C., Stefanescu G. Different profile of peripheral antioxidant enzymes and lipid peroxidation in active and non-active inflammatory bowel disease patients. Dig. Dis. Sci. 2013;58(5):1244–1249. doi: 10.1007/s10620-012-2510-z. [DOI] [PubMed] [Google Scholar]
- 17.Cadirci E., Suleyman H., Aksoy H., Halici Z., Ozgen U., Koc A., Ozturk N. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chemico-biol. Interact. 2007;170(1):40–48. doi: 10.1016/j.cbi.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 18.Oz H.S., Zhon J., de Villiers W.J.S. Pegylated arginine deiminase downregulates colitis in murine models. Mediat. Inflamm. 2012;2012:813892. doi: 10.1155/2012/813892. (7 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alzoghaibi M. a. Concepts of oxidative stress and antioxidant defense in Crohn's disease. World J. Gastroenterol. 2013;19(39):6540–6547. doi: 10.3748/wjg.v19.i39.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genser D., Kang M.H. Status of lipidsoluble antioxidants and TRAP in patients with Crohn's disease and healthy controls. Eur. J. Nutr. 1999;53(9):1999. doi: 10.1038/sj.ejcn.1600764. 1999. [DOI] [PubMed] [Google Scholar]
- 21.Marx G., Seidman E.G. Inflammatory bowel disease in pediatric patients. Curr. Opin. Gastroenterol. 1999;15(4):322. doi: 10.1097/00001574-199907000-00008. 322. [DOI] [PubMed] [Google Scholar]
- 22.Razack R., Dl S. Nutrition in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2007;23(4):400–405. doi: 10.1097/MOG.0b013e3281ddb2a3. [DOI] [PubMed] [Google Scholar]
- 23.Seidner D.L., Lashner B.A. An oral supplement enriched with fish oil, soluble fiber, and antioxidants for corticosteroid sparing in ulcerative colitis: a randomized, controlled trial. Clin. Gastroenterol. Hepatol. 2005;3(4):358–369. doi: 10.1016/s1542-3565(04)00672-x. [DOI] [PubMed] [Google Scholar]
- 24.Langmead L., Rampton D.S. Review article: complementary and alternative therapies for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006;23(3):341–349. doi: 10.1111/j.1365-2036.2006.02761.x. [DOI] [PubMed] [Google Scholar]
- 25.Ng S.C., Lam Y.T., Tsoi K.K.F., Chan F.K.L., Sung J.J.Y., Wu J.C.Y. Systematic review: the efficacy of herbal therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013;38(8):854–863. doi: 10.1111/apt.12464. [DOI] [PubMed] [Google Scholar]
- 26.Rahimi R., Shams-Ardekani M.R., Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J. Gastroenterol. 2010;16(36):4504. doi: 10.3748/wjg.v16.i36.4504. 4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debnath T., Kim D.H., Lim B.O. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules. 2013;18:7253–7270. doi: 10.3390/molecules18067253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bringiotti R., Ierardi E., Lovero R., Losurdo G., Leo A.D., Principi M. Intestinal microbiota: the explosive mixture at the origin of inflammatory bowel disease? World J. Gastrointest. Pathophysiol. 2014;5(4):550–559. doi: 10.4291/wjgp.v5.i4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P., Zhou X., Zhang L., Shan M., Bao B., Cao Y., Kang A., Ding A. Vol. 162. 2015. Anti-inflammatory effects of Huangqin tang extract in mice on ulcerative colitis; pp. 207–214. (J. Ethnopharmacol.). [DOI] [PubMed] [Google Scholar]
- 30.Rahimi R., Mozaffari S., Abdollahi M. On the use of herbal medicines in management of inflammatory bowel diseases: a systematic review of animal and human studies. Dig. Dis. Sci. 2009;54(3):471–480. doi: 10.1007/s10620-008-0368-x. [DOI] [PubMed] [Google Scholar]
- 31.Arab H.H., Salama S.A., Eid A.H., Omar H.A., Arafa E.-S.A., Maghrabi I.A. Camel's milk ameliorates TNBS-induced colitis in rats via downregulation of inflammatory cytokines and oxidative stress. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014;69:294–302. doi: 10.1016/j.fct.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 32.Aldini R., Micucci M., Cevenini M., Fato R., Bergamini C., Nanni C., Cont M., Camborata C., Spinozzi S., Montagnani M., Roda G., D’Errico-Grigioni A., Rosini F., Roda A., Mazzella G., Chiarini A., Budriesi R. Antiinflammatory effect of phytosterols in experimental murine colitis model: prevention, induction, remission study. PLoS One. 2014;9(9):e108112. doi: 10.1371/journal.pone.0108112. e108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biasi F., Astegiano M., Maina M., Leonarduzzi G., Poli G. Polyphenol Supplementation as a Complementary Medicinal Approach to Treating Inflammatory Bowel Disease. Curr. Med. Chem. 2011;18(31):4851–4865. doi: 10.2174/092986711797535263. [DOI] [PubMed] [Google Scholar]
- 34.Hur S.J., Kang S.H., Jung H.S., Kim S.C., Jeon H.S., Kim I.H., Lee J.D. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr. Res. 2012;32(11):801–816. doi: 10.1016/j.nutres.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Langhorst J., Wulfert H., Lauche R., Klose P., Cramer H., Dobos G.J., Korzenik J. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J. Crohn's Colitis. 2015;9(1):86–106. doi: 10.1093/ecco-jcc/jju007. [DOI] [PubMed] [Google Scholar]
- 36.Rana S.V., Sharma S., Prasad K.K., Sinha S.K., Singh K. Role of oxidative stress & antioxidant defence in ulcerative colitis patients from north India. Indian J. Med. Res. 2013;139:568–571. [PMC free article] [PubMed] [Google Scholar]
- 37.Saxena A., Kaur K., Hegde S., Kalekhan F.M., Baliga M.S., Fayad R. Dietary agents and phytochemicals in the prevention and treatment of experimental ulcerative colitis. J. Tradit. Complement. Med. 2014;4(4):203–217. doi: 10.4103/2225-4110.139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilardi D., Fiorino G., Genua M., Allocca M., Danese S. Complementary and alternative medicine in inflammatory bowel diseases: what is the future in the field of herbal medicine? Expert Rev. Gastroenterol. Hepatol. 2014;8(7):835–846. doi: 10.1586/17474124.2014.917954. [DOI] [PubMed] [Google Scholar]
- 39.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. C. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasconcelos S.M.L., Goulart M.O.F., Moura J.B. d F., Benfato V.M. e M. d S., Kubota L.T. Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: principais métodos analíticos para sua determinação. Quim. Nova. 2007;30(5):1323–1338. [Google Scholar]
- 41.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014;94(2):329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amasheh M., Grotjohann I., Amasheh S., Fromm A., Söderholm J.D., Zeitz M., Fromm M., Schulzke J.-D. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: a novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand. J. Gastroenterol. 2009;44(10):1226–1235. doi: 10.1080/00365520903131973. [DOI] [PubMed] [Google Scholar]
- 43.Wallace K.L., Zheng L.-B., Kanazawa Y., Shih D.Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 2014;20(1):6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis K., Caldwell J., Phan V., Prescott D., Nazil A., Wang A., Soderhölm J.D., Perdue M.H., Sherman P.M., McKay D.M. Decreased epithelial barrier function evoked by exposure to metabolic stress and nonpathogenic E. coli is enhanced by TNF-alpha. Am.J. Physiol. Gastrointest. Liver Physiol. 2008:G669–G678. doi: 10.1152/ajpgi.00382.2007. [DOI] [PubMed] [Google Scholar]
- 45.Bhardwaj P. Oxidative stress and antioxidants in gastrointestinal diseases. Trop. Gastroenterol. 2010;29(3):129–135. [PubMed] [Google Scholar]
- 46.Pravda J. Radical induction theory of ulcerative colitis. World J. Gastroenterol. 2005;11(16):2371–2384. doi: 10.3748/wjg.v11.i16.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tahan G., Aytac E., Aytekin H., Gunduz F., Dogusoy G., Aydin S., Tahan V., Uzun H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can. J. Surg. (J. Can. Chir.) 2011;54(5):333–338. doi: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keshavarzian a, Banan a, Farhadi a, Komanduri S., Mutlu E., Zhang Y., Fields J.Z. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut. 2003;52:720–728. doi: 10.1136/gut.52.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangerich A., Dedon P.C., Fox J.G., Tannenbaum S.R., Wogan G.N. Chemistry meets biology in colitis-associated carcinogenesis. Free Radic. Res. 2013;47(11):958–986. doi: 10.3109/10715762.2013.832239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banning A., Florian S., Deubel S., Thalmann S., Muller-Schmehl K., Jacobasch G., Brigelius-Flohe R. GPx2 counteracts PGE2 production by dampening COX-2 and mPGES-1 expression in human colon cancer cells. Antioxid. Redox Signal. 2008;10(9):1491–1500. doi: 10.1089/ars.2008.2047. [DOI] [PubMed] [Google Scholar]
- 51.Esworthy R.S., Yang L., Frankel P.H., Chu F.F. Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. J. Nutr. 2005;135(4):740–745. doi: 10.1093/jn/135.4.740. [DOI] [PubMed] [Google Scholar]
- 52.Thanan R., Oikawa S., Hiraku Y., Ohnishi S., Ma N. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2015;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ioannidis O., Varnalidis I., Paraskevas G., Botsios D. Nutritional modulation of the inflammatory bowel response. Digestion. 2011;84(2):89–101. doi: 10.1159/000323456. [DOI] [PubMed] [Google Scholar]
- 54.Jena G., Trivedi P.P., Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic. Res. 2012;46(11):1339–1345. doi: 10.3109/10715762.2012.717692. [DOI] [PubMed] [Google Scholar]
- 55.Trivedi P.P., Jena G.B. Role of α-lipoic acid in dextran sulfate sodium-induced ulcerative colitis in mice: studies on inflammation, oxidative stress, DNA damage and fibrosis. Food Chem. Toxicol. 2013;59:339–355. doi: 10.1016/j.fct.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 56.Wang X., Zhao L., Han T., Chen S., Wang J. Protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of polygonum multiflorum thunb, on experimental colitis in mice. Eur. J. Pharmacol. 2008;578(2–3):339–348. doi: 10.1016/j.ejphar.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Akman T., Akarsu M., Akpinar H. Erythrocyte deformability and oxidative stress in inflammatory bowel disease. Dig. Dis. Sci. 2012;57(2):458–464. doi: 10.1007/s10620-011-1882-9. [DOI] [PubMed] [Google Scholar]
