Abstract
Introduction
Mixed glandular-endocrine carcinomas are rare tumours of gastrointestinal tract (MANEC). They are more frequent in stomach and hardly one hundred cases have been described in colon. According to Lewis, they are classified into collision (side by side pattern), composite (intermingled) or amphicrine (neuroendocrine and glandular features inside a same cell). Collision tumours are related to biclonal theory: two simultaneous cancerogenic events. Conversely, multidirectional differentiation from a stem cell is accepted as origin of composite tumours.
The aim of this paper is to analyse the behaviour of these tumours, with an especial concern about how these tumours metastasise, and the different theories about carcinogenesis.
Presentation of case
We report a rare case of collision adenocarcinoma-large cell neuroendocrine tumour of colon that after a three-year period of follow-up has presented a retroperitoneal recurrence that features adenocarcinoma and large cell neuroendocrine components.
Discussion
After an exhaustive review of the English literature, we found that only two cases of collision tumour of colon with metastases showing glandular and endocrine components have been described up to date, so we report the third case, and the first happening in transverse colon.
Conclusion
We conclude that not all collision tumours follow the biclonal theory and more studies are needed to clarify the origin of these neoplasms, and consequently, to reach an adequate treatment.
Keywords: Colon, Collision, Composite, Tumour, Mixed, MANEC
Highlights
-
•
MANEC are defined as mixed adenoneuroendocrine carcinoma.
-
•
They are divided into composite and collision.
-
•
The poorest differentiated component will determine the prognosis.
-
•
Metastases occur frequently at liver and nodes.
-
•
Colon is a very rare place.
1. Introduction
Mixed tumours containing glandular and neuroendocrine components are infrequent.
The first case was noted by Cordier in 1924. According to Lewis (1987), the term “mixed” is applied when neuroendocrine component constitutes at least 30% of tumour. However, metastases from smaller neuroendocrine components have been reported, so the cutoff of 30% is controversial [1], [2], [3], [4], [5], [6], [7], [8].
Lewis classified these tumours into collision, composite and amphicrine. Composite displays glandular and neuroendocrine components intermingled; conversely, collision shows glandular and neuroendocrine components in a side by side pattern; and amphicrine features neuroendocrine and glandular characteristics inside the same cell [1], [3], [4], [9], [10].
Collision tumours are related to biclonal malignant transformation theory, so they are considered two different tumours juxtaposed. In contrast, composite tumours are believed to result from a multidirectional differentiation of a single cell [4], [9], [11]. Some authors are reluctant to accept amphicrine tumours as part of the spectrum of mixed tumours.
Immunohistochemistry is crucial for an accurate diagnosis: chromogranin A, synaptophysin, CD56, neuron-specific- enolasa are typical of neuroendocrine tumours; and CEA, keratin 20 and CDX2 of adenocarcinoma colorectal tumours [7].
The true incidence of these tumours may be underestimated due to inadequate diagnosis.
They have been described in stomach, gallbladder and pancreas [7], [12]. Other locations as duodenum [11] or colon are rare [5], [9].
When these tumours appear in colon, right colon is the most common place affected (56%) followed by left colon and, finally, transverse colon (3%).
According to Li, they are more frequent in elderly patients and male gender. Composite constitutes 42% and collision 58% of all colorectal glandular-neuroendocrine mixed tumours [3].
In 2013, we reported a 66 year old male who presented a collision adenocarcinoma-large cell neuroendocrine tumour of transverse colon with 70% neuroendocrine and 30% adenocarcinoma component. The neuroendocrine component featured more than 10 mitosis per high power fields, Ki 67 in a percentage of 20%, and chromogranin A and synaptophysin positive. It was graded as large cell neuroendocrine carcinoma (GIII). The adenocarcinoma component was noted as T3N0. Two neuroendocrine metastases in lymph nodes were reported [2].
In this paper, we analyse the behaviour of this tumour. After a three-year follow-up, a retroperitoneal recurrence was evidenced on computed tomography (CT). The patient underwent surgery and a mass of 2 × 2 cm size, surrounding the left ureter was found and excised. The frozen biopsy revealed adenocarcinoma-large cell neuroendocrine metastases with 50% of each component.
