Abstract
Current influenza virus vaccines are based on strain-specific surface glycoprotein hemagglutinin (HA) antigens and effective only when the predicted vaccine strains and circulating viruses are well-matched. The current strategy of influenza vaccination does not prevent the pandemic outbreaks and protection efficacy is reduced or ineffective if mutant strains emerge. It is of high priority to develop effective vaccines and vaccination strategies conferring a broad range of cross protection. The extracellular domain of M2 (M2e) is highly conserved among human influenza A viruses and has been utilized to develop new vaccines inducing cross protection against different subtypes of influenza A virus. However, immune mechanisms of cross protection by M2e-based vaccines still remain to be fully elucidated. Here, we review immune correlates and mechanisms conferring cross protection by M2e-based vaccines. Molecular and cellular immune components that are known to be involved in M2 immune-mediated protection include antibodies, B cells, T cells, alveolar macrophages, Fc receptors, complements, and natural killer cells. Better understanding of protective mechanisms by immune responses induced by M2e vaccination will help facilitate development of broadly cross protective vaccines against influenza A virus.
Keywords: Influenza virus, M2e, Universal vaccine, Immune mechanism
INTRODUCTION
Influenza viruses are enveloped, single-stranded, negative-sense RNA viruses and belong to the family Orthomyxoviridae. Influenza viruses are classified into main types A, B, and C. These main antigenic types are differentiated not only on the basis of antigenic differences in their nucleocapsid (NP) and matrix (M) proteins, but also with respect to the number of gene segments and viral proteins, host range and capacity to cause disease (1). Influenza B viruses are restricted to human hosts. Influenza A viruses that have many different hosts including humans, birds, and pigs are divided into subtypes based on hemagglutinin (HA) and neuraminidase (NA) proteins on the surface of the virus. At present, 18 different HA (H1-H18) and 11 different NA (N1-N11) molecules are known to exist (2).
The gene 7 among 8 segmented genes of influenza A virus is bicistronic, encodes both M1 and M2 proteins. The M2 protein is derived from the co-linear (M1) transcript by splicing. M2 is an integral membrane protein, whose membrane-spanning domain also serves as a signal for transport to the cell surface. M2 protein is also known to be a proton-selective ion channel to control the pH of the Golgi during HA synthesis and responsible for the acidification of the viral interior during uncoating and membrane fusion (3,4). The 23-amino acid sequence in the extracellular domain of M2 (M2e) is well conserved among human influenza A viruses although there are few residue changes among non-human host species such as avian and swine influenza A viruses (5,6,7). Especially, the MSLLTEVET sequence (aa 1-9) in M2e was found to be conserved at a rate of over 99% among all influenza A viruses (5,8) because these residues are shared with those of M1. Therefore, M2e-based vaccines may be developed into attractive candidates for a universal influenza vaccine with a broad spectrum of prevention.
M2e itself is known as a very poor immunogen. Even live influenza virus infection generating strong humoral and cellular immunity is not effective in inducing M2e-specific antibodies in a mouse model (9,10). Therefore, previous studies have focused on increasing the immunogenicity of M2e using a variety of carrier vehicles or in combination with various adjuvants. Examples of carrier vehicles for M2e antigens include hepatitis B virus core particles (11,12,13), human papillomavirus L proteins (14), phage Qβ-derived protein cores (15), keyhole limpet hemocyanin (16), bacterial outer membrane complexes (12,17), liposomes (18), cholera toxin subunit (19), flagellin (20), and virus-like particles (9). Adjuvant formulations that were reported with M2e antigens include complete or incomplete Freund's adjuvant (12,16,21), monophosphoryl lipid A (MPL) (18,19,22,23), cholera toxin subunits (24), heat-labile endotoxin (8,11,13,25), cholera toxin, and liposome based cationic adjuvant (26). Also, recombinant live vectors expressing M2e antigens that were tested in animal models are to utilize adenovirus (16) or modified vaccinia virus Ankara (27). Most studies described above reported that M2e-based vaccines even with potent experimental adjuvants can provide survival protection but are not capable of completely preventing weight loss after lethal challenge with different strains of influenza A virus. Thus, studies on the protective efficacies by M2e-based vaccination, involving a certain degree of body weight loss, suggest that M2e immunity-mediated cross protection is relatively weak. Despite extensive reports with M2e-based vaccines, the mechanism studies of cross protection by the immune responses induced by M2e vaccination have been relatively rare. Most licensed human vaccines are based on the induction of neutralizing antibodies. However, M2e immunity confers protection through non-neutralizing immune mechanisms. In this article, we have reviewed the possible immune mechanisms of M2e-mediated protection.