- 58.Reimund J.M., Hirth C., Koehl C., Baumann R., Duclos B. Antioxidant and immune status in active Crohn's disease. A possible relationship. Clin. Nutr. 2000;19(1):43–48. doi: 10.1054/clnu.1999.0073. [DOI] [PubMed] [Google Scholar]
- 59.Bouzid D., Gargouri B., Mansour R.B., Amouri A., Tahri N., Lassoued S. Oxidative stress markers in intestinal mucosa of tunisian inflammatory bowel disease patients. Saudi J. Gastroenterol. 2014;19(3):131–135. doi: 10.4103/1319-3767.111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinto M.A.S., Lopes M.S.-M.S., Bastos S.T.O., Reigada C.L.L., Dantas R.F., Neto J.C.B., Luna A.S., Madi K., Nunes T., Zaltman C. Does active Crohn's disease have decreased intestinal antioxidant capacity? J. Crohn's Colitis. 2013;7(9):e358–e366. doi: 10.1016/j.crohns.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60(7):1777–1788. [PubMed] [Google Scholar]
- 62.Horvath B., Liu G., Wu X., Lai K.K., Shen B., Liu X. Overexpression of p53 predicts colorectal neoplasia risk in patients with inflammatory bowel disease and mucosa changes indefinite for dysplasia. Gastroenterol. Rep. 2015 doi: 10.1093/gastro/gov022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Hussain S.P., Amstad P., Raja K., Ambs S., Nagashima M., Bennett W.P., Shields P.G., Ham A.J., Swenberg J.A., Marrogi A.J., Harris C.C. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60(13):3333–3337. [PubMed] [Google Scholar]
- 65.Staib F., Robles A.I., Varticovski L., Wang X.W., Zeeberg B.R., Sirotin M., Zhurkin V.B., Hofseth L.J., Hussain S.P., Weinstein J.N., Galle P.R., Harris C.C. The p53 tumor suppressor network is a key responder to microenvironmental components of chronic inflammatory stress. Cancer Res. 2005;65(22):10255–10264. doi: 10.1158/0008-5472.CAN-05-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kadiiska M.B., Gladen B.C., Baird D.D., Germolec D., Graham L.B., Parker C.E., Nyska A., Wachsman J.T., Ames B.N., Basu S., Brot N., Fitzgerald G.A., Floyd R.A., George M., Heinecke J.W., Hatch G.E., Hensley K., Lawson J.A., Marnett L.J., Morrow J.D., Murray D.M., Plastaras J., Roberts L.J., 2nd, Rokach J., Shigenaga M.K., Sohal R.S., Sun J., Tice R.R., Van Thiel D.H., Wellner D., Walter P.B., Tomer K.B., Mason R.P., Barrett J.C. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free. Radic. Biol. Med. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 67.Niki E. Biomarkers of lipid peroxidation in clinical material. Biochim. Biophys. Acta. 2014;1840(2):809–817. doi: 10.1016/j.bbagen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Forman H.J., Augusto O., Brigelius-Flohe R., Dennery P.A., Kalyanaraman B., Ischiropoulos H., Mann G.E., Radi R., Roberts L.J., 2nd, Vina J., Davies K.J. Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015;78:233–235. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- 69.Joo M., Kim H.S., Kwon T.H., Palikhe A., Zaw T.S., Jeong J.H. Anti-inflammatory effects of flavonoids on TNBS-induced colitis of rats. Korean J. Physiol. Pharmacol. 2015;19:43–50. doi: 10.4196/kjpp.2015.19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fonseca L.J.S.D, Nunes-souza V., Guedes G. d S., Schettino-silva G., Mota-gomes M.A., Rabelo L.A. Oxidative status imbalance in patients with metabolic syndrome: role of the myeloperoxidase/hydrogen peroxide axis. Oxidative Med. Cell. Longev. 2014;15:1–14. doi: 10.1155/2014/898501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brennan M.L., Penn M.S., Van L.F., Nambi V., Shishehbor M.H., Aviles R.J., Goormastic M., Pepoy M.L., McErlean E.S., Topol E.J., Nissen S.E., Hazen S.L. Prognostic value of myeloperoxidase in patients with chest pain. New. Engl. J. Med. 2003;349(17):1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 72.Roncucci L., Mora E., Mariani F., Bursi S., Pezzi A., Rossi G., Pedroni M., Luppi D., Santoro L., Monni S., Manenti A., Bertani A., Merighi A., Benatti P., Di Gregorio C., De Leon M.P. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 2008;17(9):2291–2297. doi: 10.1158/1055-9965.EPI-08-0224. [DOI] [PubMed] [Google Scholar]
- 73.Hartmann R.M., Fillmann H.S., Isabel M., Martins M., Meurer L., Marroni N.P. Boswellia serrata has beneficial anti-inflammatory and antioxidant properties in a model of experimental colitis. Phytother. Res. 2014;1398(February):1392–1398. doi: 10.1002/ptr.5142. [DOI] [PubMed] [Google Scholar]
- 74.Kannan N., Guruvayoorappan C. Protective effect of Bauhinia tomentosa on acetic acid induced ulcerative colitis by regulating antioxidant and inflammatory mediators. Int. Immunopharmacol. 2013;16(1):57–66. doi: 10.1016/j.intimp.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 75.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1(1):244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Witaicenis A., Luchini A.C., Hiruma-Lima C. a, Felisbino S.L., Garrido-Mesa N., Utrilla P., Gálvez J., Di Stasi L.C. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: comparison with prednisolone and sulphasalazine. Chemico-biol. Interact. 2012;195(1):76–85. doi: 10.1016/j.cbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Hall A.G. Review: the role of glutathione in the regulation of apoptosis. Eur. J. Clin. Investig. 1999;29(3):238–245. doi: 10.1046/j.1365-2362.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- 78.Tsunada S., Iwakiri R., Ootani H., Aw T.Y., Fujimoto K. Redox imbalance in the colonic mucosa of ulcerative colitis. Scand. J. Gastroenterol. 2003;38:1002–1003. doi: 10.1080/00365520310005055. [DOI] [PubMed] [Google Scholar]
- 79.Kruidenier L., Kuiper I., van Duijn W., Mieremet-Ooms M.A.C., van Hogezand R.A., Lamers C.B.H.W., Verspaget H.W. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J. Pathol. 2003;201:17–27. doi: 10.1002/path.1408. [DOI] [PubMed] [Google Scholar]
- 80.Prabhu V.V., Guruvayoorappan C. Protective effect of marine mangrove Rhizophora apiculata on acetic acid induced experimental colitis by regulating anti-oxidant enzymes, inflammatory mediators and nuclear factor-kappa B subunits. Int. Immunopharmacol. 2014;18(1):124–134. doi: 10.1016/j.intimp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Rabelo Socca E.A., Luiz-Ferreira A., De Faria F.M., De Almeida A.C., Dunder R.J., Manzo L.P., Souza Brito A.R.M. Inhibition of tumor necrosis factor-alpha and cyclooxigenase-2 by Isatin: a molecular mechanism of protection against TNBS-induced colitis in rats. Chemico-biol. Interact. 2014;209:48–55. doi: 10.1016/j.cbi.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 82.Rise C.L.V., Prabhu V.V., Guruvayoorappan C. Effect of Marine Mangrove Avicennia marina (Forssk.) Vierh against acetic acid-induced ulcerative colitis in experimental mice. J. Environ. Pathol. Toxicol. Oncol. 2012;31(2):179–192. doi: 10.1615/jenvironpatholtoxicoloncol.v31.i2.90. [DOI] [PubMed] [Google Scholar]
- 83.Sakthivel K.M., Guruvayoorappan C. Protective effect of acacia ferruginea against ulcerative colitis via modulating inflammatory mediators, cytokine profile and NF- κ B signal transduction pathways. J. Environ. Pathol. Toxicol. Oncol. 2014;33(2):83–98. doi: 10.1615/jenvironpatholtoxicoloncol.2014008425. [DOI] [PubMed] [Google Scholar]
- 84.Aleisa A.M., Al-Rejaie S.S., Abuohashish H.M., Ola M.S., Parmar M.Y., Ahmed M.M. Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement. Altern. Med. 2014;14(1):49. doi: 10.1186/1472-6882-14-49. 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y.-H., Yang X.-L., Wang L., Cui M.-X., Cai Y.-Q., Li X.-L., Wu Y.-J. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can. J. Physiol. Pharmacol. 2010;88(9):888–898. doi: 10.1139/y10-071. [DOI] [PubMed] [Google Scholar]
- 86.Xu B.-l, Zhang G.-j, Ji Y.-b. Active components alignment of Gegenqinlian decoction protects ulcerative colitis by attenuating in fl ammatory and oxidative stress. J. Ethnopharmacol. 2015;162:253–260. doi: 10.1016/j.jep.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 87.Dost T., Ozkayran H., Gokalp F., Yenisey C., Birincioglu M. The effect of Hypericum perforatum (St. John's Wort) on experimental colitis in rat. Dig. Dis. Sci. 2009;54(6):1214–1221. doi: 10.1007/s10620-008-0477-6. [DOI] [PubMed] [Google Scholar]
- 88.Mueller K., Blum N.M., Mueller A.S. Examination of the anti-inflammatory, antioxidant, and xenobiotic-inducing potential of broccoli extract and various essential oils during a Mild DSS-induced colitis in Rats. ISRN Gastroenterol. 2013;2013:710856. doi: 10.1155/2013/710856. 710856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haddad J.J. Antioxidant and prooxidant mechanisms in the regulation of redox (y) -sensitive transcription factors. Cell. Signal. 2002;14:879–897. doi: 10.1016/s0898-6568(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 90.Moura F.A., Andrade K.Q.D., Santos J.C.D.F., Oliveira M., Goulart F. Lipoic acid: its antioxidant and anti-inflammatory role and clinical applications. Curr. Top. Med. Chem. 2015;15(5):458–483. doi: 10.2174/1568026615666150114161358. [DOI] [PubMed] [Google Scholar]