We reviewed the English literature in PubMed database; for searching we used the words “mixed”, “adenoneuroendocrine”, ”glandular-neuroendocrine”, “collision tumor”, “composite tumor” and “MANEC”. We analysed the behaviour of these tumours, searching for collision tumours that had metastasised displaying both components.
We conclude that this is the third case reported in the English literature of collision tumour of colon that has metastasised showing both, glandular and neuroendocrine components, and the first case located in transverse colon. So, our case refutes the biclonal theory.
On the other hand, it must be taken into account that other cases of collision tumours and, consequently, metastases with both elements, could have been misdiagnosed, especially in the preimmunohistochemical era.
2. Presentation of case
A 66 year old male with a two-month history of abdominal pain and loss of weight was admitted to the Colorectal Surgery Department. His medical history included hypertension, diverticulitis and appendicectomy.
At the physical examination, the patient presented pain in the upper left abdominal quadrant. McBurney scar was present.
Laboratory test revealed haemoglobin level of 11 gr/dL, coagulation values in normal range, and tumour markers: CEA 3.8 ng/ml and CA 19.9: 18 U/ml.
A contrast enhanced CT was performed and revealed an irregular circumferential mass in the left half of transverse colon with fat stranding adjacent to thickened bowel wall. No evidence of metastases was reported. Multiple diverticula were noted (Fig. 1).
Fig. 1.
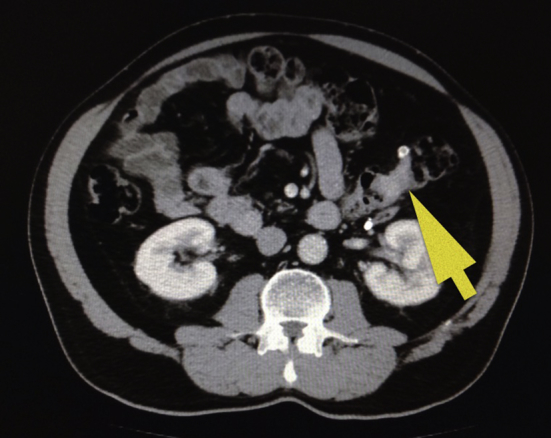
Contrast enhanced CT showed an irregular circumferential mass in the left half of transverse colon with fat stranding adjacent to thickened bowel wall.
Colonoscopy demonstrated a mass at 70 cm from the anal verge. Biopsy was taken and reported as adenocarcinoma.
The patient underwent surgery. Laparotomy was carried out. A neoplasm in the left half of transverse colon was found. The abdominal cavity and liver did not show metastases. A subtotal colectomy was chosen due to diverticulosis. The postoperative period was uneventful and the patient was discharged ten days later.
Frozen biopsy revealed a MANEC tumour of colon composed of large cell neuroendocrine, GIII, (70%) and adenocarcinoma (30%) components. Both components displayed a juxtaposed pattern, and no transition zone was observed (Fig. 2).
Fig. 2.
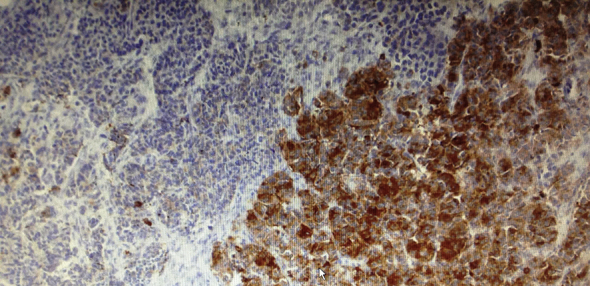
Large cell neuroendocrine carcinoma, positive for Chromogranin A, and moderately differentiated adenocarcinoma in a collision pattern.
Neuroendocrine carcinoma featured large cells with abundant eosinophilic cytoplasm and nuclei with diffuse geographic necrosis. They were positive for chromogranin A and synaptophysin, more than 10 mitoses per 10 high power fields, and Ki67 was demonstrated in a percentage of 20%.
Adenocarcinoma component was moderately differentiated and graded as T3N0. Vascular and perineural invasion were present.
Two lymph nodes metastases were identified; after an exhaustive study with immunohistochemical staining, chromogranin A and synaptophysin were positive and, finally, noted as neuroendocrine metastases.
After surgery, an octreoscan was carried out and it showed no evidence of neuroendocrine tumour in other locations. Adjuvant treatment based on chemotherapy was proposed, however, after explaining the benefits, risks and evidence from literature, the patient declined chemotherapy.