M2e ANTIBODIES AND ANTIGENIC BINDING PROPERTIES
The M2 protein is not only expressed at a low level on the virions but also shielded by the larger and more abundant surface HA and NA glycoproteins (28). Immunization with whole-inactivated or split influenza vaccine in mice was not effective in inducing M2e-specific antibodies (10,22,28,29,30), probably because shielding effects by HA and NA proteins might prevent the access of M2e to immune effector cells. In contrast, the M2 protein is expressed at high levels on the plasma membrane surfaces of virus-infected cells (31,32). Mouse anti-M2e monoclonal antibody 14C2 has been reported to reduce plaque sizes of some influenza A virus strains in vitro but not other strains (A/PR/8/34, A/WSN/34) (32). Moreover, passive immunotherapy with 14C2 monoclonal antibody reduced human influenza virus replication in the lung of mice (33).
M2e vaccines either in carrier vehicles or in adjuvant formulations were shown to induce M2e-specific antibodies conferring survival advantages but not being able to prevent weight loss upon lethal infection. M2e vaccine-induced M2e antibodies are highly effective in binding to M2e peptide antigens but show low or no reactivity to M2 protein antigens on influenza virions (9,34,35). M2e vaccine immune sera were shown to be reactive to M2 proteins expressed on the surfaces of infected cells (34,35). In general, M2 immunity provides weak protective efficacy, which might be due to the fact that anti-M2e antibodies cannot neutralize the virus in vitro (33,35,36).
IMPROVED CROSS PROTECTION BY HETEROLOGOUS M2e DOMAINS PRESENTED ON VIRUS-LIKE PARTICLES
Although M2e sequences are highly conserved in human influenza A viruses, there are minor variations in the M2e sequences derived from avian and swine influenza A viruses (5). In a strategy to overcome these M2e sequence variations, a heterologous tandem repeat of M2e epitope sequences (M2e5x) of human, swine, and avian origin influenza A viruses was expressed in a membrane-anchored form and incorporated into virus-like particles (M2e5x VLP) (9,10). The M2e epitope density of M2e5x construct on VLPs as probed by M2e specific monoclonal antibody was detected at hundreds fold higher than those in influenza virions and wild type M2 on VLPs (9,10). Recombinant M2e5x VLP vaccine has several unique features using genetic engineering techniques, which are different from other M2e vaccines. (i) The M2e5x protein contains heterologous M2e sequences with a linear tandem array of conserved M2e sequences derived from human, swine, and avian host origin influenza A viruses for broader coverages. (ii) The oligomerization domain of general control nondepressible 4 (GCN4) to stabilize oligomer formation was genetically fused to the C-terminal part of M2e5x. (iii) The signal peptide from the honeybee protein melittin was added to the N-terminus of M2e5x, which is known for efficient expression on insect cell surfaces (37). (iv) Finally, the transmembrane and cytoplasmic tail domains were replaced with those derived from HA to take advantage of its high levels on influenza virus, expecting for efficient incorporation into VLPs.
Immunization of BALB/c mice with M2e5x VLP experimental vaccines effectively induced M2e antibodies that were highly reactive to M2e antigens of human, swine, and avian influenza viruses. Serum antibodies induced by M2e5x VLP immunization were found to be highly reactive with different influenza viruses including H1N1, H3N2 and H5N1 subtypes (9,10). Compared to mono M2e or homologous tandem M2e vaccines, M2e5x VLP vaccine was demonstrated to be highly efficacious in conferring cross protection against H1N1, H3N2, and H5N1 subtype viruses by 100% protection with preventing severe weight loss in the absence of exogenous adjuvants (9,10). In addition, immune sera were found to be sufficient for conferring cross protection against H1N1, H3N2, and H5N1 influenza virues in naïve mice (9,10). It is significant to provide strong evidence that M2e-specific antibodies can have cross protective roles although these M2e antibodies lack in vitro virus neutralizing activity. However, it is difficult to compare the efficacy of different M2e-based vaccines that were reported by other laboratories under different conditions.