- 91.Thimmulappa R.K., Lee H., Rangasamy T., Reddy S.P., Yamamoto M., Kensler T.W., Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 2006;116(4):984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim J., Cha Y.N., Surh Y.J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 2010;690(1-2):12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 93.Wang K.P., Zhang C., Zhang S.G., Liu E.D., Dong L., Kong X.Z., Cao P., Hu C.P., Zhao K., Zhan Y.Q., Dong X.M., Ge C.H., Yu M., Chen H., Wang L., Yang X.M., Li C.Y. 3-(3-pyridylmethylidene)-2-indolinone reduces the severity of colonic injury in a murine model of experimental colitis. Oxid. Med. Cell. Longev. 2015;2015(959253) doi: 10.1155/2015/959253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kathiria A.S., Butcher M.A., Hansen J.M., Theiss A.L. Nrf2 is not required for epithelial prohibitin-dependent attenuation of experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304(10):G885–G896. doi: 10.1152/ajpgi.00327.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zucker S.N., Flink E.E., Bagati A., Mannava S., Bianchi-Smiraglia A., Bogner P., Wawrzyniak J.A., Foley C., Leonova K.I., Grimm M.J., Moparthy K., Ionov Y., Wang J., Liu S., Sexton S., Kandel E.S., Bakin A.V., Zhang Y., Kaminski N., Segal B.H., Nikiforov M.A. Nrf2 amplifies oxidative stress via induction of Klf9. Mol. Cell. 2014;53(6):916–928. doi: 10.1016/j.molcel.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trivedi P.P., Jena G.B. Melatonin reduces ulcerative colitis-associated local and systemic damage in mice: investigation on possible mechanisms. Dig. Dis. Sci. 2013;58(12):3460–3474. doi: 10.1007/s10620-013-2831-6. [DOI] [PubMed] [Google Scholar]
- 97.Paiotti A.P.R., Neto R.A., Marchi P., Silva R.M., Pazine V.L., Noguti J., Pastrelo M.M., Gollücke A.P.B., Miszputen S.J., Ribeiro D.A. The anti-inflammatory potential of phenolic compounds in grape juice concentrate (G8000™) on 2,4,6-trinitrobenzene sulphonic acid-induced colitis. Br. J. Nutr. 2013;110(6):973–980. doi: 10.1017/S000711451300007X. [DOI] [PubMed] [Google Scholar]
- 98.Vaziri N.D., Bai Y., Ni Z., Quiroz Y., Pandian R., Rodriguez-Iturbe B. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J. Pharmacol. Exp. Ther. 2007;323(1):85–93. doi: 10.1124/jpet.107.123638. [DOI] [PubMed] [Google Scholar]
- 99.Arab H.H., Al-Shorbagy M.Y., Abdallah D.M., Nassar N.N. Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS One. 2014;9(5):e97193. doi: 10.1371/journal.pone.0097193. e97193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guerra G.C.B., Araújo A.A., Lira G.A., Melo M.N., Souto K.K.O., Fernandes D., Silva A.L., Júnior R.F.A. Telmisartan decreases inflammation by modulating TNF-a, IL-10, and RANK/RANKL in a rat model of ulcerative colitis. Pharmacol. Rep. 2015;(3):520–526. doi: 10.1016/j.pharep.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 101.Maheshwari R.A., Balaraman R., Sailor G.U., Sen D.B. Protective effect of simvastatin and rosuvastatin on trinitrobenzene sulfonic acid-induced colitis in rats. Indian J. Pharmacol. 2015;47(1):17–21. doi: 10.4103/0253-7613.150311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oda H., Keane W.F. Recent advances in statins and the kidney. Kidney Int. Suppl. 1999;71:S2–S5. doi: 10.1046/j.1523-1755.1999.07101.x. [DOI] [PubMed] [Google Scholar]
- 103.Terry P.D., Villinger F., Bubenik G. a, Sitaraman S.V. Melatonin and ulcerative colitis: Evidence, biological mechanisms, and future research. Inflamm. Bowel Dis. 2009;15(1):134–140. doi: 10.1002/ibd.20527. [DOI] [PubMed] [Google Scholar]
- 104.Trivedi P.P., Jena G.B., Tikoo K.B., Kumar V. Melatonin modulated autophagy and Nrf2 signaling pathways in mice with colitis-associated colon carcinogenesis. Mol. Carcionog. 2015;16:1–13. doi: 10.1002/mc.22274. [DOI] [PubMed] [Google Scholar]
- 105.Takagi T., Inada Y., Naito Y. Circadian rhythm and inflammatory bowel disease. Nihon Rinsho Jpn. J. Clin. Med. 2013;71:2165–2170. [PubMed] [Google Scholar]
- 106.Galano A., Tan D.X., Reiter R.J. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 107.Dong W.G., Mei Q., Yu J.P., Xu J.M., Xiang L., Xu Y. Effects of melatonin on the expression of iNOS and COX-2 in rat models of colitis. World J. Gastroenterol. 2003;9(6):1307–1311. doi: 10.3748/wjg.v9.i6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park Y.S., Chung S.H., Lee S.K., Kim J.H., Kim J.B., Kim T.K., Kim D.S., Baik H.W. Melatonin improves experimental colitis with sleep deprivation. Int. J. Mol. Med. 2015;35(4):979–986. doi: 10.3892/ijmm.2015.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tahan G., Gramignoli R., Marongiu F., Aktolga S., Cetinkaya A., Tahan V., Dorko K. Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig. Dis. Sci. 2011;56(3):715–720. doi: 10.1007/s10620-010-1364-5. [DOI] [PubMed] [Google Scholar]
- 110.Chojnacki C., Wisniewska-Jarosinska M., Walecka-Kapica E., Klupinska G., Jaworek J., Chojnacki J. Evaluation of melatonin effectiveness in the adiuvant treatment of ulcerative colitis. J. Physiol. Pharmacol. 2011;62(4):327–334. [PubMed] [Google Scholar]
- 111.Samuni Y., Goldstein S., Dean O.M., Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. acta. 2013;1830(8):4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 112.Siddiqui A., Ancha H., Tedesco D., Lightfoot S., Stewart C. a, Harty R.F. Antioxidant therapy with N-acetylcysteine plus mesalamine accelerates mucosal healing in a rodent model of colitis. Dig. Dis. Sci. 2006;51(4):698–705. doi: 10.1007/s10620-006-3194-z. [DOI] [PubMed] [Google Scholar]
- 113.Guijarro L.G., Mate J., Gisbert J.P., Perez-Calle J.L., Marin-Jimenez I., Arriaza E., Olleros T., Delgado M., Castillejo M.S., Prieto-Merino D., Gonzalez Lara V., Pena A.-S. N-acetyl-l-cysteine combined with mesalamine in the treatment of ulcerative colitis: randomized, placebo-controlled pilot study. World J. Gastroenterol. 2008;14(18):2851–2857. doi: 10.3748/wjg.14.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baker W.L., Anglade M.W., Baker E.L., White C.M., Kluger J., Coleman C.I. Vol. 35. 2009. Use of N-acetylcysteine to reduce post-cardiothoracic surgery complications: a meta-analysis; pp. 521–527. (Eur. J. Cardio-thorac. Surg.). [DOI] [PubMed] [Google Scholar]
- 115.Sadowska A.M., Manuel-y-Keenoy B., De Backer W. a. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: a review. Pulm. Pharmacol. Ther. 2005;20(2007):9–22. doi: 10.1016/j.pupt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 116.Atkuri K.R., Mantovani J.J., Herzenberg L. a, Herzenberg L. a. N-Acetylcysteine-a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007;7:355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dröge W., Eck H.P., Mihm S. HIV-induced cysteine deficiency and T-cell dysfunction--a rationale for treatment with N-acetylcysteine. Immunol. Today. 1992;13(6):211–214. doi: 10.1016/0167-5699(92)90156-2. [DOI] [PubMed] [Google Scholar]
- 118.Berk M., Malhi G.S., Gray L.J., Dean O.M. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol. Sci. 2013;34(3):167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 119.Lasram M.M., El-Golli N., Lamine A.J., Douib I.B., Bouzid K., Annabi A., El Fazaa S., Abdelmoula J., Gharbi N. Changes in glucose metabolism and reversion of genes expression in the liver of insulin-resistant rats exposed to malathion. The protective effects of N-acetylcysteine. Gen. Comp. Endocrinol. 2014 doi: 10.1016/j.ygcen.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 120.Amrouche-Mekkioui I., Djerdjouri B. N-acetylcysteine improves redox status, mitochondrial dysfunction, mucin-depleted crypts and epithelial hyperplasia in dextran sulfate sodium-induced oxidative colitis in mice. Eur. J. Pharmacol. 2012;691(1-3):209–217. doi: 10.1016/j.ejphar.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 121.Kasperczyk S., Dobrakowski M., Kasperczyk A., Machnik G., Ewa B. Effect of N-acetylcysteine administration on the expression and activities of antioxidant enzymes and the malondialdehyde level in the blood of lead-exposed workers. Environ. Toxicol. Pharmacol. 2014;37(2):638–647. doi: 10.1016/j.etap.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 122.Porfire A.S., Leucuţa S.E., Kiss B., Loghin F., Pârvu A.E. Investigation into the role of Cu/Zn-SOD delivery system on its antioxidant and antiinflammatory activity in rat model of peritonitis. Pharmacol. Rep. 2014;66:670–676. doi: 10.1016/j.pharep.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 123.Manni M.L., Tomai L.P., Norris C. a, Thomas L.M., Kelley E.E., Salter R.D., Crapo J.D., Chang L.Y.L., Watkins S.C., Piganelli J.D., Oury T.D. Extracellular superoxide dismutase in macrophages augments bacterial killing by promoting phagocytosis. Am. J. Pathol. 2011;178(6):2752–2759. doi: 10.1016/j.ajpath.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ishihara T., Tanaka K.-i, Tasaka Y., Namba T., Suzuki J., Ishihara T., Okamoto S., Hibi T., Takenaga M., Igarashi R., Sato K., Mizushima Y., Mizushima T. Therapeutic Effect of Lecithinized Superoxide Dismutase against colitis. J. Pharmacol. Exp. Ther. 2009;328(1):152–164. doi: 10.1124/jpet.108.144451. [DOI] [PubMed] [Google Scholar]
- 125.Suzuki Y., Matsumoto T., Matsumoto S., Hibi T. A lecithinized superoxide dismutase (PC-SOD) improves ulcerative colitis. Colorectal Dis. Off. J. Assoc. Coloproctol. G. B. Irel. 2008;10(9):931–934. doi: 10.1111/j.1463-1318.2008.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hou C.L., Zhang J., Liu X.T., Liu H., Zeng X.F., Qiao S.Y. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-κB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J. Appl. Microbiol. 2014;116(6):1621–1631. doi: 10.1111/jam.12461. [DOI] [PubMed] [Google Scholar]
- 127.Mikhailova T.L., Sishkova E., Poniewierka E., Zhidkov K.P., Bakulin I.G., Kupcinskas L., Lesniakowski K., Grinevich V.B., Malecka-Panas E., Ardizzone S., D’Arienzo A., Valpiani D., Koch M., Denapiene G., Vago G., Fociani P., Zerbi P., Ceracchi M., Camerini R., Gasbarrini G. Randomised clinical trial: The efficacy and safety of propionyl- l -carnitine therapy in patients with ulcerative colitis receiving stable oral treatment. Aliment. Pharmacol. Ther. 2011;34(September):1088–1097. doi: 10.1111/j.1365-2036.2011.04844.x. [DOI] [PubMed] [Google Scholar]