Follow-up was performed with CT, colonoscopy, tumoral markers and chromogranin A in blood.
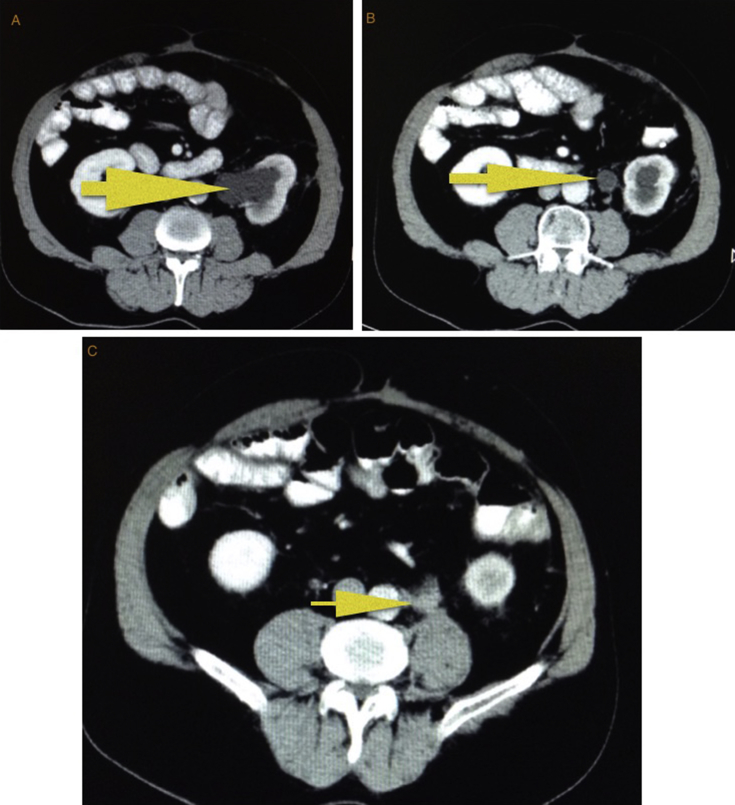
Three years after surgery, a mass of 2 × 2 cm size across surrounding the left ureter and causing ureterohydronephrosis, was identified on CT (Fig. 3). This lesion was suggestive of retroperitoneal recurrence. No other lesions were evidenced. The peritoneal carcinomatosis index was stated 2/39. Colonoscopy did not show endoluminal lesion. Tumoral markers were in normal range: CEA 3.2 ng/dL, CA 19.9: 8.4 U/mL and chromogranin A 3.6 U/mL.
Fig. 3.
CT demonstrated a retroperitoneal mass surrounding the left lumbar urether and causing ureterohydronefrosis: A and B. This lesion was suggestive of recurrence: C.
FDG -PET/CT was realised and showed a pathological caption at the level of the left ureter (SUV max 10), no evidence of other metastases.
Finally, the patient underwent surgery. Previously, a double J stent had been placed. Middle laparotomy was performed, and a careful adhesiolysis was needed. A 2 × 2 cm size across mass was found adjacent to the left lumbar ureter and excised. Then, an ureterectomy of 1 cm longitudinal of the lumbar ureter was performed, and repaired with end to end anastomosis.
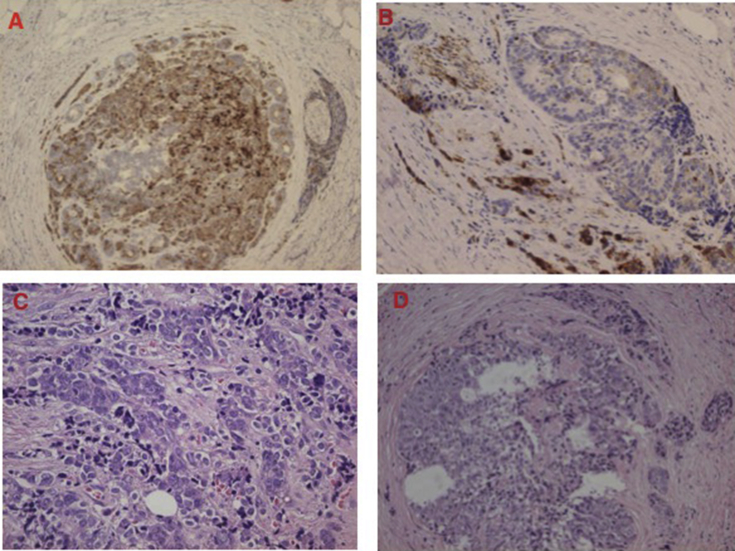
Frozen biopsy demonstrated a yellowish retroperitoneal mass with a size of 2 × 2 cm across composed of large cell neuroendocrine carcinoma and adenocarcinoma, in a proportion of 50% each of them. Neuroendocrine component was positive for chromogranin A and negative for synaptophysin (Fig. 4).