MECHANISMS OF M2e-BASED VACCINES IN CONFERRING CROSS PROTECTION AGAINST INFLUENZA
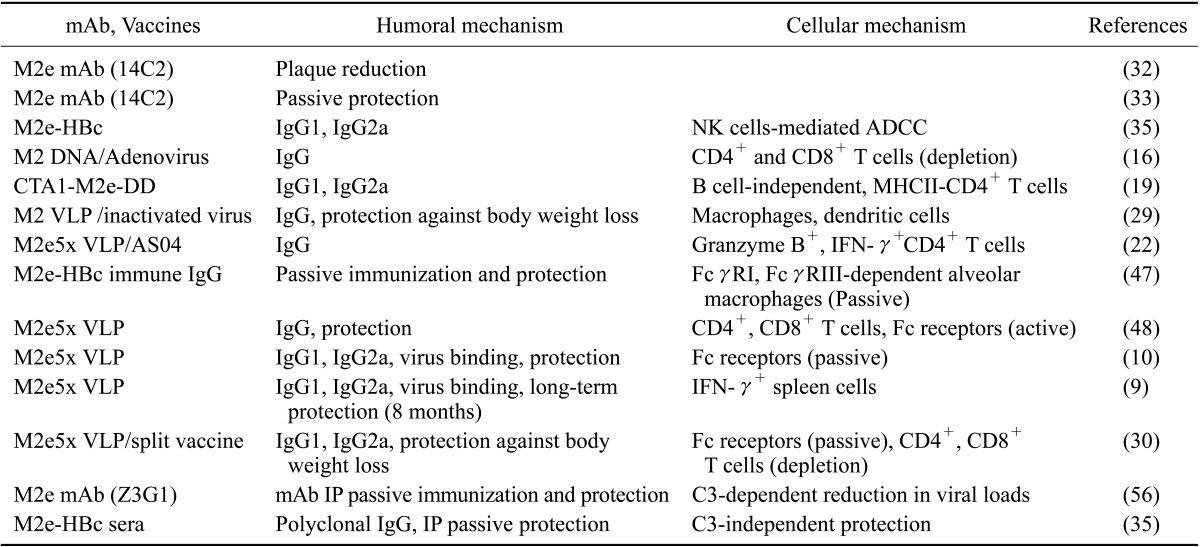
Multiple mechanisms might be involved in conferring protection by M2e-specific antibodies. It was suggested that M2e-specific antibodies could perturb crucial interactions between the M1 and M2 proteins and subsequently interfere with the interaction of the M1 protein with the HA protein, the NA protein, and the nucleocapsid complexes, thus interfering with virus assembly and causing growth restriction (7). Moreover, anti-M2e antibodies bind strongly to the virus-infected cells, proposing indirect mechanisms of protection. Non-neutralizing antibody-mediated protective mechanisms are antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytolysis (CDC), preventing the release of viral particles into the extracellular fluids, and/or enhancing the uptake by phagocytic cells. Potential immune mechanisms by which M2e-based vaccines or M2e antibodies confer protection are summarized in the Table 1.
Table I. Humoral and cellular immune mechanisms for M2e immunity.
M2e mAb, M2e specific monoclonal antibody; M2e-HBc, M2e-hepatitis B virus core fusion protein; M2 VLP, virus-like particles (VLP) presenting the wild type M2 protein; M2e5x VLP, VLP presenting a heterologous tandem repeat of M2e epitope sequences (M2e5x) of human, swine, and avian origin influenza A viruses; NK cells, natural killer cells; ADCC, antibody-dependent cellular cytotoxicity; CTA1-M2e-DD, M2e fusion protein vaccine to cholera toxin subunit A1 and a dimer of the D-fragment of Staphylococcus aureus protein A; AS04, adjuvant of alum plus monophosphoryl lipid A (MPL), Protection against body weight loss indicates significantly improved cross protection by preventing severe weight loss.
Roles of Fc receptors in conferring cross protection by M2e antibodies
Neutralization of virions is believed to be the primary function of antibodies in anti-viral immunity (38,39,40,41,42,43). It is also known that Fc receptor (FcR)-mediated phagocytosis by macrophages plays a pivotal role in clearance of influenza A virus-infected cells (44,45). Previous studies have shown that anti-M2e antibodies passively transferred could mediate protection against influenza infection in vivo (13,46). Given the fact that anti-M2e antibodies are nonneutralizing antibodies, FcR-mediated opsonophagocytosis by macrophages could be essential for M2e-specific antibody-mediated immune protection. El Bakkouri et al. demonstrated that alveolar macrophages and FcR-dependent elimination of influenza A virus-infected cells are crucial for protection by anti-M2e IgG using passive immunization experiments in wild-type and FcR common γ chain deficient (FcR γ-/-) mice models as well as a conditional cell depletion and adoptive transfer protocol (47). Moreover, Song et al. also reported that cross protection against influenza A virus by M2e-immune sera is mediated by dendritic cells and macrophages as shown by depletion experiments using clodronate-liposomes (20).
To better understand M2e immune-mediated protection, antibody levels and protective efficacy were compared between FcR γ-/- and wild type BALB/c mice that were immunized with M2e5x VLP (48). M2e antibodies in FcR γ-/- mice were induced at similar levels and showed equal protective capacity as observed in wild type BALB/c mice. However, M2e5x VLP-immunized FcR γ-/- mice were much less protected than M2e5x VLP-immunized wild type mice after lethal challenge. In contrast, FcR γ-/- mice that were immunized with inactivated influenza virus effectively induced hemagglutination inhibition (virus neutralizing) activity and were well protected. These studies indicate that HA-based vaccines inducing virus neutralizing antibodies confer strain-specific protection in an Fc receptor independent mechanism whereas M2e antibodymediated cross protection requires Fc receptors (48). In line with this mechanism, immune sera with M2e antibodies showed much less protective efficacies in FcR γ-/- mice compared to those in wild type mice using a modified passive immunization approach (10,30). Taken together, a possible explanation is that M2e-specific antibodies are contributing to protection via FcR expressing host immune cell-dependent mechanisms because these antibodies cannot directly neutralize influenza A virus. Alternatively, anti-M2e antibodies may promote the adaptive response by enhancing uptake of antibody-bound virions via dendritic cells and macrophages which are not by themselves sufficient for protection. It is also possible that immune-complex activated dendritic cells and macrophages further contribute to increasing antiviral IFN-γ-secreting T cell responses.