- 128.Scioli M.G., Stasi M.A., Passeri D., Doldo E., Costanza G., Camerini R., Fociani P., Arcuri G., Lombardo K., Pace S., Borsini F., Orlandi A. Propionyl-l-carnitine is efficacious in ulcerative colitis through its action on the immune function and microvasculature. Clin. Transl. Gastroenterol. 2014;5(3):e55. doi: 10.1038/ctg.2014.4. e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang H., Xue Y., Zhang H., Huang Y., Yang G., Du M., Zhu M.J. Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL10-deficient mice. Mol. Nutr. Food Res. 2013;57(12):2253–2257. doi: 10.1002/mnfr.201300146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Romier B., Schneider Y.-J., Larondelle Y., During A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr. Rev. 2009;67(7):363–378. doi: 10.1111/j.1753-4887.2009.00210.x. [DOI] [PubMed] [Google Scholar]
- 131.Shigeshiro M., Tanabe S., Suzuki T. Dietary polyphenols modulate intestinal barrier defects and inflammation in a murine model of colitis. J. Funct. Foods. 2013;5:949–955. [Google Scholar]
- 132.Piechota-Polanczyk A., Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg's Arch. Pharmacol. 2014;387(7):605–620. doi: 10.1007/s00210-014-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Das S., Chaudhury A., Ng K.Y. Preparation and evaluation of zinc-pectin-chitosan composite particles for drug delivery to the colon: role of chitosan in modifying in vitro and in vivo drug release. Int. J. Pharm. 2011;406(1–2):11–20. doi: 10.1016/j.ijpharm.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 134.Abdin A.A, Sarhan N.I. Intervention of mitochondrial dysfunction-oxidative stress-dependent apoptosis as a possible neuroprotective mechanism of α-lipoic acid against rotenone-induced parkinsonism and l-dopa toxicity. Neurosci. Res. 2011;71(4):387–395. doi: 10.1016/j.neures.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 135.Samsami-Kor M., Daryani N.E., Asl P.R., Hekmatdoost A. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch. Med. Res. 2015 doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 136.Ullah M.F., Bhat S.H., Husain E., Abu-Duhier F., Hadi S.M., Sarkar F.H., Ahmad A. Pharmacological Intervention through dietary nutraceuticals in gastrointestinal neoplasia. Crit. Rev. Food Sci. Nutr. 2014;0 doi: 10.1080/10408398.2013.772091. [DOI] [PubMed] [Google Scholar]
- 137.Abdin A. a. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur. J. Pharmacol. 2013;718(1–3):145–153. doi: 10.1016/j.ejphar.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 138.Larrosa M., Tomé-Carneiro J., Yáñez-Gascón M.J., Alcántara D., Selma M.V., Beltrán D., García-Conesa M.T., Urbán C., Lucas R., Tomás-Barberán F., Morales J.C., Espín J.C. Preventive oral treatment with resveratrol pro-prodrugs drastically reduce colon inflammation in rodents. J. Med. Chem. 2010;53(20):7365–7376. doi: 10.1021/jm1007006. [DOI] [PubMed] [Google Scholar]
- 139.Yao J., Wang J.-Y., Liu L., Zeng W.-S., Li Y.-X., Xun A.-Y., Zhao L., Jia C.-H., Feng J.-L., Wei X.-X., Wang L.-S. Polydatin ameliorates DSS-induced colitis in mice through inhibition of nuclear factor-kappaB activation. Planta Med. 2011;77(5):421–427. doi: 10.1055/s-0030-1250462. [DOI] [PubMed] [Google Scholar]
- 140.Singh U.P., Singh N.P., Singh B., Hofseth L.J., Price R.L., Nagarkatti M., Nagarkatti P.S. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J. Pharmacol. Exp. Ther. 2010;332(3):829–839. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abdallah D.M., Ismael N.R. Resveratrol abrogates adhesion molecules and protects against TNBS-induced ulcerative colitis in rats. Can. J. Physiol. Pharmacol. 2011;89(11):811–818. doi: 10.1139/y11-080. [DOI] [PubMed] [Google Scholar]
- 142.Sánchez-Fidalgo S., Cárdeno A., Villegas I., Talero E., de la Lastra C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010;633(1-3):78–84. doi: 10.1016/j.ejphar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 143.Youn J., Lee J.-S., Na H.-K., Kundu J.K., Surh Y.-J. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr. cancer. 2009;61(August):847–854. doi: 10.1080/01635580903285072. [DOI] [PubMed] [Google Scholar]
- 144.Rocha K.K.R., Souza G. a, Ebaid G.X., Seiva F.R.F., Cataneo a C., Novelli E.L.B. Resveratrol toxicity: Effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem. Toxicol. 2009;47(6):1362–1367. doi: 10.1016/j.fct.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 145.Posadino A.M., Cossu A., Giordo R., Zinellu A., Sotgia S., Vardeu A., Hoa P.T., Nguyen L.H.V., Carru C., Pintus G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015:1–7. doi: 10.1016/j.fct.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 146.Epstein J., Sanderson I.R., Macdonald T.T. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br. J. Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 147.Hanai H., Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr. Pharm. Des. 2009;15(18):2087–2094. doi: 10.2174/138161209788489177. [DOI] [PubMed] [Google Scholar]
- 148.McCann M.J., Johnston S., Reilly K., Men X., Burgess E.J., Perry N.B., Roy N.C. The effect of turmeric (Curcuma longa) extract on the functionality of the solute carrier protein 22 A4 (SLC22A4) and interleukin-10 (IL-10) variants associated with inflammatory bowel disease. Nutrients. 2014:4178–4190. doi: 10.3390/nu6104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hanai H., Iida T., Takeuchi K., Watanabe F., Maruyama Y., Andoh A., Tsujikawa T., Fujiyama Y., Mitsuyama K., Sata M., Yamada M., Iwaoka Y., Kanke K., Hiraishi H., Hirayama K., Arai H., Yoshii S., Uchijima M., Nagata T., Koide Y. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 150.Jurenka J.S., Ascp M.T. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa : a review of preclinical and clinical research. Altern. Med. Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 151.Arafa H.M.M., Hemeida R. a, El-Bahrawy A.I.M., Hamada F.M. a. Prophylactic role of curcumin in dextran sulfate sodium (DSS)-induced ulcerative colitis murine model. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2009;47(6):1311–1317. doi: 10.1016/j.fct.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 152.Topcu-Tarladacalisir Y., Akpolat M., Uz Y.H., Kizilay G., Sapmaz-Metin M., Cerkezkayabekir A., Omurlu I.K. Effects of curcumin on apoptosis and oxidoinflammatory regulation in a rat model of acetic acid-induced colitis: the roles of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. J. Med. Food. 2013;16(4):296–305. doi: 10.1089/jmf.2012.2550. [DOI] [PubMed] [Google Scholar]
- 153.Motawi T.K., Rizk S.M., Shehata A.H. Effects of curcumin and Ginkgo biloba on matrix metalloproteinases gene expression and other biomarkers of inflammatory bowel disease. J. Physiol. Biochem. 2012;68(4):529–539. doi: 10.1007/s13105-012-0168-9. [DOI] [PubMed] [Google Scholar]
- 154.Mouzaoui S., Rahim I., Djerdjouri B. Aminoguanidine and curcumin attenuated tumor necrosis factor (TNF)-α-induced oxidative stress, colitis and hepatotoxicity in mice. Int. Immunopharmacol. 2012;12(1):302–311. doi: 10.1016/j.intimp.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 155.Dodda D., Chhajed R., Mishra J., Padhy M. Targeting oxidative stress attenuates trinitrobenzene sulphonic acid induced inflammatory bowel disease like symptoms in rats: role of quercetin. Indian J. Pharmacol. 2014;46(3):286–291. doi: 10.4103/0253-7613.132160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guazelli C.F.S., Fattori V., Colombo B.B., Georgetti S.R., Vicentini F.T.M.C., Casagrande R., Baracat M.M., Verri W.A. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J. Nat. Prod. 2013;76(2):200–208. doi: 10.1021/np300670w. [DOI] [PubMed] [Google Scholar]
- 157.Mouria M., Gukovskaya A.S., Jung Y., Buechler P., Hines O.J., Reber H. a, Pandol S.J. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome c release and apoptosis. Int. J. Cancer. 2002;98:761–769. doi: 10.1002/ijc.10202. October 2001. [DOI] [PubMed] [Google Scholar]
- 158.Suzuki T., Hara H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011;22(5):401–408. doi: 10.1016/j.jnutbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 159.Suzuki T., Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009;139:965–974. doi: 10.3945/jn.108.100867. [DOI] [PubMed] [Google Scholar]
- 160.Graham H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 161.Salah N., Miller N.J., Paganga G., Tijburg L., Bolwell G.P., Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995;322:339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- 162.Lambert J.D., Sang S., Hong J., Kwon S.J., Lee M.J., Ho C.T., Yang C.S. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug. Metab. Dispos. 2006;34:2111–2116. doi: 10.1124/dmd.106.011460. [DOI] [PubMed] [Google Scholar]
- 163.Chiou Y.-s, Sang S., Ho C.-t, Wang Y.-j, Pan M.-h. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J. Agric. Food Chem. 2012;60:3441–3451. doi: 10.1021/jf300441p. [DOI] [PubMed] [Google Scholar]
- 164.Katiyar S.K. Proanthocyanidins from grape seeds inhibit UV radiation-induced immune suppression in mice: detection and analysis of molecular and cellular targets. Photochem. Photobiol. 2014;91:156–162. doi: 10.1111/php.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li X., Yang X., Cai Y., Qin H., Wang L., Wang Y., Huang Y., Wang X., Yan S., Wang L., Zhao X., Li W., Li S., Chen J., Wu Y. Proanthocyanidins from grape seeds modulate the NF-κB signal transduction pathways in rats with TNBS-induced ulcerative colitis. Molecules. 2011;16:6721–6731. doi: 10.3390/molecules16086721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Y.-H., Ge B., Yang X.-L., Zhai J., Yang L.-N., Wang X.-X., Liu X., Shi J.-C., Wu Y.-J. Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int. Immunopharmacol. 2011;11(10):1620–1627. doi: 10.1016/j.intimp.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 167.Baghaei A., Esmaily H., Abdolghaffari A.H., Baeeri M. Efficacy of Setarud (IMOD®), a novel drug with potent anti-toxic stress potential in rat inflammatory bowel disease and comparison with dexamethasone and infliximab. Indian J. Biochem. Biophys. 2010;47(August):219–226. [PubMed] [Google Scholar]