Fig. 4.
Recurrence retroperitoneal mass with a size of 2 × 2 cm size composed of adenocarcinoma and neuroendocrine (GIII). Immunohistochemical study revealed neuroendocrine cells positive for chromogranin A (A and B). Hematoxylin eosin staining showed glandular component (C and D).
Ureter fragment of 1 × 0.6 cm was infiltrated by mixed tumour composed of adenocarcinoma and large cell neuroendocrine carcinoma, also in a proportion of 50% each component. The neuroendocrine component was positive for both, chromogranin A and synaptophysin.
3. Discussion
Twenty-three years after Lewis' classification, a new term was introduced and recognized by World Health Organisation in an attempt to achieve a proper diagnosis and treatment of these tumours.
In 2010, World Health Organization (WHO) defined MANEC as mixed adeno-neuroendocrine carcinoma. Each component must constitute at least 30% of the tumour. In addition, both components must be malignant and, consequently, must be graded separately [5], [7], [13].
MANECs were, definitively, classified into composite and collision. As a result, amphicrine tumours were excluded from the spectrum.
MANECs account for 3–9.6% of all colorectal tumours [7].
It is important to highlight that neuroendocrine cells appear within conventional adenocarcinoma in 20–50% of cases, but they do not reach MANEC criteria [1], [14].
The origin of these tumours is not clear. Why two different neoplastic cells, with different origin and different behaviour can coexist in a single tumour? This still remains a challenge for researchers.
Neuroendocrine component ranges from well-differentiated neuroendocrine tumours (low and intermediate grade, G1 and G2, respectively) to poorly differentiated neuroendocrine carcinoma (large and small cells, G3) [8]. Most cases are non functioning. The grading of neuroendocrine tumours (NET) depends on Ki 67 labelling index and mitotic rate according to WHO [10].
Similarly, glandular component has been reported as adenoma, well-differentiated, moderately differentiated or poorly differentiated adenocarcinoma, and the sequence adenoma to adenocarcinoma is accepted [3].
However, to be considered as a MANEC tumour, both components must be malignant. Consequently, some authors have suggested to subdivide these tumours according to the differentiation degree of each component: high grade malignant, intermediate grade malignant or mixed adenoendocrine tumour [5], [8].
Collision tumours are thought to be two different neoplasms that coexist in a side by side pattern with no transition zone. Their origin is explained by the biclonal malignant transformation theory that consists of two carcinogenesis events occurring simultaneously. This is the so called “double primaries” theory [3], [4], [7].
On the other hand, in the case of composite tumours, a single histogenic event is accepted on basis of multidirectional theory from a single cell: “monoclonal origin” [1], [3], [4], [7], [9], [12].
Each component will have a differentiation degree and the poorest differentiated component will determine the prognosis.
In the same way, each component can metastasise separately. Consequently, metastases from composite tumours have been noted as glandular component alone, neuroendocrine alone or both elements. In contrast, in the case of collision tumours, if we accept they are two different neoplasms, then metastases should show only neuroendocrine or glandular component, but not both of them.
About location, colon is a very rare place and no more than a hundred of cases have been reported. In addition, transverse colon is a very rare place of presentation. To date, no more than five cases have been reported in the English literature [12], [13], [15], [16]. So, our case is indeed, a very uncommon location and the sixth case of mixed glandular-neuroendocrine tumour in this location.
The most common places of metastases are regional lymph nodes and liver. Rarely, some cases of peritoneal spread have been described [3].
Colorectal MANECs have a very poor prognosis and high grade neuroendocrine tumours are difficult to treat.
Surgery is the only option for cure.
The effectivity of adjuvant chemotherapy after resection is not clear [6].
