Abstract
Sweating events occur in response to mental stress (psychogenic) or with increased body temperature (thermogenic). We previously found that both were linked to activation of common brain stem regions, suggesting that they share the same output pathways: a putative common premotor nucleus was identified in the rostral-lateral medulla (Farrell MJ, Trevaks D, Taylor NA, McAllen RM. Am J Physiol Regul Integr Comp Physiol 304: R810–R817, 2013). We therefore looked in higher brain regions for the neural basis that differentiates the two types of sweating event. Previous work has identified hemispheric activations linked to psychogenic sweating, but no corresponding data have been reported for thermogenic sweating. Galvanic skin responses were used to measure sweating events in two groups of subjects during either psychogenic sweating (n = 11, 35.3 ± 11.8 yr) or thermogenic sweating (n = 11, 34.4 ± 10.2 yr) while regional brain activation was measured by BOLD signals in a 3-Tesla MRI scanner. Common regions activated with sweating events in both groups included the anterior and posterior cingulate cortex, insula, premotor cortex, thalamus, lentiform nuclei, and cerebellum (Pcorrected < 0.05). Psychogenic sweating events were associated with significantly greater activation in the dorsal midcingulate cortex, parietal cortex, premotor cortex, occipital cortex, and cerebellum. No hemispheric region was found to show statistically significantly greater activation with thermogenic than with psychogenic sweating events. However, a discrete cluster of activation in the anterior hypothalamus/preoptic area was seen only with thermogenic sweating events. These findings suggest that the expected association between sweating events and brain regions implicated in “arousal” may apply selectively to psychogenic sweating; the neural basis for thermogenic sweating events may be subcortical.
Keywords: body temperature/physiology, brain mapping, functional neuroimaging, humans, sweating
sweating in humans at rest may occur in response to increased body temperature (thermogenic) or during mental or emotional stress (psychogenic). Although early views considered that unique neural pathways (Chalmers and Keele 1952; Iwase et al. 1997; List and Peet 1938), different neurotransmitters (Nakazato et al. 2004; Noppen et al. 1997; Robertshaw 1977) and even sweat glands from separate skin regions (Darrow 1937; Kuno 1956; Ogawa 1975) were responsible for thermogenic and psychogenic sweating, recent studies indicate that these sweating events are all cholinergically driven phenomena (Machado-Moreira et al. 2012) that are expressed across the entire body surface (Machado-Moreira and Taylor 2012a,b). Bursts of the sudomotor nerve activity that drives sweating events are also highly correlated in time and amplitude between nerves innervating different skin regions (Bini et al. 1980a). In line with this evidence, we recently found that common brain stem pathways were activated in association with both thermogenic and psychogenic sweating events in humans (Farrell et al. 2013). The brain mechanisms generating the two categories of sweating event cannot be identical, however. To underline this point, volleys of cutaneous vasoconstrictor activity are coactivated with psychogenic sweating events, but not with thermogenic sweating events (Bini et al. 1980a,b; Delius et al. 1972; Macefield and Wallin 1996). So, if their brain stem pathways are indistinguishable, it makes sense to look for differences in higher brain regions.
Hemispheric brain regions driving psychogenic sweat events have been studied previously and appear to be relatively stable, irrespective of the psychogenic stress that gives rise to sudomotor activity. For instance, studies of brain activity associated with skin conductance responses during different mental tasks (gambling and working memory) have demonstrated common neural activation across those tasks (Patterson et al. 2002). Brain regions showing sweating-related activation are widely distributed and include the anterior and posterior cingulate cortices, ventromedial prefrontal cortex, premotor and motor areas, visual cortex, thalamus, and cerebellum (Craig et al. 2000; Fredrikson et al. 1998; James et al. 2013; Nagai et al. 2004; Patterson et al. 2002; Williams et al. 2000). Additionally, event-related EEG dipoles have implicated the inferior frontal gyrus, amygdala, and hippocampus in the generation of sweating events during mental arithmetic (Homma et al. 1998). However, the hemispheric regions associated with thermogenic sweating are essentially unknown.