- 168.Cirulis J.T., Scott J.A., Ross G.M. Management of oxidative stress by microalgae. Can. J. Physiol. Pharmacol. 2013;91(1):15–21. doi: 10.1139/cjpp-2012-0249. [DOI] [PubMed] [Google Scholar]
- 169.Haller D., Antoine J.-M., Bengmark S., Enck P., Rijkers G.T., Lenoir-Wijnkoop I. Guidance for substantiating the evidence for beneficial effects of probiotics: probiotics in chronic inflammatory bowel disease and the functional disorder irritable bowel syndrome. J. Nutr. 2010;140:690S–697SS. doi: 10.3945/jn.109.113746. [DOI] [PubMed] [Google Scholar]
- 170.Al-Rejaie S.S., Abuohashish H.M., Al-Enazi M.M., Al-Assaf A.H., Parmar M.Y., Ahmed M.M. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J. Gastroenterol. 2013;19(34):5633–5644. doi: 10.3748/wjg.v19.i34.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Farombi E.O., Adedara I. a, Ajayi B.O., Ayepola O.R., Egbeme E.E. Kolaviron, a natural antioxidant and anti-inflammatory phytochemical prevents dextran sulphate sodium-induced colitis in rats. Basic Clin. Pharmacol. Toxicol. 2013;113(1):49–55. doi: 10.1111/bcpt.12050. [DOI] [PubMed] [Google Scholar]
- 172.Zhao J., Hong T., Dong M., Meng Y., Mu J. Protective effect of myricetin in dextran sulphate sodium-induced murine ulcerative colitis. Mol. Med. Rep. 2013;7(2):565–570. doi: 10.3892/mmr.2012.1225. [DOI] [PubMed] [Google Scholar]
- 173.Schweigert N., Zehnder A.J.B., Eggen R.I.L. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2001;3:81–91. doi: 10.1046/j.1462-2920.2001.00176.x. In. [DOI] [PubMed] [Google Scholar]
- 174.Rahimi R., Nikfar S., Abdollahi M. Induction of clinical response and remission of inflammatory bowel disease by use of herbal medicines: a meta-analysis. World J. Gastroenterol. 2013;19(34):5738–5749. doi: 10.3748/wjg.v19.i34.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Carter M.J., Lobo A.J., Travis S.P.L. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;60:571–607. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kornbluth A., Sachar D.B. Ulcerative colitis practice guidelines in adults: american college of gastroenterology, practice parameters committee. Am. J. Gastroenterol. 2010;105(3):501–523. doi: 10.1038/ajg.2009.727. quiz 524. [DOI] [PubMed] [Google Scholar]
- 177.Tesoriere L., Allegra M., Gentile C. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-κB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1β-exposed Caco-2 cells. Br. J. Nutr. 2014;111(3):415–423. doi: 10.1017/S0007114513002663. [DOI] [PubMed] [Google Scholar]
- 178.Lee I.-A., Hyun Y.-J., Kim D.-H. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur. J. Pharmacol. 2010;648(1–3):162–170. doi: 10.1016/j.ejphar.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 179.Borrelli F., Fasolino I., Romano B., Capasso R., Maiello F., Coppola D., Orlando P., Battista G., Pagano E., Di Marzo V., Izzo A. a. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013;85(9):1306–1316. doi: 10.1016/j.bcp.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 180.Borrelli F., Aviello G., Romano B., Orlando P., Capasso R., Maiello F., Guadagno F., Petrosino S., Capasso F., Di Marzo V., Izzo A. a. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J. Mol. Med. 2009;87(11):1111–1121. doi: 10.1007/s00109-009-0512-x. [DOI] [PubMed] [Google Scholar]
- 181.Jowett S.L., Seal C.J., Phillips E., Gregory W., Barton J.R., Welfare M.R. Dietary beliefs of people with ulcerative colitis and their effect on relapse and nutrient intake. Clin. Nutr. 2004;23:161–170. doi: 10.1016/S0261-5614(03)00132-8. [DOI] [PubMed] [Google Scholar]
- 182.Darwish H. a, Abd Raboh N.R., Mahdy A. Camel's milk alleviates alcohol-induced liver injury in rats. Food Chem. Toxicol. 2012;50(5):1377–1383. doi: 10.1016/j.fct.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 183.Al Haj O. a, Al Kanhal H. a. Compositional, technological and nutritional aspects of dromedary camel milk. Int. Dairy J. 2010;20(12):811–821. [Google Scholar]
- 184.Barrett J.S., Irving P.M., Shepherd S.J., Muir J.G., Gibson P.R. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment. Pharmacol. Ther. 2009;30:165–174. doi: 10.1111/j.1365-2036.2009.04018.x. April. [DOI] [PubMed] [Google Scholar]
- 185.Wiecek S., Wos H., Radziewicz-winnicki I., Komraus M., Grzybowska-chlebowczyk U. Disaccharidase activity in children with inflammatory bowel disease. Turk. J. Gastroenterol. 2014;25:185–191. doi: 10.5152/tjg.2014.3994. [DOI] [PubMed] [Google Scholar]
- 186.Legrand D., Elass E., Carpentier M., Mazurier J. Lactoferrin: A modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2005;62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sánchez-Fidalgo S., Villegas I., Cárdeno a, Talero E., Sánchez-Hidalgo M., Motilva V., Alarcón de la Lastra C. Extra-virgin olive oil-enriched diet modulates DSS-colitis-associated colon carcinogenesis in mice. Clin. Nutr. 2010;29(5):663–673. doi: 10.1016/j.clnu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 188.Sánchez-Fidalgo S., Cárdeno A., Sánchez-Hidalgo M., Aparicio-Soto M., Villegas I., Rosillo M.A., De La Lastra C.A. Dietary unsaponifiable fraction from extra virgin olive oil supplementation attenuates acute ulcerative colitis in mice. Eur. J. Pharm. Sci. 2013;48:572–581. doi: 10.1016/j.ejps.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 189.Oliveras-López M.-J., Berná G., Jurado-Ruiz E., López-García de la Serrana H., Martín F. Consumption of extra-virgin olive oil rich in phenolic compounds has beneficial antioxidant effects in healthy human adults. J. Funct. Foods. 2014;10:475–484. [Google Scholar]
- 190.Oliveras-López M.-J., Berná G., Carneiro E.M., López-García de la Serrana H., Martín F., López M.C. An extra-virgin olive oil rich in polyphenolic compounds has antioxidant effects in OF1 mice. J. Nutr. 2008;138:1074–1078. doi: 10.1093/jn/138.6.1074. August 2007. [DOI] [PubMed] [Google Scholar]
- 191.Condelli N., Carmela M., Galgano F., Russo D., Milella L., Favati F. Prediction of the antioxidant activity of extra virgin olive oils produced in the Mediterranean area. Food Chem. 2015;177:233–239. doi: 10.1016/j.foodchem.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 192.Itzkowitz S.H. Molecular Biology of Dysplasia and Cancer in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2006;35:553–571. doi: 10.1016/j.gtc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 193.Davidson L.A., Nguyen D.V., Hokanson R.M., Callaway E.S., Isett R.B., Turner N.D., Dougherty E.R., Wang N., Lupton J.R., Carroll R.J., Chapkin R.S. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–6804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Owen R.W., Haubner R., Würtele G., Hull E., Spiegelhalder B., Bartsch H. Olives and olive oil in cancer prevention. Eur. J. Cancer Prev.: Off. J. Eur. Cancer Prev. Organ. 2004;13(1999):319–326. doi: 10.1097/01.cej.0000130221.19480.7e. [DOI] [PubMed] [Google Scholar]
- 195.Takashima T., Sakata Y., Iwakiri R., Shiraishi R., Oda Y., Inoue N., Nakayama A., Toda S., Fujimoto K. Feeding with olive oil attenuates inflammation in dextran sulfate sodium-induced colitis in rat. J. Nutr. Biochem. 2014;25(2):186–192. doi: 10.1016/j.jnutbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 196.Müller-Decker K., Fürstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol. Carcinog. 2007;46(February):705–710. doi: 10.1002/mc.20326. [DOI] [PubMed] [Google Scholar]
- 197.Kawanishi S., Hiraku Y., Pinlaor S., Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. April. [DOI] [PubMed] [Google Scholar]
- 198.Servili M., Esposto S., Fabiani R., Urbani S., Taticchi a, Mariucci F., Selvaggini R., Montedoro G.F. Phenolic compounds in olive oil: antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology. 2009;17:76–84. doi: 10.1007/s10787-008-8014-y. [DOI] [PubMed] [Google Scholar]
- 199.Sánchez-Fidalgo S., Villegas I., Aparicio-Soto M., Cárdeno A., Rosillo M.Á., González-Benjumea A., Marset A., López Ó., Maya I., Fernández-Bolaños J.G., l. Lastra C.A. d. Effects of dietary virgin olive oil polyphenols: hydroxytyrosyl acetate and 3,4- dihydroxyphenylglycol on DSS-induced acute colitis in mice. J. Nutr. Biochem. 2015;28:1–33. doi: 10.1016/j.jnutbio.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 200.Seifried H.E., Anderson D.E., Fisher E.I., Milner J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 201.Vrolijk M.F., Opperhuizen A., Jansen E.H.J.M., Godschalk R.W., Schooten F.J.V., Bast A., Haenen G.R.M.M. The shifting perception on antioxidants: The case of vitamin E and β-carotene. Redox Biol. 2015;4:272–278. doi: 10.1016/j.redox.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Joshipura K.J., Hu F.B., Manson J.E., Stampfer M.J., Rimm E.B., Speizer F.E., Colditz G., Ascherio A., Rosner B., Spiegelman D., Willett W.C. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Int. Med. 2001;134:1106–1114+I. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 203.Bostick R.M., Potter J.D., McKenzie D.R., Sellers T.A., Kushi L.H., Steinmetz K.A., Folsom A.R. Reduced risk of colon cancer with high intake of vitamin E: the Iowa Women's Health Study. Cancer Res. 1993;53:4230–4237. [PubMed] [Google Scholar]
- 204.Kamal-Eldin A., Appelqvist L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 205.Packer L., Weber S.U., Rimbach G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J. Nutr. 2001;131:369S–373SS. doi: 10.1093/jn/131.2.369S. [DOI] [PubMed] [Google Scholar]
