Abstract
We attempted to investigate molecular mechanisms underlying phenotypic change of vascular smooth muscle cells (VSMCs) by determining signaling molecules involved in chemokine production. Treatment of human aortic smooth muscle cells (HAoSMCs) with thrombin resulted not only in elevated transcription of the (C-C motif) ligand 11 (CCL11) gene but also in enhanced secretion of CCL11 protein. Co-treatment of HAoSMCs with GF109230X, an inhibitor of protein kinase C, or GW5074, an inhibitor of Raf-1 kinase, caused inhibition of ERK1/2 phosphorylation and significantly attenuated expression of CCL11 at transcriptional and protein levels induced by thrombin. Both Akt phosphorylation and CCL11 expression induced by thrombin were attenuated in the presence of pertussis toxin (PTX), an inhibitor of Gi protein-coupled receptor, or LY294002, a PI3K inhibitor. In addition, thrombin-induced production of CCL11 was significantly attenuated by pharmacological inhibition of Akt or MEK which phosphorylates ERK1/2. These results indicate that thrombin is likely to promote expression of CCL11 via PKC/Raf-1/ERK1/2 and PTX-sensitive protease-activated receptors/PI3K/Akt pathways in HAoSMCs. We propose that multiple signaling pathways are involved in change of VSMCs to a secretory phenotype.
Keywords: CCL11, Secretory phenotype, Signaling pathway, Thrombin, Vascular smooth muscle cell
INTRODUCTION
Thrombin, a serine protease, is released during tissue damage and converts fibrinogen to fibrin at the final step of the blood coagulation cascade [1]. The released thrombin molecules not only contribute to repair of damaged blood vessels but also are associated with progression of vascular diseases [2]. Interaction of thrombin molecules with platelets at sites of vascular injury ensures rapid formation of haemostatic plugs [2,3]. Binding of thrombin to its receptors, protease-activated receptors (PARs), leads to molecular and cellular events that occur atherosclerosis. PARs, a subfamily of related seven transmembrane G-protein-coupled receptors (GPCRs), are expressed on surface of multiple vascular cells, including endothelial cells and vascular smooth muscle cells (VSMCs) [4,5], and activation of PARs cause production of mediators, including platelet-derived growth factor and inflammatory chemokines [3], and migration and proliferation of the cells [6,7,8].
Among CC chemokines whose expression is elevated in atherosclerotic lesions and injured arteries, expression of C-C motif chemokines 11 (CCL11) is of interest because this chemokine is believed to be involved remodeling of blood vessels [9,10]. CCL11, which is also known as eosinophil chamotactic protein and eotaxin-1, is a protein of the CC family chemokines that in human is encoded by the CCL11 gene with a high degree of amino acid sequence homology with monocyte chemotactic proteins (MCPs) [11]. Overexpression of CCL11 protein has been detected in smooth muscle cells (SMCs) of human atheroma, with negligible expression in normal vessels, and CCL11 mRNA is up-regulated in SMCs of rat aortic allografts exposed to prolonged ischemic storage [12,13]. However, it is not clear which factors induce expression of CCL11 in VSMCs.
In the normal arteries expression of PARs is restricted mainly to the endothelial layer whereas their expression is significantly elevated in areas rich in SMCs within atherosclerotic lesions, indicating activation of seine protease-mediated pathways in atherosclerosis [14]. As thrombin is released during injury or inflammation, subendothelial SMCs will be come into contact with thrombin after endothelial injury and thrombus formation [15]. Moreover, due to protection from inactivation by circulating inhibitors, thrombin bound to the subendothelial extracellular matrix remains functionally active [16], and SMCs are likely to be exposed to thrombin in atherosclerotic arteries. Therefore, to better understand underlying mechanisms associated with changes in VSMCs in the injured artery, it is necessary to determine molecular pathways via which thrombin exerts its effects on VSMCs because activation of thrombin receptors cause proliferation, migration, and production of chemokines and extracellular matrix synthesis of VSMCs [7,17,18].
In the current study, we attempted to determine signaling molecules below PARs whose activity is necessary for thrombin to induce production of CCL11 using human aortic smooth muscle cells (HAoSMCs) in order to clarify molecular mechanisms involved in change of VSMCs to secretory phenotype in the artery.
METHODS
Cell culture and reagents
HAoSMCs purchased from American Type Culture Collection were grown in ATCC-formulated F-12K medium supplemented with vascular smooth muscle growth kit, 50 units/ml penicillin and 50 µg/ml streptomycin, as previously reported [8]. The cells in between passage 6 and 9 were used. Thrombin, pertussis toxin (PTX), GF109203X, LY294002, N-acetylcysteine (NAC) and diphenyleneiodonium (DPI) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). U0126, Akt Inhibitor IV (AktiIV), and anti-phosphorylated Akt antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). GW5074 and anti-phosphorylated ERK1/2 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Enzyme-linked immunosorbent assay (ELISA) of CCL11
The amount of CCL11 protein released from HAoSMC was determined using commercially available ELISA kit according to the manufacturer's instructions (R&D systems, Minneapolis, MN, USA). HAoSMCs were incubated for 12 h in the presence of 1% fetal bovine serum (FBS) and exposed to thrombin prior to isolation of culture medium. The isolated culture medium and standard dilutions of CCL11 protein were added to each well. After incubation for 2 h, each well was washed and the Conjugate was added. After incubation for 1 h, each well was washed and the Substrate Solution was added. After incubation for 30 min, the Stop Solution was added and color intensity was measured at 450 nm.