Patta reported a high response rate to cisplatin + ectoposide in patients with high grade neuroendocrine colorectal tumours [17].
NCCN recommends cisplatin or carboplatin and etoposide (based on protocols for small cell lung carcinoma) [18]. Other protocols are based on cetuximab + FOLFOX + octreotide; or bevacizumab + FOLFOX6 [13].
In the case of hepatic metastases, TACE with doxorubicin has been reported [13]. Other agents as mitomicin C or streptozocin have been used with different success rates.
The role of new drugs such as everolimus or sunitinib need to be defined. Radiotherapy could be considered in patients at high risk of local recurrence [19].
In 2007, Pecorella reported a case of collision tumour of cecum (moderately differentiated adenocarcinoma and carcinoid) that metastasised, at the time of surgery, in a lymph node featuring neuroendocrine and glandular components juxtaposed. So, this refutes the theory of “double primaries” theory [4].
Later, in 2014, Mesina noted a case of collision tumour in the rectosigmoid junction (adenocarcinoma and small cell neuroendocrine carcinoma) that also metastasised in a lymph node showing both components, at the time of surgery [9].
Eventually, we report a case of collision tumour located in transverse colon (adenocarcinoma and large cell neuroendocrine carcinoma) that after a 3 year follow up, presented a retroperitoneal metastasis with 50% neuroendocrine carcinoma and 50% adenocarcinoma.
So, this is the third case in the English literature of collision tumour that has metastasised displaying glandular and neuroendocrine component.
In addition, we highlight that our patient did not received chemotherapy and he still benefited from a three year disease-free survival, which is unusual for these tumours and does not coincide with the previous statistics of survival [2].
Moreover, the metastasis featuring both components did not appear at the time of surgery, as the two previous cases, but three year later; and it presented as a retroperitoneal mass with 50% adenocarcinoma and 50% neuroendocrine component instead of metastatic lymph nodes.
Therefore, our case also rebuts the biclonal theory of collision tumours.
4. Conclusion
We present the first case of MANEC type collision located in transverse colon that has metastasised displaying glandular and neuroendocrine cells. Furthermore, this is also the first case that showed a metastasis with both components not at the time of surgery but three years later. Moreover, it is the first case of metastasis with these characteristics that presents as retroperitoneal mass instead of metastatic lymph nodes.
We highlight that not all collision tumours follow the biclonal theory, there must be a common chain between the two tumoral components that may explain this peculiar behaviour. Therefore, we conclude that more studies are needed to clarify the origin of these neoplasms, and consequently, to reach an adequate treatment.
Ethical approval
No required.
Sources of funding
No sources of funding.
Author contribution
Ana Maria Minaya Bravo: design of the paper, draft and review of literature.
Julio César Garcia Mahillo: search of bibliography and translation.
Fernando Mendoza Moreno: review of literature.
Fernando Noguerales Fraguas: review of the paper.
Javier Granell: review of the paper.
Conflicts of interest
No conflict of interest.
Consent of patient
Provided.
Guarantor
Ana María Minaya Bravo.