- 206.Özkan Y., Yilmaz Ö., Öztürk A.I., Erşan Y. Effects of triple antioxidant combination (vitamin E, vitamin C and α-lipoic acid) with insulin on lipid and cholesterol levels and fatty acid composition of brain tissue in experimental diabetic and non-diabetic rats. Cell. Biol. Int. 2005:754–760. doi: 10.1016/j.cellbi.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 207.Rouaki F., Mazari A., Kanane A., Errahmani M.B., Ammouche A. Cardiotoxicity induced by dietary oxidized sunflower oil in rats: Pro- and antioxidant effects of α-tocopherol. Int. J. Vitam. Nutr. Res. 2013;83(6):367–376. doi: 10.1024/0300-9831/a000178. [DOI] [PubMed] [Google Scholar]
- 208.Mirbagheri S.-A. Rectal administration of D-alpha tocopherol for active ulcerative colitis: A preliminary report. World J. gastroenterol. 2008;14(39):5990. doi: 10.3748/wjg.14.5990. 5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bitiren M., Karakilcik A.Z., Zerin M., Ozardali I., Selek S., Nazligül Y., Ozgonul A., Musa D., Uzunkoy A. Protective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol. Trace Elem. Res. 2010;136(1):87–95. doi: 10.1007/s12011-009-8518-3. [DOI] [PubMed] [Google Scholar]
- 210.Schepens M. a a, Vink C., Schonewille A.J., Roelofs H.M.J., Brummer R.J., Van Der Meer R., Bovee-Oudenhoven I.M.J. Supplemental antioxidants do not ameliorate colitis development in HLA-B27 transgenic rats despite extremely low glutathione levels in colonic mucosa. Inflamm. Bowel Dis. 2011;17(10):2065–2075. doi: 10.1002/ibd.21584. [DOI] [PubMed] [Google Scholar]
- 211.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. J. Am. Med. Assoc. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 212.Myung S.K., Kim Y., Ju W., Choi H.J., Bae W.K. Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010;21:166–179. doi: 10.1093/annonc/mdp286. [DOI] [PubMed] [Google Scholar]
- 213.Perera R.M., Bardeesy N. When antioxidants are bad on towering heights. Nature. 2011;475:43–44. doi: 10.1038/475043a. [DOI] [PubMed] [Google Scholar]
- 214.Barollo M., Medici V., D’Incà R., Banerjee A., Ingravallo G., Scarpa M., Patak S., Ruffolo C., Cardin R., Sturniolo G.C. Antioxidative potential of a combined therapy of anti TNFα and Zn acetate in experimental colitis. World J. Gastroenterol. 2011;17(36):4099–4103. doi: 10.3748/wjg.v17.i36.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Aghdassi E., Wendland B.E., Steinhart A.H., Wolman S.L., Jeejeebhoy K., Allard J.P. Antioxidant vitamin supplementation in Crohn's disease decreases oxidative stress. a randomized controlled trial. Am. J. Gastroenterol. 2003;98(2):348. doi: 10.1111/j.1572-0241.2003.07226.x. 53. [DOI] [PubMed] [Google Scholar]
- 216.Roggenbuck C., Lammert F., Berthold H.K., Giese T., Stallmach A., Stehle P., Ellinger S. High-dose oral supplementation of antioxidants and glutamine improves the antioxidant status in patients with Crohn's disease: A pilot study. e-SPEN, Eur. e-J. Clin. Nutr. Metab. 2008;3(5):e246–e253. [Google Scholar]
- 217.Di Stasi L.C., Costa C.A., Witaicenis A. Products for the treatment of inflammatory bowel disease: a patent review (2013–2014) Expert Opin. Ther. Pat. 2015;25(6):629–642. doi: 10.1517/13543776.2015.1041921. [DOI] [PubMed] [Google Scholar]
- 218.Lin M.Y., Yen C.L. Antioxidative ability of lactic acid bacteria. J. Agric. Food Chem. 1999;47:1460–1466. doi: 10.1021/jf981149l. [DOI] [PubMed] [Google Scholar]
- 219.Talwalkar A., Kailasapathy K. Metabolic and biochemical responses of probiotic bacteria to oxygen. J. Dairy Sci. 2003;86:2537–2546. doi: 10.3168/jds.S0022-0302(03)73848-X. [DOI] [PubMed] [Google Scholar]
- 220.LeBlanc J.G., del Carmen S., Miyoshi A., Azevedo V., Sesma F., Langella P., Bermúdez-Humarán L.G., Watterlot L., Perdigon G., de Moreno de LeBlanc A. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn's disease in mice. J. Biotechnol. 2011;151(3):287–293. doi: 10.1016/j.jbiotec.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 221.Songisepp E., Kals J., Kullisaar T., Mändar R., Hütt P., Zilmer M., Mikelsaar M. Evaluation of the functional efficacy of an antioxidative probiotic in healthy volunteers. Nutr. J. 2005;4:22. doi: 10.1186/1475-2891-4-22. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Naruszewicz M., Johansson M.-L., Zapolska-Downar D., Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 2002;76:1249–1255. doi: 10.1093/ajcn/76.6.1249. [DOI] [PubMed] [Google Scholar]
- 223.Yang M., Gong S., Ye S.Q., Lyman B., Geng L., Chen P., Li D.-y. Non-alcoholic fatty liver disease in children: focus on nutritional interventions. Nutrients. 2014;28(6):4691–4705. doi: 10.3390/nu6114691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Amaretti A., Di Nunzio M., Pompei A., Raimondi S., Rossi M., Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013;97:809–817. doi: 10.1007/s00253-012-4241-7. [DOI] [PubMed] [Google Scholar]
- 225.Cammarota G., Ianiro G., Cianci R., Bibbo S., A G., Curro D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy. Pharmacol. Ther. 2015:1–22. doi: 10.1016/j.pharmthera.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 226.Rahimi R., Nikfar S., Rahimi F., Elahi B., Derakhshani S., Vafaie M., Abdollahi M. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn's disease. Dig. Dis. Sci. 2008;53:2524–2531. doi: 10.1007/s10620-007-0171-0. [DOI] [PubMed] [Google Scholar]
- 227.Sengül N., Işık S., Aslım B., Uçar G., Demirbağ A.E. The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Dig. Dis. Sci. 2011;56(3):707–714. doi: 10.1007/s10620-010-1362-7. [DOI] [PubMed] [Google Scholar]
- 228.Toumi R., Abdelouhab K., Rafa H., Soufli I., Raissi-Kerboua D., Djeraba Z., Touil-Boukoffa C. Beneficial role of the probiotic mixture Ultrabiotique on maintaining the integrity of intestinal mucosal barrier in DSS-induced experimental colitis. Immunopharmacol. Immunotoxicol. 2013;35(3):403–409. doi: 10.3109/08923973.2013.790413. [DOI] [PubMed] [Google Scholar]
- 229.Mañé J., Lorén V., Pedrosa E., Ojanguren I., Xaus J., Cabré E., Domènech E., Gassull M. a. Lactobacillus fermentum CECT 5716 prevents and reverts intestinal damage on TNBS-induced colitis in mice. Inflamm. Bowel Dis. 2009;15(8):1155–1163. doi: 10.1002/ibd.20908. [DOI] [PubMed] [Google Scholar]
- 230.Gardlik R., Palffy R., Celec P. Recombinant probiotic therapy in experimental colitis in mice. Folia Biol. 2012;58:238–245. doi: 10.14712/fb2012058060238. [DOI] [PubMed] [Google Scholar]
- 231.Mowat C., Cole A., Windsor A., Ahmad T., Arnott I., Driscoll R., Mitton S., Orchard T., Rutter M., Younge L., Lees C., Ho G.-T., Satsangi J., Bloom S. : Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 232.Jonkers D., Penders J., Masclee A., Pierik M. Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs. 2012;72(6):803–823. doi: 10.2165/11632710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 233.Nagib M.M., Tadros M.G., ElSayed M.I., Khalifa A.E. Anti-inflammatory and anti-oxidant activities of olmesartan medoxomil ameliorate experimental colitis in rats. Toxicol. Appl. Pharmacol. 2013;271(1):106–113. doi: 10.1016/j.taap.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 234.Sayyed H.G., Jaumdally R.J., Idriss N.K., El Sers D. a, Blann A. The effect of melatonin on plasma markers of inflammation and on expression of nuclear factor-kappa beta in acetic acid-induced colitis in the rat. Dig. Dis. Sci. 2013;58:3156–3164. doi: 10.1007/s10620-013-2811-x. [DOI] [PubMed] [Google Scholar]
- 235.Ancha H.R., Kurella R.R., McKimmey C.C., Lightfoot S., Harty R.F. Effects of N-acetylcysteine plus mesalamine on prostaglandin synthesis and nitric oxide generation in TNBS-induced colitis in rats. Dig. Dis. Sci. 2009;54:758–766. doi: 10.1007/s10620-008-0438-0. [DOI] [PubMed] [Google Scholar]
- 236.Romagnoli C., Marcucci T., Picariello L., Tonelli F., Vincenzini M.T., Iantomasi T. Role of N-acetylcysteine and GSH redox system on total and active MMP-2 in intestinal myofibroblasts of Crohn's disease patients. Int. J. Colorectal Dis. 2013;28(7):915–924. doi: 10.1007/s00384-012-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Uraz S., Tahan G., Aytekin H., Tahan V. N-acetylcysteine expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic acid-induced colitis in rats. Scand. J. Clin. Lab. Investig. 2013;73(1):61–66. doi: 10.3109/00365513.2012.734859. [DOI] [PubMed] [Google Scholar]
- 238.You Y., Fu J.-J., Meng J., Huang G.-D., Liu Y.-H. Effect of N-acetylcysteine on the murine model of colitis induced by dextran sodium sulfate through up-regulating PON1 activity. Dig. Dis. Sci. 2009;54(8):1643–1650. doi: 10.1007/s10620-008-0563-9. [DOI] [PubMed] [Google Scholar]
- 239.Vasina V. Non-peptidyl low molecular weight radical scavenger IAC attenuates DSS-induced colitis in rats. World J. Gastroenterol. 2010;16(29):3642. doi: 10.3748/wjg.v16.i29.3642. 3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vasina V., Broccoli M., Ursino M.G., Bellot S.F., Soleti A., Paolini M., De Ponti F. Effects of the non-peptidyl low molecular weight radical scavenger IAC in DNBS-induced colitis in rats. Eur. J. Pharmacol. 2009;614(1-3):137–145. doi: 10.1016/j.ejphar.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 241.Xing J., Sun J., You H., Lv J., Sun J., Dong Y. Anti-inflammatory effect of 3,4-oxo-isopropylidene-shikimic acid on acetic acid-induced colitis in rats. Inflammation. 2012;35(6):1872–1879. doi: 10.1007/s10753-012-9509-7. [DOI] [PubMed] [Google Scholar]
- 242.Xing J., You C., Dong K., Sun J., You H., Dong Y., Sun J. Ameliorative effects of 3,4-oxo-isopropylidene-shikimic acid on experimental colitis and their mechanisms in rats. Int. Immunopharmacol. 2013;15(3):524–531. doi: 10.1016/j.intimp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 243.Xing J.-F., Sun J.-N., Sun J.-Y., You C.-Y., Dong K., Lv J., Dong Y.-L. Protective effects of 3,4-oxo-isopropylidene-shikimic acid on experimental colitis induced by trinitrobenzenesulfonic acid in rats. Dig. Dis. Sci. 2012;57(8):2045–2054. doi: 10.1007/s10620-012-2155-y. [DOI] [PubMed] [Google Scholar]