Reverse transcription (RT) - polymerase chain reaction (PCR)
Total RNAs were reverse-transcribed to complementary DNA (cDNA) for 1 h at 42℃ with Moloney Murine Leukemia Virus reverse transcriptase using the oligod(T)15 primer, followed by non-quantitative and quantitative real-time PCR. For non-quantitative PCR, transcripts of genes of interest were amplified using Hot Start Taq Polymerase. The cDNA was denatured at 90℃ for 5 min followed by 25 cycles of PCR (95℃ for 30 sec, 55℃ for 30 sec, 72℃ for 30 sec) in the presence of forward and reverse primers of the genes. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as an internal control. Real-time quantitative PCR was performed in triplicate; each 20-µl reaction consisted of 10 µl of SYBR Green Master Mix, 2 µl of forward and reverse primers (10 pM each) of genes to be analyzed, and cDNA template. Thermal cycling conditions were as follow: 95℃ for 10 min, and 45 cycles at 95℃ for 10 sec, 50℃ for 10 sec, and an elongation period for 10 sec at 72℃. The relative expression of each gene was then calculated as a ratio to GAPDH. The primers were CCL11: 5'-aaccacctgctgctttaacc-3' (forward) and 5'-tggctttggagttggagatt-3' (reverse); and GAPDH: 5'-gagtcaacggatttggtcct-3' (forward) and 5'-tgtggtcatgagtccttcca-3' (reverse).
Western blot analysis
Cells were lysed with the lysis buffer (1% SDS, 1 mM NaVO3, 10 mM Tris-HCl, pH 7.4) containing protease inhibitors and centrifuged (15,000 xg) for 5 min at 4℃. Supernatants were isolated, and protein concentration of the lyaste was determined. An equal amount of protein was separated by 12% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking for an hour in 5% skim milk in 0.1% Tween 20/Tris-buffered saline (TBS) (pH 7.6), the membranes were incubated with indicated primary antibodies diluted in the blocking solution at 4℃ overnight. After washing three times with 0.1% Tween 20/TBS for 10 min each, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies diluted in the blocking solution (1 : 5,000) for an hour at room temperature. After washing three times with washing buffer for 10 min each, bands were detected using the Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific Inc., Rockford, IL, USA).
Statistics
Statistical analyses were performed using GraphPad PRISM (version 5.0) (GraphPad Software Inc., San Diego, CA, USA), and p<0.05 was considered statistically significant.
RESULTS
Up-regulated expression of CCL11 in human VSMCs by treatment with thrombin
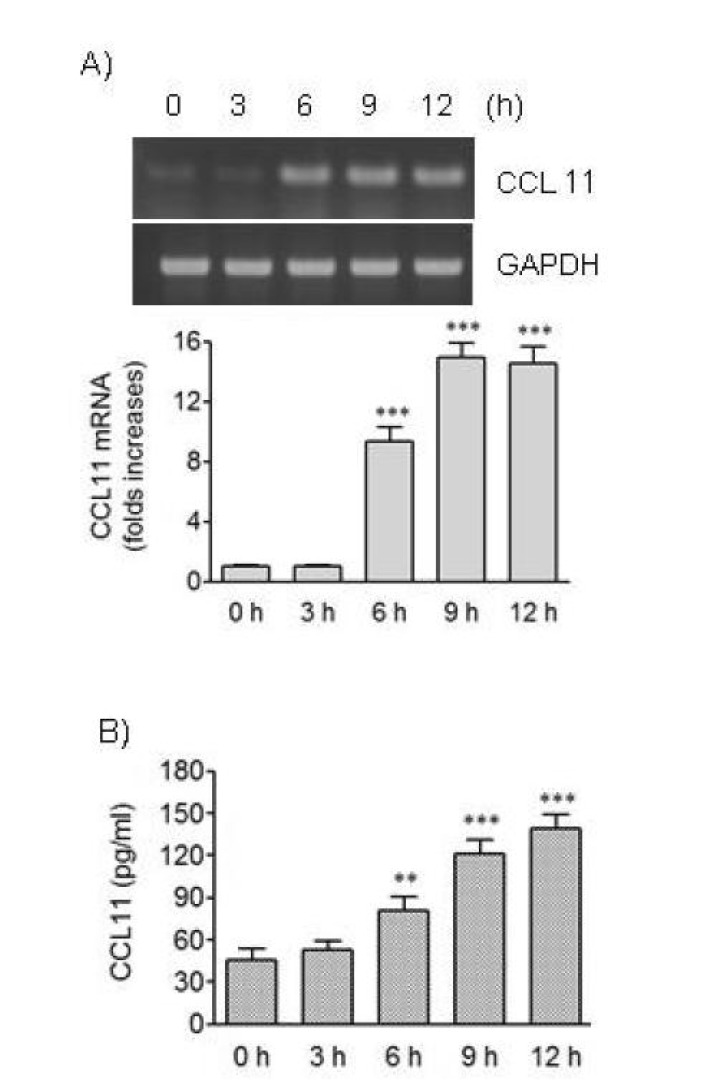
We investigated the possibility of change of VSMCs to secretory phenotype in the presence of thrombin, a PARs ligand, by determining production of CCL11 chemokine. Treatment of HAoSMCs with thrombin resulted in an increased expression of the CCL11 gene. The increase was observed at 6 h post-treatment and persisted up to 12 h post-treatment with thrombin (Fig. 1A). We also examined the effects of thrombin on CCL11 at protein level. Treatment with thrombin resulted in significantly elevated secretion of CCL11 from HAoSMC in proportion to the treatment duration with thrombin up to 12 h (Fig. 1B).