Roles of natural killer cells in conferring cross protection by M2 immune responses
The FcR γ subunit is known to be required for antibody-mediated phagocytosis by macrophages and for natural killer (NK) cell-dependent ADCC (35). In mice, contradictory findings have been reported with regards to the involvement of ADCC by NK and/or NK T cells in M2e-mediated protection (35,49). Jegerlehner et al. proposed an important role for ADCC by NK cells in acquiring anti-M2e immunity (35). Meanwhile, other studies (49,50) argued for a different immune mechanism by M2e-mediated protection rather than NK cell-mediated ADCC. Considering several differences in experimental protocols, it is difficult to reconcile contradictory findings on the role of NK cells in the M2e-mediated protection in mice. Recently, it has been reported that human NK cell-mediated ADCC against M2-expressing cells as well as pro-inflammatory cytokine and chemokine secretion from NK cells were enhanced in the presence of human anti-M2e monoclonal antibody Ab1-10 (51). Thus, it is likely that NK cells may contribute to M2e immune mediated protection via multiple mechanisms.
Roles of complement protein C3 in conferring cross protection by M2e vaccines
Complement can also play an essential role in virus elimination (52,53,54). Beebe et al. reported that complement can bind to influenza virus in the presence of virus-specific antibodies (55). Moreover, the classical complement pathway can be activated by antigen-antibody immune complexes, ultimately leading to CDC. However, the role of complement in M2e-mediated immune response is also controversial. Jegerlehner et al. demonstrated that the complement component C3 is not critical for anti-M2e antibody-mediated protection by intraperitoneal passive transfer of M2e-immune sera (35). In contrast, Wang et al. suggested that complement is required for reducing lung viral loads in infected mice using passive immunization of human anti-M2e monoclonal antibodies in C3-/- mice model (56). This discrepancy might be due to the differences in experimental protocols. The IgG2a isotype antibody is known to be involved in binding to FcRs more effectively than IgG1 isotype (44,57,58,59). Mice were immunized by M2-hepatitis B core (M2-HBc) particles used in Jegerlehner's experiments showed that endpoint titers of M2e-specific IgG1 isotype antibody were approximately 10-fold higher than that of M2e-specific IgG2a isotype antibody (23). It is possible that a profile of specific IgG isotypes might play a role in differential effects on the dependency of FcRs or complement. Further studies are needed to better understand the possible roles of complement C3 in conferring protection by using an appropriate vaccine that can elicit a T helper type 1-biased antibody immune response.
Roles of M2e-specific T cell responses in conferring M2e immunity by vaccination
Optimum protection against influenza A virus requires antibody production by B cells as well as cytotoxic cellular and soluble cytokine mechanisms mediated by T cells (60). Activated influenza-specific T cells have been shown to be associated with protection against influenza in human studies (61,62). Several studies have shown that M2e-specific T cells can mediate protection against influenza infection in vivo (16,19,30,48). Eliasson et al. have demonstrated that an M2e-specific CD4+ T cell but not CD8+ T cell response was induced in BALB/c mice that were intranasally immunized with protein fusion vaccine (CTA1-M2e-DD of cholera toxin subunit A1 and a dimer of the D-fragment of Staphylococcus aureus protein A) in the presence of immune stimulatory complex ISCOMS adjuvant (19). Anti-influenza CD8+ cytotoxic T lymphocyte activity is also known to contribute to recovery from influenza infection in mice (63). Other studies have demonstrated the presence of MHC class I or II restricted epitopes in M2e in mice or human (64,65,66). Although it is not clear why there is discrepancy in T cell responses among different studies, differences in immunization protocols such as the platforms of vaccines and adjuvants, strains of animals, routes of immunization and with or without adjuvants may affect the differential outcomes of immune responses.