- 244.Yao J., Wang J.-Y., Liu L., Li Y.-X., Xun A.-Y., Zeng W.-S., Jia C.-H., Wei X.-X., Feng J.-L., Zhao L., Wang L.-S. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch. Med. Res. 2010;41(4):288–294. doi: 10.1016/j.arcmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 245.Witaicenis A., Seito L.N., da Silveira Chagas A., de Almeida L.D., Luchini A.C., Rodrigues-Orsi P., Cestari S.H., Di Stasi L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine: Int. J. phytother. phytopharm. 2014;21(3):240–246. doi: 10.1016/j.phymed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 246.Witaicenis A., Seito L.N., Di Stasi L.C. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chemico -Biol. Interact. 2010;186(2):211–218. doi: 10.1016/j.cbi.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 247.Abdel-daim M.M., Farouk S.M., Madkour F.F., Azab S.S., Farouk S.M., Madkour F.F., Azab S.S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015;8:1–14. doi: 10.3109/08923973.2014.998368. [DOI] [PubMed] [Google Scholar]
- 248.Abdelkhalek N.K.M., Ghazy E.W., Abdel-Daim M.M. Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ. Sci. Pollut. Res. Int. 2014;9:1–9. doi: 10.1007/s11356-014-3578-0. [DOI] [PubMed] [Google Scholar]
- 249.Piganelli J.D., Delmastro M.M. Oxidative stress and redox modulation potential in type 1 diabetes. Clin. Dev. Immunol. 2011;2011:1–15. doi: 10.1155/2011/593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Koláček M., Muchová J., Dvořáková M., Paduchová Z., Žitňanová I., Čierna I., Országhová Z., Székyová D., Jajcaiová-Zedníčková N., Kovács L., Ďuračková Z. Effect of natural polyphenols (Pycnogenol) on oxidative stress markers in children suffering from Crohn's disease – a pilot study. Free Radic. Res. 2013;47(8):624–634. doi: 10.3109/10715762.2013.807508. [DOI] [PubMed] [Google Scholar]
- 251.Battershill A.J., Scott L.J. Telmisartan: a review of its use in the management of hypertension. Drugs. 2006;66:51–83. doi: 10.2165/00003495-200666010-00004. [DOI] [PubMed] [Google Scholar]
- 252.Shifrin H., Nadler-Milbauer M., Shoham S., Weinstock M. Rivastigmine alleviates experimentally induced colitis in mice and rats by acting at central and peripheral sites to modulate immune responses. PLoS One. 2013;8(2):e57668. doi: 10.1371/journal.pone.0057668. e57668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Iseri S.O., Ersoy Y., Ercan F., Yuksel M., Atukeren P., Gumustas K., Alican I. The effect of sildenafil, a phosphodiesterase-5 inhibitor, on acetic acid-induced colonic inflammation in the rat. J. Gastroenterol. Hepatol. 2009;24(6):1142–1148. doi: 10.1111/j.1440-1746.2009.05797.x. [DOI] [PubMed] [Google Scholar]
- 254.Karakoyun B., Uslu U., Ercan F., Aydin M.S., Yuksel M., Ogunc A.V., Alican I. The effect of phosphodiesterase-5 inhibition by sildenafil citrate on inflammation and apoptosis in rat experimental colitis. Life Sci. 2011;89(11–12):402–407. doi: 10.1016/j.lfs.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 255.Fatani A.J., Al-Hosaini K.A., Ahmed M.M., Abuohashish H.M., Parmar M.Y., Al-Rejaie S.S. Carvedilol Attenuates Inflammatory Biomarkers and Oxidative Stress in a Rat Model of Ulcerative colitis. Drug. Dev. Res. 2015;76(4):204–214. doi: 10.1002/ddr.21256. [DOI] [PubMed] [Google Scholar]
- 256.Sehirli A.O., Cetinel S., Ozkan N., Selman S., Tetik S., Yuksel M., Dulger G. St. John's wort may ameliorate 2,4,6-trinitrobenzenesulfonic acid colitis off rats through the induction of pregnane X receptors and/or P-glycoproteins. J. Physiol. Pharmacol. 2015;66(2):203–214. [PubMed] [Google Scholar]
- 257.Minaiyan M., Hajhashemi V., Rabbani M., Fattahian E., Mahzouni P. Evaluation of anti-colitic effect of fluvoxamine against acetic acid-induced colitis in normal and reserpinized depressed rats. Eur. J. Pharmacol. 2015;746:293–300. doi: 10.1016/j.ejphar.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 258.Liu W., Guo W., Wu J., Luo Q., Tao F., Gu Y., Shen Y., Li J., Tan R., Xu Q., Sun Y. A novel benzo[d]imidazole derivate prevents the development of dextran sulfate sodium-induced murine experimental colitis via inhibition of NLRP3 inflammasome. Biochem. Pharmacol. 2013;85(10):1504–1512. doi: 10.1016/j.bcp.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 259.El Morsy E.M., Kamel R., Ahmed M.A. Attenuating effects of coenzyme Q10 and amlodipine in ulcerative colitis model in rats. Immunopharmacol. Immunotoxicol. 2015;37(3):244–251. doi: 10.3109/08923973.2015.1021357. [DOI] [PubMed] [Google Scholar]
- 260.Margonis G.A., Christoloukas N., Antoniou E., Arkadopoulos N., Theodoropoulos G., Agrogiannis G., Pikoulis E., Patsouris E.S., Zografos G.C., Papalois A.E. Effectiveness of sildenafil and U-74389G in a rat model of colitis. J. Surg. Res. 2015;193(2):667–674. doi: 10.1016/j.jss.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 261.Konturek P.C., Brzozowski T., Engel M., Burnat G., Gaca P., Kwiecien S., Pajdo R., Konturek S.J. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J. physiol. Pharmacol. 2009;60:41–47. [PubMed] [Google Scholar]
- 262.Talero E., Alvarez de Sotomayor M., Sánchez-Fidalgo S., Motilva V. Vascular contribution of adrenomedullin to microcirculatory improvement in experimental colitis. Eur. J. Pharmacol. 2011;670(2–3):601–607. doi: 10.1016/j.ejphar.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 263.Azuma K., Osaki T., Kurozumi S., Kiyose M., Tsuka T., Murahata Y., Imagawa T., Itoh N., Minami S., Sato K., Okamoto Y. Anti-inflammatory effects of orally administered glucosamine oligomer in an experimental model of inflammatory bowel disease. Carbohydr. Polym. 2015;115:448–456. doi: 10.1016/j.carbpol.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 264.Sobczak M., Zakrzewski P.K., Cygankiewicz A.I., Mokrowiecka A., Chen C., Salaga M., Malecka-Panas E., Kordek R., Krajewska W.M., Fichna J. Anti-inflammatory action of a novel orally available peptide 317 in mouse models of inflammatory bowel diseases. Pharmacol. Rep. 2014;66(5):741–750. doi: 10.1016/j.pharep.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 265.Ek R.O., Serter M., Ergin K., Cecen S., Unsal C., Yildiz Y., Mehmet D. Protective effects of citicoline on TNBS-induced experimental colitis in rats. Int. J. Clin. Exp. Med. 2014;7(4):989–997. [PMC free article] [PubMed] [Google Scholar]
- 266.Moeinian M., Ghasemi-Niri S.F., Mozaffari S., Abdolghaffari A.H., Baeeri M., Navaea-Nigjeh M., Abdollahi M. Beneficial effect of butyrate, Lactobacillus casei and l-carnitine combination in preference to each in experimental colitis. World J. Gastroenterol. 2014;20(31):10876–10885. doi: 10.3748/wjg.v20.i31.10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Amirshahrokhi K., Bohlooli S., Chinifroush M.M. The effect of methylsulfonylmethane on the experimental colitis in the rat. Toxicol. Appl. Pharmacol. 2011;253(3):197–202. doi: 10.1016/j.taap.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 268.Singh R.S., Bhari R., Rana V., Tiwary A.K. Immunomodulatory and therapeutic potential of a mycelial lectin from Aspergillus nidulans. Appl. Biochem. Biotechnol. 2011;165(2):624–638. doi: 10.1007/s12010-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 269.Mishra S.K., Kang J.-H., Kim D.-K., Oh S.H., Kim M.K. Orally administered aqueous extract of Inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. J. Ethnopharmacol. 2012;143(2):524–532. doi: 10.1016/j.jep.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 270.Martínez-Moya P., Romero-Calvo I., Requena P., Hernández-Chirlaque C., Aranda C.J., González R., Zarzuelo A., Suárez M.D., Martínez-Augustin O., Marín J.J.G., De Medina F.S. Dose-dependent antiinflammatory effect of ursodeoxycholic acid in experimental colitis. Int. Immunopharmacol. 2013;15(2):372–380. doi: 10.1016/j.intimp.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 271.Ye Z., Liu Z., Henderson A. Increased CYP4B1 mRNA is associated with the inhibition of dextran sulfate sodium–induced colitis by caffeic acid in mice. Exp. Biol. 2009;234(6):605–616. doi: 10.3181/0901-RM-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mazzon E., Esposito E., Di Paola R., Riccardi L., Caminiti R., Dal Toso R., Pressi G., Cuzzocrea S. Effects of verbascoside biotechnologically produced by Syringa vulgaris plant cell cultures in a rodent model of colitis. Naunyn-Schmiedeberg's Arch. Pharmacol. 2009;380(1):79–94. doi: 10.1007/s00210-009-0400-5. [DOI] [PubMed] [Google Scholar]
- 273.Rosillo M. a, Sanchez-Hidalgo M., Cárdeno a, de la Lastra C.A. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn's disease. Biochem. Pharmacol. 2011;82(7):737–745. doi: 10.1016/j.bcp.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 274.Marín M., María Giner R., Ríos J.L., Carmen Recio M. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013;150(3):925–934. doi: 10.1016/j.jep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 275.D’Argenio G., Mazzone G., Tuccillo C., Ribecco M.T., Graziani G., Gravina A.G., Caserta S., Guido S., Fogliano V., Caporaso N., Romano M. Apple polyphenols extract (APE) improves colon damage in a rat model of colitis. Dig. Liver Dis. 2012;44(7):555–562. doi: 10.1016/j.dld.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 276.Oz H.S., Chen T., de Villiers W.J.S. Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front. Immunol. 2013;4:132. doi: 10.3389/fimmu.2013.00132. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Brückner M., Westphal S., Domschke W., Kucharzik T., Lügering A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohn's Colitis. 2012;6(2):226–235. doi: 10.1016/j.crohns.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 278.Gupta R.A., Motiwala M.N., Dumore N.G., Danao K.R., Ganjare A.B. Effect of piperine on inhibition of FFA induced TLR4 mediated inflammation and amelioration of acetic acid induced ulcerative colitis in mice. J. Ethnopharmacol. 2015;164:239–246. doi: 10.1016/j.jep.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 279.Mochizuki M., Hasegawa N. (-)-Epigallocatechin-3-gallate reduces experimental colon injury in rats by regulating macrophage and mast cell. Phytother. Res. 2010;24(S1):S120–S122. doi: 10.1002/ptr.2862. [DOI] [PubMed] [Google Scholar]