Fig. 1. Effects of thrombin on expression of CCL11 in human VSMCs. (A, B) HAoSMCs were treated for the indicated time periods with thrombin (1 U/ml). (A) CCL11 transcripts were amplified by RT-PCR (upper panel) and the levels of CCL11 transcripts were determined by realtime PCR (lower panel). (B) The levels of CCL11 protein secreted into culture media were determined by ELISA. Data are expressed as mean±SD (n=3 replicates for each group). **p<0.01 vs. control cultured in the absence of thrombin (0 h); ***p<0.001 vs. 0 h.
Active roles of ERK and Akt pathways in CCL11 expression induced by thrombin
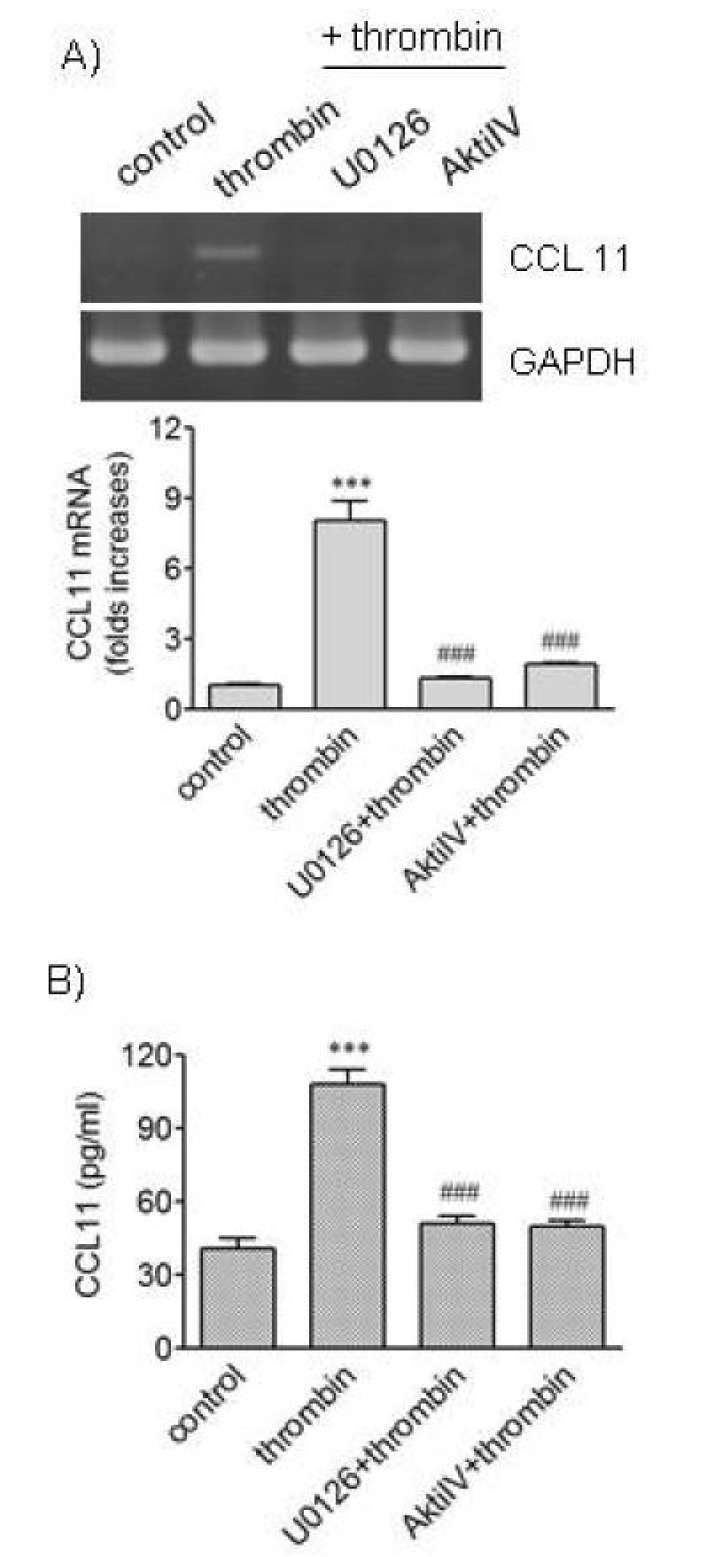
Treatment of VSMCs with thrombin caused elevated phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt [8]. We investigated whether ERK and Akt pathways are involved in CCL11 production using U0126 and AktiIV, respectively (Fig. 2). U0126 inhibits mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) which activates ERK1/2, and AktiIV controls activity of Akt. Thrombin increased transcription of the CCL11 gene, and pharmacological inhibition of ERK kinase and Akt pathways resulted in blockage of the CCL11 gene transcription induced by thrombin. Secretion of CCL11 increased by thrombin was also abrogated when ERK kinase or Akt pathway was inhibited using the inhibitors. These results suggest participation of ERK and Akt pathways in production of CCL11 in response to thrombin.