Specific T cell depletion approaches in vaccinated mice are often used to determine the roles of T cells in conferring protection. In a previous study of M2-DNA and adenoviral vector vaccines (16), BALB/c mice that were immunized with M2-DNA prime and M2-recombinant adenovirus boost were treated with CD4, CD8, or CD4 plus CD8 antibodies prior to viral challenge. M2-DNA/adenovirus immune mice with depletion of both CD4+ and CD8+ T cell but not either single CD4+ or CD8+ T cell showed significant lower efficacy of survival protection indicating the roles of CD4+ and CD8+ T cells in conferring M2 immunity (16). Using a similar approach, Kim et al. have reported differential effects on the roles of CD4+ and CD8+ T cells in mice that were immunized with split vaccine plus M2e5x VLP (30). Split-M2e5x VLP immune mice with depletion of CD4+ T cells showed significant more weight loss than the CD8+ T cell depleted mice, indicating that CD4+ T cells appeared to play a greater role than CD8+ T cells in conferring cross protection (30). Of course, severe weight loss was observed in Split-M2e5x VLP immune mice with depletion of both CD4+ T and CD8+ T cells, providing further evidence that both CD4+ T and CD8+ T cells play an important role conferring cross protection via M2e-based vaccines. In line with these above studies, M2e5x VLP immune FcR γ-/- mice induced higher frequency of M2e-specific IFN-γ-producing CD4+ and CD8+ T cells as well as lower lung viral titers compared to those of naïve FcR γ-/- mice post challenge (48). Therefore, it is likely that M2e-specific CD4+ and CD8+ T cell immunity plays an important role during recovery from illness caused by influenza virus infection if immunogenic M2e vaccines are used to induce T cell immunity.
SUPPLEMENTED VACCINATIONS WITH A REALISTIC GOAL OF CONFERRING BROADER CROSS PROTECTION
Developing a truly cross protective single shot universal vaccine might not be a realistic goal due to the extremely high genetic and antigenic differences among influenza viruses as well as the relatively low immunogenicity and efficacy of conserved antigenic targets. To overcome the low immunogenicity and efficacy of M2e, most animal studies were carried out using M2e-conjugate vaccines in a range of high doses (40 to 100 µg per mouse) and multiple immunizations in the presence of potent adjuvants inappropriate for human use.
A more realistic approach for inducing broader cross protection would be to supplement current platforms of strain-sepcific HA-based influenza vaccines with a highly conserved antigenic target vaccines such as M2e. Song et al. (2011) demonstrated that an inactivated influenza vaccine supplemented with M2 VLPs can significantly improve cross protective efficacy by preventing weight loss and conferring protection against H1N1, H3N2, and H5N1 influenza viruses (29). In a recent study to further support the concept of supplemented vaccination, co-immunization with licensed split vaccine plus M2e5x VLP significantly improved the efficacy of cross protection compared to that of split vaccine alone (30). Also, immune sera from supplemented vaccination were shown to confer improved cross protection in naïve mice (30). In real world, most humans have a certain level of pre-existing immunity to influenza either by vaccination or natural infection. To mimic this heterogeneity with pre-existing immunity, mice that were previously immunized with split vaccine were subsequently immunized with M2e5x VLPs and evaluated to assess the cross protective efficacy against heterosubtypic viruses (22). Subsequent supplemental immunization with M2e5x VLPs significantly enhanced the cross protective efficacy of pre-existing HA immunity (22). To further move forward this concept of supplementation with a conserved M2e epitope vaccine to clinical trials, the efficacy of M2e5x VLP supplementation should be tested in a more relevant ferret animal model.
CONCLUSION
Influenza viruses are continuously evolving, introducing various mutations to the surface glycoproteins HA and NA, or transferring new HA and/or NA genes from other species stains to human influenza viruses. Current vaccination is based on immunity to HA, mainly aiming to induce vaccine strain-specific neutralizing antibodies. When the prediction of a circulating strain in the next season is well matched with a chosen vaccine strain, the efficacy is sufficiently high. However, current strategy has a major drawback of being unable to protect against a new strain with an antigenically different HA.
The concept behind developing universal vaccines is to utilize the highly conserved antigenic target such as M2e and to make it immunogenic sufficient enough for inducing protective immunity. However, the protective immune mechanisms of the immune responses induced by M2e vaccination remain to be fully elucidated and further studies are needed for a better understanding. It will provide highly informative insight into developing novel new vaccines if we better understand the immunological mechanisms of how universal vaccines work and what immune components involve in the protection against different strains of influenza A virus. Based on mechanistic understanding, it is expected that effective and safe M2e-specific influenza vaccines will be developed for prevention of seasonal and pandemic influenza in near future.
A caveat is that most human vaccines were licensed by proven criteria of inducing neutralizing antibodies. As reviewed above, protective immune mechanisms by which M2e immunity works rely on the induction of cross protective non-neutralizing antibodies and T cell responses. Due to the nature of M2 immune-mediated protection, developing standalone broadly cross protective universal influenza vaccines to completely replace the current annually updating influenza vaccination program would be an ideal goal. Instead, an achievable approach will be to develop simple vaccination of supplementing strain-specific current influenza vaccines with a highly conserved M2e antigenic target in an effective platform such as VLPs.