- 280.Joo M., Kim H.S., Kwon T.H., Palikhe A., Zaw T.S., Jeong J.H., Sohn U.D. Anti-inflammatory effects of flavonoids on TNBS-induced colitis of rats. Korean J. Physiol. Pharmacol. 2015;19(1):43–50. doi: 10.4196/kjpp.2015.19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Vochyánová Z., Barto L., Bujdáková V., Fictum P., Husník R., Suchý P., Šmejkal K., Hošek J. Diplacone and mimulone ameliorate dextran sulfate sodium-induced colitis in rats. Fitoterapia. 2015:1–8. doi: 10.1016/j.fitote.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 282.Seito L.N., Sforcin J.M., Bastos J.K., Di Stasi L.C. Zeyheria montana Mart. (Bignoniaceae) as source of antioxidant and immunomodulatory compounds with beneficial effects on intestinal inflammation. J. Pharm. Pharmacol. 2015;67(4):597–604. doi: 10.1111/jphp.12354. [DOI] [PubMed] [Google Scholar]
- 283.Lei X., Liu M., Yang Z., Ji M., Guo X., Dong W. Thymoquinone prevents and ameliorates dextran sulfate sodium-induced colitis in mice. Dig. Dis. Sci. 2012;57(9):2296–2303. doi: 10.1007/s10620-012-2156-x. [DOI] [PubMed] [Google Scholar]
- 284.Kondamudi P.K., Kovelamudi H., Mathew G., Nayak P.G., Rao C.M., Shenoy R.R. Modulatory effects of sesamol in dinitrochlorobenzene-induced inflammatory bowel disorder in albino rats. Pharmacol. Rep. 2013;65(3):658–665. doi: 10.1016/s1734-1140(13)71043-0. [DOI] [PubMed] [Google Scholar]
- 285.Yang X.-L., Guo T.-K., Wang Y.-H., Gao M.-T., Qin H., Wu Y.-J. Therapeutic effect of ginsenoside Rd in rats with TNBS-induced recurrent ulcerative colitis. Arch. Pharmacal Res. 2012;35(7):1231–1239. doi: 10.1007/s12272-012-0714-6. [DOI] [PubMed] [Google Scholar]
- 286.Liu X., Wang J. Anti-inflammatory effects of iridoid glycosides fraction of Folium syringae leaves on TNBS-induced colitis in rats. J. Ethnopharmacol. 2011;133(2):780–787. doi: 10.1016/j.jep.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 287.Kumar K., Dhamotharan G., R., Kulkarni N.M., Honnegowda S., Murugesan S. Embelin ameliorates dextran sodium sulfate-induced colitis in mice. Int. Immunopharmacol. 2011;11(6):724–731. doi: 10.1016/j.intimp.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 288.Thippeswamy B.S., Mahendran S., Biradar M.I., Raj P., Srivastava K., Badami S., Veerapur V.P. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur. J. Pharmacol. 2011;654(1):100–105. doi: 10.1016/j.ejphar.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 289.Niu X., Fan T., Li W., Huang H., Zhang Y., Xing W. Protective effect of sanguinarine against acetic acid-induced ulcerative colitis in mice. Toxicol. Appl. Pharmacol. 2013;267(3):256–265. doi: 10.1016/j.taap.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 290.He J., Liang J., Zhu S., Zhao W., Zhang Y., Sun W. Protective effect of taurohyodeoxycholic acid from Pulvis Fellis Suis on trinitrobenzene sulfonic acid induced ulcerative colitis in mice. Eur. J. Pharmacol. 2011;670(1):229–235. doi: 10.1016/j.ejphar.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 291.Huang Z., Zuo L., Zhang Z., Liu J., Chen J., Dong L., Zhang J. 3,3′-Diindolylmethane decreases VCAM-1 expression and alleviates experimental colitis via a BRCA1-dependent antioxidant pathway. Free Radic. Biol. Med. 2011;50(2):228–236. doi: 10.1016/j.freeradbiomed.2010.10.703. [DOI] [PubMed] [Google Scholar]
- 292.Miroliaee A.E., Esmaily H., Vaziri-Bami A., Baeeri M., Shahverdi A.R., Abdollahi M. Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol. Mech. Methods. 2011;21(3):200–208. doi: 10.3109/15376516.2010.547887. [DOI] [PubMed] [Google Scholar]
- 293.Park M.-Y., Kwon H.-J., Sung M.-K. Dietary aloin, aloesin, or aloe-gel exerts anti-inflammatory activity in a rat colitis model. Life Sci. 2011;88(11-12):486–492. doi: 10.1016/j.lfs.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 294.Yum H.-W., Zhong X., Park J., Na H.-K., Kim N., Lee H.S., Surh Y.-J. Oligonol inhibits dextran sulfate sodium-induced colitis and colonic adenoma formation in mice. Antioxid. Redox Signal. 2013;19(2):102–114. doi: 10.1089/ars.2012.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Niu X., Zhang H., Li W., Wang Y., Mu Q., Wang X., He Z., Yao H. Protective effect of cavidine on acetic acid-induced murine colitis via regulating antioxidant, cytokine profile and NF-kappaB signal transduction pathways. Chem. Biol. Interact. 2015;239:34–45. doi: 10.1016/j.cbi.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 296.Wu X.F., Ouyang Z.J., Feng L.L., Chen G., Guo W.J., Shen Y., Wu X.D., Sun Y., Xu Q. Suppression of NF-kappaB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol. Appl. Pharmacol. 2014;281(1):146–156. doi: 10.1016/j.taap.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 297.Guo W., Liu W., Jin B., Geng J., Li J., Ding H., Wu X., Xu Q., Sun Y., Gao J. Asiatic acid ameliorates dextran sulfate sodium-induced murine experimental colitis via suppressing mitochondria-mediated NLRP3 inflammasome activation. Int. Immunopharmacol. 2015;24(2):232–238. doi: 10.1016/j.intimp.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 298.Pandurangan A.K., Ismail S., Saadatdoust Z., Esa N.M. Allicin alleviates dextran sodium sulfate- (DSS-) induced ulcerative colitis in BALB/c mice. Oxid. Med. Cell. Longev. 2015;2015:605208. doi: 10.1155/2015/605208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sánchez-Fidalgo S., Cárdeno A., Sánchez-Hidalgo M., Aparicio-Soto M., De la Lastra C.A. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J. Nutr. Biochem. 2013;24(7):1401–1413. doi: 10.1016/j.jnutbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 300.Sánchez-Fidalgo S., Sánchez de Ibargüen L., Cárdeno a, de la Lastra C. Alarcón. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur. J. Nutr. 2012;51(4):497–506. doi: 10.1007/s00394-011-0235-y. [DOI] [PubMed] [Google Scholar]
- 301.Hamer H.M., Jonkers D.M. a E., Vanhoutvin S. a L.W., Troost F.J., Rijkers G., de Bruïne A., Bast A., Venema K., Brummer R.-J.M. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin. Nutr. 2010;29(6):738–744. doi: 10.1016/j.clnu.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 302.Harisa G.E.I., Abo-Salem O.M., El-Sayed E.-S.M., Taha E.I., El-Halawany N. l-Arginine augments the antioxidant effect of garlic against acetic acid-induced ulcerative colitis in rats. Pak. J. Pharm. Sci. 2009;22:373–380. [PubMed] [Google Scholar]
- 303.Philippe D., Brahmbhatt V., Foata F., Saudan Y., Serrant P., Blum S., Benyacoub J., Vidal K. Anti-inflammatory effects of Lacto-Wolfberry in a mouse model of experimental colitis. World J. Gastroenterol. 2012;18(38):5351–5359. doi: 10.3748/wjg.v18.i38.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nishitani Y., Tanoue T., Yamada K., Ishida T., Yoshida M., Azuma T., Mizuno M. Lactococcus lactis subsp. cremoris FC alleviates symptoms of colitis induced by dextran sulfate sodium in mice. Int. Immunopharmacol. 2009;9(12):1444–1451. doi: 10.1016/j.intimp.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 305.Jeong J.J., Woo J.Y., Ahn Y.T., Shim J.H., Huh C.S., Im S.H., Han M.J., Kim D.H. The probiotic mixture IRT5 ameliorates age-dependent colitis in rats. Int. Immunopharmacol. 2015;26(2):416–422. doi: 10.1016/j.intimp.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 306.Jeong J.J., Kim K.A., Jang S.E., Woo J.Y., Han M.J., Kim D.H. Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS One. 2015;10(2):e0116533. doi: 10.1371/journal.pone.0116533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ballal S.A., Veiga P., Fenn K., Michaud M., Kim J.H., Gallini C.A., Glickman J.N., Quere G., Garault P., Beal C., Derrien M., Courtin P., Kulakauskas S., Chapot-Chartier M.P., van Hylckama Vlieg J., Garrett W.S. Host lysozyme-mediated lysis of Lactococcus lactis facilitates delivery of colitis-attenuating superoxide dismutase to inflamed colons. Proc. Natl. Acad. Sci. U. S. A. 2015;112(25):7803–7808. doi: 10.1073/pnas.1501897112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Toumi R., Soufli I., Rafa H., Belkhelfa M., Biad A., Touil-Boukoffa C. Probiotic bacteria lactobacillus and bifidobacterium attenuate inflammation in dextran sulfate sodium-induced experimental colitis in mice. Int. J. Immunopathol. Pharmacol. 2014;27(4):615–627. doi: 10.1177/039463201402700418. [DOI] [PubMed] [Google Scholar]
- 309.Satish Kumar C.S., Kondal Reddy K., Reddy A.G., Vinoth A., Ch S.R., Boobalan G., Rao G.S. Protective effect of Lactobacillus plantarum 21, a probiotic on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. Int. Immunopharmacol. 2015;25(2):504–510. doi: 10.1016/j.intimp.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 310.Eeckhaut V., Machiels K., Perrier C., Romero C., Maes S., Flahou B., Steppe M., Haesebrouck F., Sas B., Ducatelle R., Vermeire S., Van Immerseel F. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. 2013;62(12):1745–1752. doi: 10.1136/gutjnl-2012-303611. [DOI] [PubMed] [Google Scholar]
- 311.Tomé-Carneiro J., Larrosa M., González-Sarrías A., Tomás-Barberán F.A., García-Conesa M.T., Espín J.C. Resveratrol and Clinical Trials: The Crossroad from In Vitro Studies to Human Evidence. Curr. Pharm. Des. 2013;19:6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]