Fig. 2. Effects of inhibitors of MEK and Akt in thrombin-induced expression of CCL11. (A, B) HAoSMCs were incubated with or without U0126 and Akti IV (10 µM each) for 2 h followed by stimulation with thrombin for 9 h. (A) CCL11 transcripts were amplified by RT-PCR (upper panel) and the levels of CCL11 transcripts were determined by realtime PCR (lower panel). (B) The levels of CCL11 protein secreted into culture media were determined by ELISA. Data are expressed as mean±SD (n=3 replicates for each group). ***p<0.001 vs. control; ###p<0.001 vs. thrombin.
Involvement of PKC-mediated pathway in ERK1/2 phosphorylation and CCL11 expression induced by thrombin
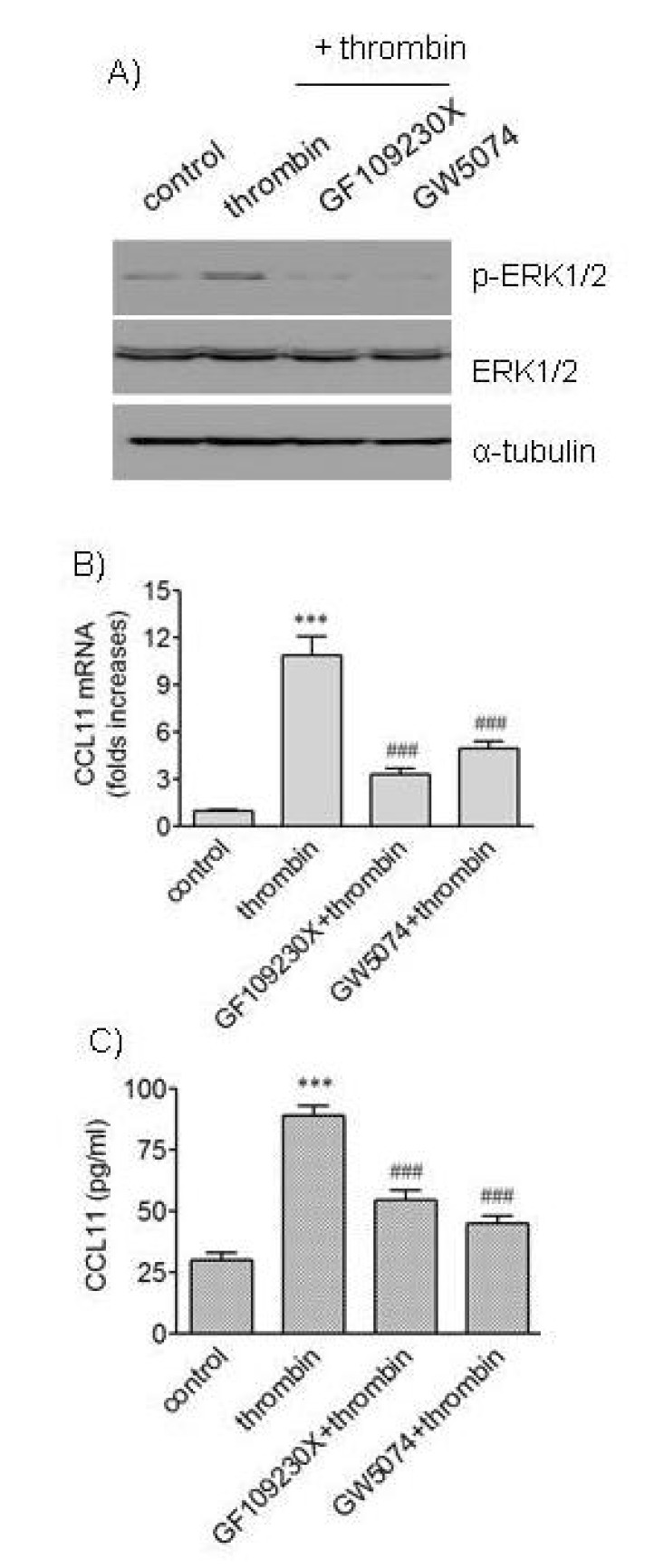
Thrombin increases activity of protein kinase C (PKC), and PKC can activate ERK pathway [8,19]. Therefore, it was investigated whether PKC played roles in ERK1/2 phosphorylation and CCL11 production induced by thrombin (Fig. 3). Consistent with the previous study, treatment of HAoSMCs with thrombin resulted in increased phosphorylation of ERK1/2. An addition of GF109230X, a potent inhibitor of PKC isoforms, resulted in abrogation of the ERK1/2 phosphorylation. Treatment with GF109230X also led to significantly attenuated transcription of the CCL11 gene and secretion of CCL11 protein induced by thrombin. Because Raf-1 kinase participates in ERK cascade [19], it was investigated whether the kinase was involved in the effects of thrombin on VSMCs. Exposure of HAoSMCs to GW5074, an inhibitor of Raf-1 kinase, resulted in attenuated phosphorylation of ERK1/2 and reduced expression of CCL11 at transcriptional and protein levels. These results suggest requirement of PKC and Raf-1 kinase in thrombin-induced phosphorylation of ERK1/2 and expression of CCL11 in VSMCs.
Fig. 3. Effects of inhibitors of PKC and Raf-1 on phosphorylation of ERK and expression of CCL11 induced by thrombin. (A) HAo-SMCs were stimulated with thrombin for 5 min after incubation with or without GF109203X (3 µM) and GW5074 (25 nM) for 2 h. An equal amount of protein was subjected to Western blot analysis with antibodies against α-tubulin and phosphorylated and unphosphorylated forms of ERK1/2. (B, C) HAoSMCs were incubated with or without GF109203X (3 µM) and GW5074 (25 nM) for 2 h followed by stimulation with thrombin for 9 h. The levels of CCL11 transcripts were determined by realtime PCR (B), and the levels of CCL11 protein secreted into culture media were determined by ELISA (C). Data are expressed as mean±SD (n=3 replicates for each group). ***p<0.001 vs. control; ###p<0.001 vs. thrombin.