ACKNOWLEDGEMENTS
This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI093772 (S.M.K.), and AI119366 (S.M.K).
Abbreviations
- HA
hemagglutinin
- M2e
extracellular domain of M2
- NP
nucleocapsid
- M
matrix
- NA
neuraminidase
- M2e5x
a heterologous tandem repeat of M2e epitope sequences
- VLP
virus-like particle
- GCN4
general control; nondepressible 4
- MPL
monophosphoryl lipid A
- ADCC
antibody-dependent cellular cytotoxicity
- CDC
complement-dependent cytolysis
- FcR γ-/-
FcR common γ chain deficient
- M2-HBc
M2-hepatitis; B core
Footnotes
CONFLICTS OF INTEREST: The authors have no financial conflict of interest.
References
- 1.Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication.: Fields virology. 5th ed. Philadelphia: Williams and Wilkins; 2007. [Google Scholar]
- 2.Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9:e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chizhmakov IV, Geraghty FM, Ogden DC, Hayhurst A, Antoniou M, Hay AJ. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J Physiol. 1996;494(Pt 2):329–336. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 5.Long JX, Wang QZ, Lu JH, Liu YL, Liu XF. [Cloning of full-length genes of H5N1 subtype Avian influenza virus strain A/duck/Shandong/093/2004 and analysis of the sequences] Wei Sheng Wu Xue Bao. 2005;45:690–696. [PubMed] [Google Scholar]
- 6.Ito T, Gorman OT, Kawaoka Y, Bean WJ, Webster RG. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J Virol. 1991;65:5491–5498. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zebedee SL, Lamb RA. Nucleotide sequences of influenza A virus RNA segment 7: a comparison of five isolates. Nucleic Acids Res. 1989;17:2870. doi: 10.1093/nar/17.7.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiers W, De FM, Birkett A, Neirynck S, Min JW. A "universal" human influenza A vaccine. Virus Res. 2004;103:173–176. doi: 10.1016/j.virusres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 9.Kim MC, Song JM, E O, Kwon YM, Lee YJ, Compans RW, Kang SM. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2013;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MC, Lee JS, Kwon YM, E O, Lee YJ, Choi JG, Wang BZ, Compans RW, Kang SM. Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antiviral Res. 2013;99:328–335. doi: 10.1016/j.antiviral.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De FM, Ramne A, Birkett A, Lycke N, Lowenadler B, Min JW, Saelens X, Fiers W. The universal influenza vaccine M2e-HBc administered intranasally in combination with the adjuvant CTA1-DD provides complete protection. Vaccine. 2006;24:544–551. doi: 10.1016/j.vaccine.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 12.Fan J, Liang X, Horton MS, Perry HC, Citron MP, Heidecker GJ, Fu TM, Joyce J, Przysiecki CT, Keller PM, Garsky VM, Ionescu R, Rippeon Y, Shi L, Chastain MA, Condra JH, Davies ME, Liao J, Emini EA, Shiver JW. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004;22:2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 14.Ionescu RM, Przysiecki CT, Liang X, Garsky VM, Fan J, Wang B, Troutman R, Rippeon Y, Flanagan E, Shiver J, Shi L. Pharmaceutical and immunological evaluation of human papillomavirus viruslike particle as an antigen carrier. J Pharm Sci. 2006;95:70–79. doi: 10.1002/jps.20493. [DOI] [PubMed] [Google Scholar]
- 15.Bessa J, Schmitz N, Hinton HJ, Schwarz K, Jegerlehner A, Bachmann MF. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. Eur J Immunol. 2008;38:114–126. doi: 10.1002/eji.200636959. [DOI] [PubMed] [Google Scholar]
- 16.Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T, Ye Z, Hogan RJ, Wu Z, Benton KA, Tumpey TM. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis. 2007;13:426–435. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu TM, Grimm KM, Citron MP, Freed DC, Fan J, Keller PM, Shiver JW, Liang X, Joyce JG. Comparative immunogenicity evaluations of influenza A virus M2 peptide as recombinant virus like particle or conjugate vaccines in mice and monkeys. Vaccine. 2009;27:1440–1447. doi: 10.1016/j.vaccine.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Ernst WA, Kim HJ, Tumpey TM, Jansen AD, Tai W, Cramer DV, dler-Moore JP, Fujii G. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24:5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Eliasson DG, El BK, Schon K, Ramne A, Festjens E, Lowenadler B, Fiers W, Saelens X, Lycke N. CTA1-M2e-DD: a novel mucosal adjuvant targeted influenza vaccine. Vaccine. 2008;26:1243–1252. doi: 10.1016/j.vaccine.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 20.Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, Tang J, McDonald W, Song L, Evans RK, Umlauf S, Tussey L, Powell TJ. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008;26:201–214. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 21.Wu F, Yuan XY, Li J, Chen YH. The co-administration of CpG-ODN influenced protective activity of influenza M2e vaccine. Vaccine. 2009;27:4320–4324. doi: 10.1016/j.vaccine.2009.04.075. [DOI] [PubMed] [Google Scholar]