References
- 1.Makino A., Serra S., Chetty R. Composite adenocarcinoma and large cell neuroendocrine carcinoma of the rectum. Virchows Arch. 2006;448(5):644–647. doi: 10.1007/s00428-006-0169-6. [DOI] [PubMed] [Google Scholar]
- 2.Minaya A., Vera C., Hernández F., Noguerales F., Granell J. Collision adenocarcinoma-large cell neuroendocrine tumour of colon: a case report and review of literature. World J. Colorectal Surg. 2013;3(1):19. [Google Scholar]
- 3.Li Y., Yau A., Schaeffer D., Magliocco A., Gui X., Urbanski S., Waghray R., Owen D., Gao Z.H. Colorectal glandular-neuroendocrine mixed tumor: pathologic spectrum and clinical implications. Am. J. Surg. Pathol. 2011;35(3):413–425. doi: 10.1097/PAS.0b013e3182093657. [DOI] [PubMed] [Google Scholar]
- 4.Pecorella I., Memeo L., Ciardi A., Rotterdam H. An unusual case of colonic mixed adenoendocrine carcinoma: collision versus composite tumor. A case report and review of the literature. Ann. Diagn. Pathol. 2007;11(4):285–290. doi: 10.1016/j.anndiagpath.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Liu X.J., Feng J.S., Xiang W.Y., Kong B., Wang L.M., Zeng J.C., Liang Y.F. Clinicopathological features of an ascending colon mixed adenoneuroendocrine carcinoma with clinical serosal invasion. Int. J. Clin. Exp. Pathol. 2014;7(9):6395–6398. [PMC free article] [PubMed] [Google Scholar]
- 6.Yamauchi H., Sakurai S., Tsukagoshi R., Suzuki M., Tabe Y., Fukasawa T., Kiriyama S., Fukuchi M., Naitoh H., Kuwano H. A case of very well- differentiated adenocarcinoma with carcinoid tumor in the ascending colon. Int. Surg. 2014;99:132–136. doi: 10.9738/INTSURG-D-13-00041.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurzu S., Kadar Z., Bara T., Bara T., Jr., Tamasi A., Azamfirei L., Jung I. Mixed adenoneuroendocrine carcinoma of gastrointestinal tract: report of two cases. World J. Gastroenterol. 2015;21(4):1329–1333. doi: 10.3748/wjg.v21.i4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Rosa S., Marando A., Sessa F., Capella C. Mixed adenoneuroendocrine carcinomas (MANECs) gastrointestinal tract: an update. Cancers. 2012;4(1):11–30. doi: 10.3390/cancers4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesina C., Vasile I., Ciobanu D., Calota F., Gruia C.L., Streba L., Mogoanta S.S., Parvanescu H., Georgescu C.V., Tarnita D.N. Collision tumor of recto-sigmoidian junction – case presentation. Rom. J. Morphol. Embryol. 2014;55(2 Suppl.):643–647. [PubMed] [Google Scholar]
- 10.Hui C.K. Collision adenoma-carcinoid tumour of the colon complicated by carcinoid syndrome. Singap. Med. J. 2012;53(9):e195–197. [PubMed] [Google Scholar]
- 11.Peng L., Schwarz R.E. Collision tumor in form of primary adenocarcinoma and neuroendocrine carcinoma of the duodenum. Rare Tumors. 2012;4(2):e20. doi: 10.4081/rt.2012.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain A., Singla S., Jagdeesh K.S., Vishnumurthy H.Y. Mixed adenoneuroendocrine carcinoma of cecum: a rare entity. J. Clin. Imaging Sci. 2013;3:10. doi: 10.4103/2156-7514.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito H., Kudo A., Matsumura S., Ban D., Irie T., Ochiai T., Nakamura N., Tanaka S., Tanabe M. Mixed adenoneuroendocrine carcinoma of the colon progressed rapidly after hepatic rupture: report of a case. Int. Surg. 2014;99(1):40–44. doi: 10.9738/INTSURG-D-13-00161.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bruine A.P., Wiggers T., Beek C., Volovics A., von Meyenfeldt M., Arends J.W., Bosman F.T. Endocrine cells in colorectal adenocarcinomas: incidence, hormone profile and prognostic relevance. Int. J. Cancer. 1993;54(5):765–771. doi: 10.1002/ijc.2910540510. [DOI] [PubMed] [Google Scholar]
- 15.Furlan D., Cerutti R., Genasetti A., Pelosi G., Uccella S., La Rosa S., Capella C. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab. Invest. 2003;83(7):963–971. doi: 10.1097/01.lab.0000079006.91414.be. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez F.J., Reid J.D. Mixed carcinoid and mucus-secreting intestinal tumors. Arch. Pathol. 1969;88(5):489–496. [PubMed] [Google Scholar]
- 17.Patta A., Fakih M. First-line cisplatin plus etoposide in high- grade metastatic neuroendocrine tumors of colon and rectum (MCRC NET): a review of 8 cases. Anti Cancer Res. 2011;31(3):975–978. [PubMed] [Google Scholar]
- 18.Neuroendocrine Tumors. NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines; 2015. http://www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf Version 1.2015. [Google Scholar]
- 19.Strosberg J.R., Coppola D., Klimstra D.S., Phan A.T., Kulke M.H., Wiseman G.A., Kvols L.K. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799–800. doi: 10.1097/MPA.0b013e3181ebb56f. North American Neuroendocrine Tumor Society NANETS. [DOI] [PMC free article] [PubMed] [Google Scholar]