Involvement of PTX-sensitive PARs in Akt phosphorylation and CCL11 expression induced by thrombin
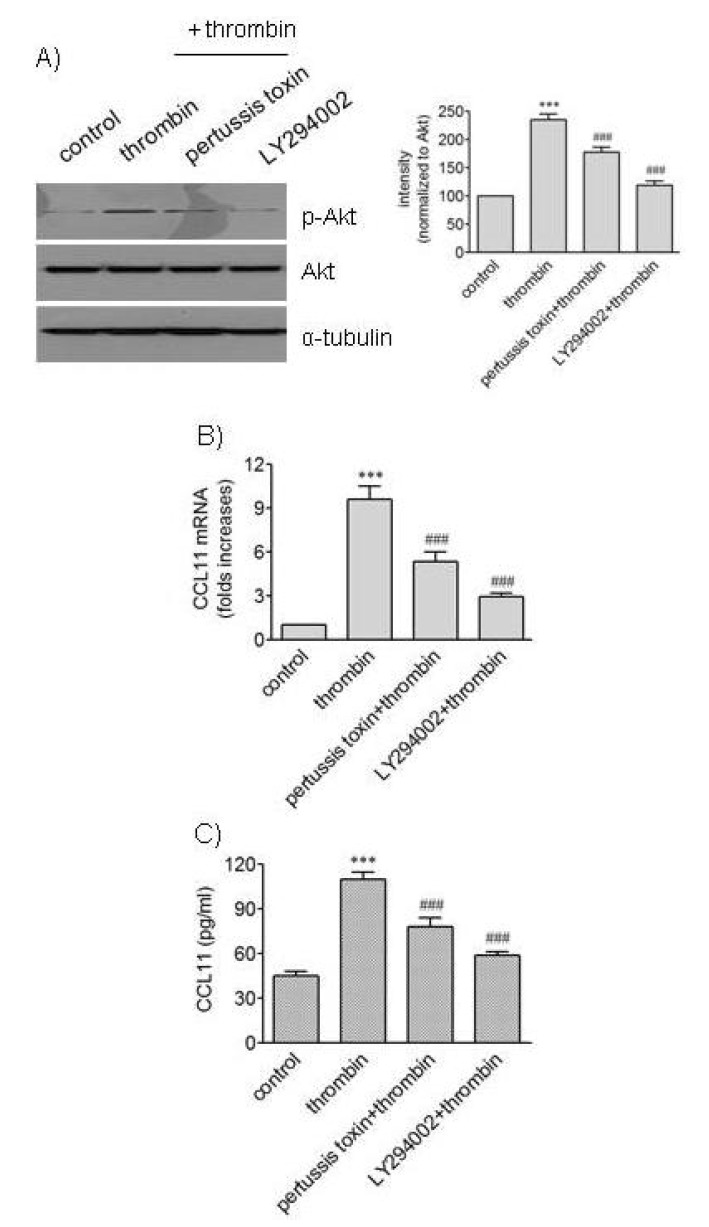
Akt is activated via phosphorylation by phosphoinositide-3-kinase (PI3K) [20]. We investigated the possibility that PI3K inhibition affected action of thrombin in VSMCs (Fig. 4). Exposure of HAoSMCs to LY294002, an inhibitor of PI3K, resulted in significantly reduced phosphorylation of Akt and attenuated expression of CCL11 at transcriptional and protein levels. PTX-sensitive G protein is involved in thrombin signaling on human VSMCs [21]. We investigated whether PTX affected Akt phosphorylation and CCL11 expression. Treatment of HAoSMCs with thrombin resulted in increased phosphorylation of Akt, and Akt phosphorylation was attenuated by exposure to PTX. The presence of PTX also resulted in attenuated significantly attenuated transcription of the CCL11 gene and secretion of CCL11 protein induced by thrombin. These results suggest involvement of PTX-sensitive G-protein-coupled PARs in phosphorylation of Akt and production of CCL11 induced by thrombin although the inhibitory effects of PTX were not as strong as those of LY294002.
Fig. 4. Effects of pertussis toxin and LY294002 on phosphorylation of Akt and expression of CCL11 induced by thrombin. (A) HAo-SMCs were stimulated with thrombin for 5 min after incubation with or without pertussis toxin (100 ng/ml) and LY294002 (10 µM) for 2 h. An equal amount of protein was subjected to Western blot analysis with antibodies against α-tubulin and phosphorylated and unphosphorylated forms of Akt. The mean band intensity of p-Akt was normalized to that of Akt. (B, C) HAoSMCs were incubated with or without pertussis toxin (100 ng/ml) and LY294002 (10 µM) for 2 h followed by stimulation with thrombin for 9 h. The levels of CCL11 transcripts were determined by realtime PCR (B), and the levels of CCL11 protein secreted into culture media were determined by ELISA (C). Data are expressed as mean±SD (n=3 replicates for each group). ***p<0.001 vs. control; ###p<0.001 vs. thrombin.