- 22.Kim MC, Lee YN, Hwang HS, Lee YT, Ko EJ, Jung YJ, Cho MK, Kim YJ, Lee JS, Ha SH, Kang SM. Influenza M2 virus-like particles confer a broader range of cross protection to the strain-specific pre-existing immunity. Vaccine. 2014;32:5824–5831. doi: 10.1016/j.vaccine.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De FM, Martens W, Roose K, Deroo T, Vervalle F, Bentahir M, Vandekerckhove J, Fiers W, Saelens X. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;283:11382–11387. doi: 10.1074/jbc.M800650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Peng Z, Liu Z, Lu Y, Ding J, Chen YH. High epitope density in a single recombinant protein molecule of the extracellular domain of influenza A virus M2 protein significantly enhances protective immunity. Vaccine. 2004;23:366–371. doi: 10.1016/j.vaccine.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Heinen PP, Rijsewijk FA, de Boer-Luijtze EA, Bianchi AT. Vaccination of pigs with a DNA construct expressing an influenza virus M2-nucleoprotein fusion protein exacerbates disease after challenge with influenza A virus. J Gen Virol. 2002;83:1851–1859. doi: 10.1099/0022-1317-83-8-1851. [DOI] [PubMed] [Google Scholar]
- 26.Andersson AM, Hakansson KO, Jensen BA, Christensen D, Andersen P, Thomsen AR, Christensen JP. Increased immunogenicity and protective efficacy of influenza M2e fused to a tetramerizing protein. PLoS One. 2012;7:e46395. doi: 10.1371/journal.pone.0046395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hessel A, Savidis-Dacho H, Coulibaly S, Portsmouth D, Kreil TR, Crowe BA, Schwendinger MG, Pilz A, Barrett PN, Falkner FG, Schafer B. MVA vectors expressing conserved influenza proteins protect mice against lethal challenge with H5N1, H9N2 and H7N1 viruses. PLoS One. 2014;9:e88340. doi: 10.1371/journal.pone.0088340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zebedee SL, Richardson CD, Lamb RA. Characterization of the influenza virus M2 integral membrane protein and expression at the infected-cell surface from cloned cDNA. J Virol. 1985;56:502–511. doi: 10.1128/jvi.56.2.502-511.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song JM, Van RN, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A. 2011;108:757–761. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MC, Lee YN, Ko EJ, Lee JS, Kwon YM, Hwang HS, Song JM, Song BM, Lee YJ, Choi JG, Kang HM, Quan FS, Compans RW, Kang SM. Supplementation of influenza split vaccines with conserved M2 ectodomains overcomes strain specificity and provides long-term cross protection. Mol Ther. 2014;22:1364–1374. doi: 10.1038/mt.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris A, Cardone G, Winkler DC, Heymann JB, Brecher M, White JM, Steven AC. Influenza virus pleiomorphy characterized by cryoelectron tomography. Proc Natl Acad Sci U S A. 2006;103:19123–19127. doi: 10.1073/pnas.0607614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988;62:2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treanor JJ, Tierney EL, Zebedee SL, Lamb RA, Murphy BR. Passively transferred monoclonal antibody to the M2 protein inhibits influenza A virus replication in mice. J Virol. 1990;64:1375–1377. doi: 10.1128/jvi.64.3.1375-1377.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JM, Wang BZ, Park KM, Van RN, Quan FS, Kim MC, Jin HT, Pekosz A, Compans RW, Kang SM. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004;172:5598–5605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- 36.Mozdzanowska K, Maiese K, Furchner M, Gerhard W. Treatment of influenza virus-infected SCID mice with nonneutralizing antibodies specific for the transmembrane proteins matrix 2 and neuraminidase reduces the pulmonary virus titer but fails to clear the infection. Virology. 1999;254:138–146. doi: 10.1006/viro.1998.9534. [DOI] [PubMed] [Google Scholar]
- 37.Wang BZ, Liu W, Kang SM, Alam M, Huang C, Ye L, Sun Y, Li Y, Kothe DL, Pushko P, Dokland T, Haynes BF, Smith G, Hahn BH, Compans RW. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. J Virol. 2007;81:10869–10878. doi: 10.1128/JVI.00542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ada GL, Jones PD. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- 39.Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 40.Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PM, Wraith DC, Askonas BA. Control of immune interferon release by cytotoxic T-cell clones specific for influenza. Immunology. 1985;54:607–614. [PMC free article] [PubMed] [Google Scholar]
- 42.Virelizier JL, Allison AC, Schild GC. Immune responses to influenza virus in the mouse, and their role in control of the infection. Br Med Bull. 1979;35:65–68. doi: 10.1093/oxfordjournals.bmb.a071544. [DOI] [PubMed] [Google Scholar]
- 43.Bachmann MF, Lutz MB, Layton GT, Harris SJ, Fehr T, Rescigno M, Ricciardi-Castagnoli P. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996;26:2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 44.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 45.Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 46.Zharikova D, Mozdzanowska K, Feng J, Zhang M, Gerhard W. Influenza type A virus escape mutants emerge in vivo in the presence of antibodies to the ectodomain of matrix protein 2. J Virol. 2005;79:6644–6654. doi: 10.1128/JVI.79.11.6644-6654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El BK, Descamps F, De FM, Smet A, Festjens E, Birkett A, Van RN, Verbeek S, Fiers W, Saelens X. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol. 2011;186:1022–1031. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- 48.Lee YN, Lee YT, Kim MC, Hwang HS, Lee JS, Kim KH, Kang SM. Fc receptor is not required for inducing antibodies but plays a critical role in conferring protection after influenza M2 vaccination. Immunology. 2014;143:300–309. doi: 10.1111/imm.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu TM, Freed DC, Horton MS, Fan J, Citron MP, Joyce JG, Garsky VM, Casimiro DR, Zhao Q, Shiver JW, Liang X. Characterizations of four monoclonal antibodies against M2 protein ectodomain of influenza A virus. Virology. 2009;385:218–226. doi: 10.1016/j.virol.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 50.Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat Rev Immunol. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simhadri VR, Dimitrova M, Mariano JL, Zenarruzabeitia O, Zhong W, Ozawa T, Muraguchi A, Kishi H, Eichelberger MC, Borrego F. A Human Anti-M2 Antibody Mediates Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) and Cytokine Secretion by Resting and Cytokine-Preactivated Natural Killer (NK) Cells. PLoS One. 2015;10:e0124677. doi: 10.1371/journal.pone.0124677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anders EM, Hartley CA, Reading PC, Ezekowitz RA. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J Gen Virol. 1994;75(Pt 3):615–622. doi: 10.1099/0022-1317-75-3-615. [DOI] [PubMed] [Google Scholar]
- 53.Hirsch RL, Winkelstein JA, Griffin DE. The role of complement in viral infections. III. Activation of the classical and alternative complement pathways by Sindbis virus. J Immunol. 1980;124:2507–2510. [PubMed] [Google Scholar]
- 54.Cooper NR, Jensen FC, Welsh RM, Jr, Oldstone MB. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976;144:970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beebe DP, Schreiber RD, Cooper NR. Neutralization of influenza virus by normal human sera: mechanisms involving antibody and complement. J Immunol. 1983;130:1317–1322. [PubMed] [Google Scholar]
- 56.Wang R, Song A, Levin J, Dennis D, Zhang NJ, Yoshida H, Koriazova L, Madura L, Shapiro L, Matsumoto A, Yoshida H, Mikayama T, Kubo RT, Sarawar S, Cheroutre H, Kato S. Therapeutic potential of a fully human monoclonal antibody against influenza A virus M2 protein. Antiviral Res. 2008;80:168–177. doi: 10.1016/j.antiviral.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 58.Heusser CH, Anderson CL, Grey HM. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J Exp Med. 1977;145:1316–1327. doi: 10.1084/jem.145.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neuberger MS, Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur J Immunol. 1981;11:1012–1016. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- 60.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 61.Heidema J, Rossen JW, Lukens MV, Ketel MS, Scheltens E, Kranendonk ME, van Maren WW, van Loon AM, Otten HG, Kimpen JL, van Bleek GM. Dynamics of human respiratory virus-specific CD8+ T cell responses in blood and airways during episodes of common cold. J Immunol. 2008;181:5551–5559. doi: 10.4049/jimmunol.181.8.5551. [DOI] [PubMed] [Google Scholar]
- 62.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 63.Bender BS, Croghan T, Zhang L, Small PA., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jameson J, Cruz J, Ennis FA. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J Virol. 1998;72:8682–8689. doi: 10.1128/jvi.72.11.8682-8689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gianfrani C, Oseroff C, Sidney J, Chesnut RW, Sette A. Human memory CTL response specific for influenza A virus is broad and multispecific. Hum Immunol. 2000;61:438–452. doi: 10.1016/s0198-8859(00)00105-1. [DOI] [PubMed] [Google Scholar]
- 66.Mozdzanowska K, Feng J, Eid M, Kragol G, Cudic M, Otvos L, Jr, Gerhard W. Induction of influenza type A virus-specific resistance by immunization of mice with a synthetic multiple antigenic peptide vaccine that contains ectodomains of matrix protein 2. Vaccine. 2003;21:2616–2626. doi: 10.1016/s0264-410x(03)00040-9. [DOI] [PubMed] [Google Scholar]