Participation of ROS in thrombin-induced secretion of CCL11
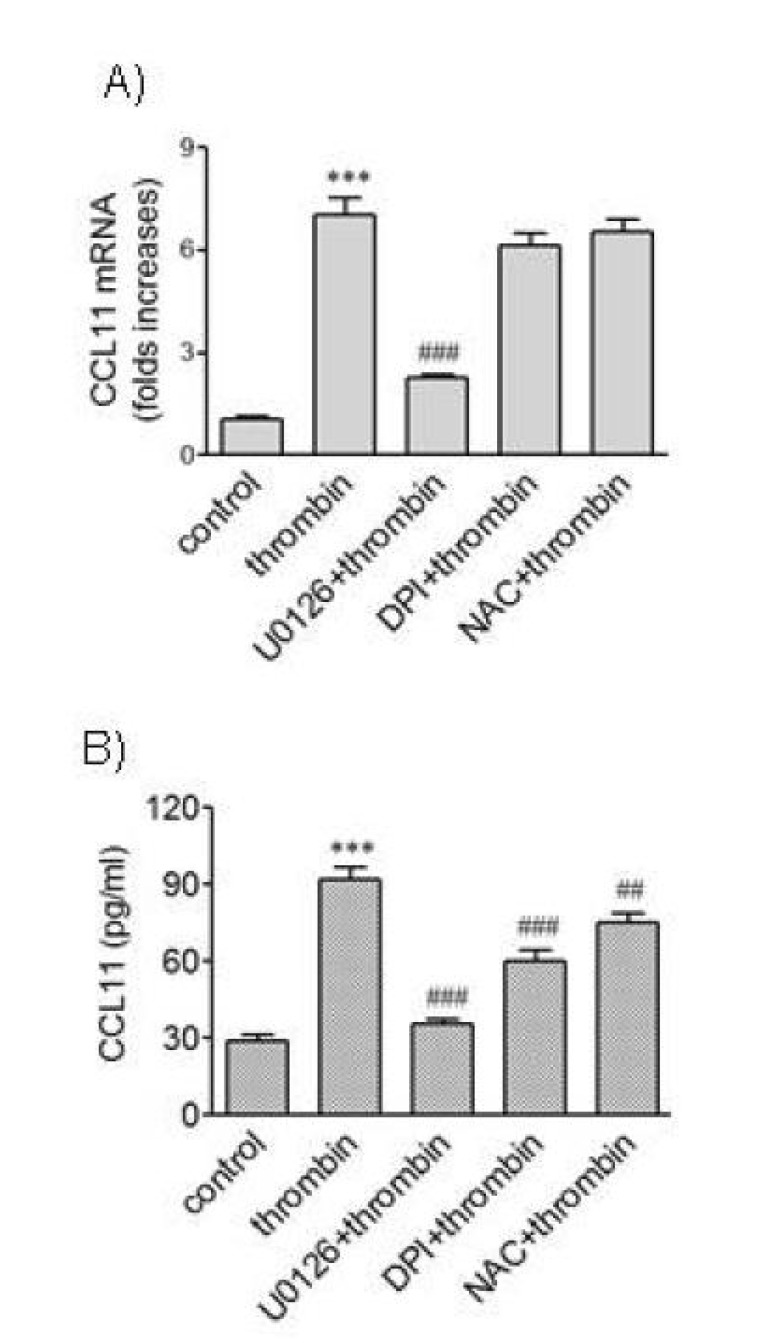
Thrombin stimulates production of reactive oxygen species (ROS) which control thrombin-mediates responses of VSMCs [22]. We investigated the possibility that ROS participated in thrombin-induced expression of CCL11 using the antioxidant NAC and the flavoprotein enzyme NAD(P)H oxidase inhibitor DPI, in parallel with U0126 (Fig. 5). Exposure of HAoSMCs to U0126 resulted in significantly attenuated transcription of the CCL11 gene induced by thrombin whereas DPI and NAC did not affect CCL11 gene transcription. However, exposure to U0126, DPI or NAC resulted in significantly reduced secretion of CCL11 protein from HAoSMCs. The inhibitory effect of DPI or NAC, however, was not as potent as that observed with U0126. These results suggest involvement of ROS-sensitive mechanisms in thrombin-mediated secretion of CCL11 in VSMCs.
Fig. 5. Effects of ROS quenchers on expression of CCL11 induced by thrombin. (A, B) HAoSMCs were incubated with or without DPI (10 µM) and NAC (5 mM) for 2 h followed by stimulation with thrombin for 9 h. The levels of CCL11 transcripts were determined by realtime PCR (A), and the levels of CCL11 protein secreted into culture media were determined by ELISA (B). Data are expressed as mean±SD (n=3 replicates for each group). ***p<0.001 vs. control; #p<0.05 vs. thrombin; ###p<0.001 vs. thrombin.
DISCUSSION
CCL11 exerts its effects not only to leukocytes expressing CCR3 receptor in atherosclerotic plaques but also to non-leukocytic cells. CCL11 induces chemotaxis of microvascular endothelial cells and promotes endothelial sprouting from rat aortic rings [23]. CCL11 also enhance SMC migration and induce blood vessel formation in chick chorioallantoic membranes and in matrigel plugs in association with an eosinophilic infiltrate [23,24]. We demonstrated increased transcription of the CCL11 gene and enhanced secretion of CCL11 protein after exposure of HAoSMC to thrombin. These findings, taken together, indicate that thrombin may be involved in neovascularization in ischemic tissues characterized by endothelial and SMC migration and proliferation via upregulation of CCL11. Therefore, identification of factors that are involved in thrombin-induced CCL11 expression in VSMCs will broaden current understandings of molecular mechanisms underlying vascular remodeling.
Cellular responses to thrombin are determined by thrombin-responsive PARs expressed by cells [2]. SMCs of the saphenous vein expressed PAR-1, -3, and -4 [6,25], and exposure of thrombin causes migration and proliferation of the cells [22,25]. HAoSMCs expresse PAR-1, -2 and -3, and treatment with thrombin leads to increased expression of chemokines, tissue factor, platelet-derived growth factor, and vascular endothelial growth factor and synthesis of proteoglycan by SMCs of the artery [8,17,26,27]. Among the 3 types of PARs expressed by HAoSMCs, PAR-1 and -3 will be associated with CCL11 expression in response to thrombin because PAR-2 is activated by trypsin, but not by thrombin [28]. Of the two PARs, PAR-1 is likely to play key roles in CCL11 production due to its much higher affinity for thrombin, compared with PAR-3 [29]. However, the contribution of each PAR to CCL11 expression needs to be assessed by specific inhibition of individual receptors in further study.
We investigated signaling molecules involved in enhanced phosphorylation of ERK1/2 in response to thrombin in conjunction with CCL11 expression. The ERK pathway, which is also known as the Ras-Raf-MEK-ERK pathway, is a chain of proteins in cells that transmits a signal from a receptor on the surface to the nucleus to induce gene expression [30]. When a ligand binds to the receptor on the cell surface, Ras (a GTPase) swaps its GDP for a GTP. Then, it activates Raf kinase which activates MEK, and MEK catalyzes phosphorylation of ERK1/2, leading to its activation [30,31]. We demonstrated significantly reduced expression of CCL11 when PKC, Raf-1 or MEK was inhibited, indicating involvement of PKC and ERK pathway in thrombin-induced CCL11 expression. We also demonstrated that phosphorylation of ERK enhanced by thrombin was completely inhibited in the presence of an inhibitor of PKC or Raf-1, which indicates involvement of PKC in phosphorylation of ERK. Collectively, these results indicate that intact ERK signaling pathway seems to be necessary for thrombin-induced CCL11 expression and that thrombin mediates CCL11 expression via PKC-ERK pathway in human VSMCs.
Because PARs are G protein-coupled receptors, the signaling triggered after thrombin binding will be transmitted through G-proteins [2]. For instance, PAR-1 can couple to members of the G12/13, Gq, and Gi families and activate a large number of downstream intracellular effectors, including Akt [2,8]. In the current study, we attempted to determine whether G-proteins are involved in phosphorylation of Akt and expression of CCL11 in response to thrombin. We demonstrated significantly decreased expression of CCL11 when Akt and PI3K was inhibited, indicating involvement of PI3K/Akt pathway in thrombin action. A significantly attenuated phosphorylation of Akt and expression of CCL11 enhanced by thrombin also occurred in the presence of PTX, as determined in parallel with LY294002. Since PTX inactivates Gi proteins, these results, taken together, indicate that PI3K/Akt signaling pathway seems to be necessary for thrombin-induced CCL11 expression and that certain types of PARs sensitive to PTX are likely to participate in thrombin-induced expression of CCL11 via Akt in human VSMCs.
We investigated involvement of ROS in thrombin-induced expression of CCL11 using DPI and NAC because treatment of VSMCs with thrombin resulted in generation ROS in VSMCs [22,26]. The ROS quenching agents did not influence transcription of the CCL11 gene whereas secretion of CCL11 was significantly inhibited by treatment with DPI or NAC. These results are in line with the report that ROS are involved in pathophysiological processes of VSMCs, including secretion of inflammatory molecules [32]. We, however, did not determine whether ROS and the ERK pathway or the PI3K/Akt pathway act in a linked or independent manner; therefore, elucidation of the type of correlation that may occur between ROS and the signaling pathways is necessary for a better understanding of molecular mechanisms underlying elevated secretion of CCL11 in response to thrombin.
Previously, increased transcription of CCL11 and activation of PKC, ERK1/2, and Akt have been reported after treatment of HAoSMCs with thrombin [8]. In the current study we demonstrated participation of ERK and PI3K/Akt pathways in CCL11 secretion induced by thrombin. In addition, the current study also demonstrated roles of PKC/raf-1 kinase in ERK phosphorylation and involvement of PTX-sensitive GPCR in Akt phosphorylation to induce CCL11 secretion from HAoSMCs after treatment with thrombin. Because expression of receptors for thrombin is significantly elevated in atherosclerotic lesions, we presume that thrombin, in addition to remodeling after injury of blood vessels, will be involved in change of VSMCs to secretory phenotype in the atherosclerotic arteries via multiple pathways.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A4A01006878).
ABBREVIATIONS
- AktiIV
Akt inhibitor IV
- CCL11
C-C motif ligand 11
- ERK
extracellular signal-regulated kinase
- HAoSMCs
human aortic smooth muscle cells
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein/extracellular signal-regulated kinase kinase
- PI3K
phosphoinositide 3 kinase
- PTX
pertussis toxin
- PKC
protein kinase C
- VSMCs
vascular smooth muscle cells
Footnotes
CONFLICT OF INTERESTS: Authors do not have any conflicts of interest.
References
- 1.Jakubowski HV, Owen WG. Macromolecular specificity determinants on thrombin for fibrinogen and thrombomodulin. J Biol Chem. 1989;264:11117–11121. [PubMed] [Google Scholar]
- 2.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 3.Chambers RC, Laurent GJ. Coagulation cascade proteases and tissue fibrosis. Biochem Soc Trans. 2002;30:194–200. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 5.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 6.Bretschneider E, Kaufmann R, Braun M, Nowak G, Glusa E, Schrör K. Evidence for functionally active protease-activated receptor-4 (PAR-4) in human vascular smooth muscle cells. Br J Pharmacol. 2001;132:1441–1446. doi: 10.1038/sj.bjp.0703947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bretschneider E, Kaufmann R, Braun M, Wittpoth M, Glusa E, Nowak G, Schrör K. Evidence for proteinase-activated receptor-2 (PAR-2)-mediated mitogenesis in coronary artery smooth muscle cells. Br J Pharmacol. 1999;126:1735–1740. doi: 10.1038/sj.bjp.0702509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung SW, Park JW, Lee SA, Eo SK, Kim K. Thrombin promotes proinflammatory phenotype in human vascular smooth muscle cell. Biochem Biophys Res Commun. 2010;396:748–754. doi: 10.1016/j.bbrc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Aukrust P, Halvorsen B, Yndestad A, Ueland T, Øie E, Otterdal K, Gullestad L, Damås JK. Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2008;28:1909–1919. doi: 10.1161/ATVBAHA.107.161240. [DOI] [PubMed] [Google Scholar]
- 10.Schober A, Zernecke A. Chemokines in vascular remodeling. Thromb Haemost. 2007;97:730–737. [PubMed] [Google Scholar]
- 11.Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Akyürek LM, Fellström B, Häyry P, Paul LC. Eotaxin and capping protein in experimental vasculopathy. Am J Pathol. 1998;153:81–90. doi: 10.1016/S0002-9440(10)65548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haley KJ, Lilly CM, Yang JH, Feng Y, Kennedy SP, Turi TG, Thompson JF, Sukhova GH, Libby P, Lee RT. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000;102:2185–2189. doi: 10.1161/01.cir.102.18.2185. [DOI] [PubMed] [Google Scholar]
- 14.Nelken NA, Soifer SJ, O'Keefe J, Vu TK, Charo IF, Coughlin SR. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992;90:1614–1621. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrör K, Bretschneider E, Fischer K, Fischer JW, Pape R, Rauch BH, Rosenkranz AC, Weber AA. Thrombin receptors in vascular smooth muscle cells - function and regulation by vasodilatory prostaglandins. Thromb Haemost. 2010;103:884–890. doi: 10.1160/TH09-09-0627. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Shavit R, Eldor A, Vlodavsky I. Binding of thrombin to subendothelial extracellular matrix. Protection and expression of functional properties. J Clin Invest. 1989;84:1096–1104. doi: 10.1172/JCI114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivey ME, Little PJ. Thrombin regulates vascular smooth muscle cell proteoglycan synthesis via PAR-1 and multiple downstream signalling pathways. Thromb Res. 2008;123:288–297. doi: 10.1016/j.thromres.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Marutsuka K, Hatakeyama K, Sato Y, Yamashita A, Sumiyoshi A, Asada Y. Protease-activated receptor 2 (PAR2) mediates vascular smooth muscle cell migration induced by tissue factor/factor VIIa complex. Thromb Res. 2002;107:271–276. doi: 10.1016/s0049-3848(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 19.Ueda Y, Hirai Si, Osada Si, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 20.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Kanthou C, Kanse SM, Kakkar VV, Benzakour O. Involvement of pertussis toxin-sensitive and -insensitive G proteins in alpha-thrombin signalling on cultured human vascular smooth muscle cells. Cell Signal. 1996;8:59–66. doi: 10.1016/0898-6568(95)02018-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Castresana MR, Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004;36:49–56. doi: 10.1016/j.yjmcc.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, Oppenheim JJ. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166:7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 24.Kodali RB, Kim WJ, Galaria II, Miller C, Schecter AD, Lira SA, Taubman MB. CCL11 (Eotaxin) induces CCR3-dependent smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2004;24:1211–1216. doi: 10.1161/01.ATV.0000131654.90788.f5. [DOI] [PubMed] [Google Scholar]
- 25.Bretschneider E, Spanbroek R, Lötzer K, Habenicht AJ, Schrör K. Evidence for functionally active protease-activated receptor-3 (PAR-3) in human vascular smooth muscle cells. Thromb Haemost. 2003;90:704–709. doi: 10.1160/TH03-04-0203. [DOI] [PubMed] [Google Scholar]
- 26.Bassus S, Herkert O, Kronemann N, Görlach A, Bremerich D, Kirchmaier CM, Busse R, Schini-Kerth VB. Thrombin causes vascular endothelial growth factor expression in vascular smooth muscle cells: role of reactive oxygen species. Arterioscler Thromb Vasc Biol. 2001;21:1550–1555. doi: 10.1161/hq0901.095148. [DOI] [PubMed] [Google Scholar]
- 27.Wu SQ, Aird WC. Thrombin, TNF-alpha, and LPS exert overlapping but nonidentical effects on gene expression in endothelial cells and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289:H873–H885. doi: 10.1152/ajpheart.00993.2004. [DOI] [PubMed] [Google Scholar]
- 28.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg SF. The cardiovascular actions of protease-activated receptors. Mol Pharmacol. 2005;67:2–11. doi: 10.1124/mol.104.003103. [DOI] [PubMed] [Google Scholar]
- 30.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 31.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]