The mental and emotional stimuli that are typically used to elicit psychogenic sweating also evoke a wide range of autonomic responses that collectively are part of the arousal response (Critchley et al. 2013). Cerebral regions associated with arousal have been extensively studied. In this context, the dorsal midcingulate cortex has been identified by Critchley and others as the common region associated with arousal-related activation of a wide range of sympathetic nerves (Critchley et al. 2013). By contrast, body heating is associated with drowsiness (Gilbert et al. 2004; Qian et al. 2014), which could have implications for regional brain activation during thermal sweating. However, the few studies investigating responses in the hemispheres to whole body heating have failed to produce a consensus on the brain regions that are activated during periods of either increased thermoafferent or thermoefferent flow (Egan et al. 2005; Fechir et al. 2010; Nunneley et al. 2002).
This study was designed to compare regional activation during both psychogenic and thermogenic sweating events, focusing on regions above the midbrain. As in our previous study (Farrell et al. 2013), we sought brain regions that were selectively activated in association with sweating events rather than with mean ongoing sweating levels. Our aim was to look for similarities and differences in the cerebral mechanisms driving human sudomotor function under these different forms of external stress.
MATERIALS AND METHODS
Participants
The study was undertaken according to procedures approved by the Melbourne Health Human Research Ethics Committee (no. 2008.147). Participants provided written, informed consent before enrolment. A total of 22 people contributed data for the study, of which there were two groups of 11, aged 34.4 ± 10.2 yr (Thermogenic Group) and 35.3 ± 11.8 yr (Psychogenic Group), and including the same proportions of men and women (91% male). Some of the data collected from these participants has been reported previously (Farrell et al. 2014; Farrell et al. 2013). The data presented herein from the Psychogenic Group have not previously been reported, and all outcomes reported from the current analyses of the Thermogenic Group data are also novel. The Thermogenic Group data were previously used in an analysis focused on the preoptic area and investigated the sweating-related activation and functional connectivity of that region based on contrasts with a normothermic resting-state data set (Farrell et al. 2014).
Recording of Sweating Events and Skin Temperature
Sweating was monitored by recording galvanic skin responses with Ag-AgCl electrodes (TSD203 electrodes, Biopac Systems) fixed on the palmar surfaces of the right index and middle fingers. Cabling (MECMRI-3 MRI Cable, Biopac Systems) and filters (MRIRFIF interference filter set, Biopac Systems) connected the electrodes to a constant-voltage amplifier (GSR100C Galvanic Skin Response Amplifier, Biopac Systems). The signal was digitized at 1 kHz (Power 1401, Cambridge Electronic Design) and recorded to computer [Spike2 (ver.7), Cambridge Electronic Design]. Gradient artefacts related to magnetic resonance image acquisition were excised from the GSR recording. The electrodermal signal was recorded as an AC signal (0.5-Hz high-pass filter) to identify discrete electrodermal events independently of any shift in mean signal level (Kunimoto et al. 1991). Output signals from the scanner control panel were used to trigger recordings of electrodermal data so that it could be matched with synchronously acquired functional brain images.
Type T (copper-constantan) thermocouples were attached to three different sites on each participant's trunk (left, right, midline) and recorded via an electronic thermometer (TH-5, Physitemp Instruments). Signals from the thermocouples were filtered, digitized and recorded to computer using the same procedures and equipment as those described above for the recording of electrodermal events. Finally, those data were averaged to yield an unweighted, mean torso skin temperature.
Sweating-Related Stimulation
Thermogenic sweating.
Heating was induced by means of a water-perfused garment (LCG)(Med-Eng BCS4 Body Cooling System, Allen Vanguard, ON, Canada) as previously described (Farrell et al. 2014; Farrell et al. 2013). Temperature-controlled water (40–50°C) was circulated through a network of small-diameter tubes sewn into the garment, which covered the torso, arms, and legs, but excluded the head, hands, and feet. Layers of insulating fabric were added to minimize heat loss to the environment. Passive heating was sustained until regular sweating events were detected electrodermally, at which time functional brain image acquisition commenced with heating maintained throughout. As previously described (Farrell et al. 2013), the water temperature was then adjusted to sustain a low mean rate of sweating events.
Psychogenic sweating.
Psychogenic sweating was achieved through the imposition of a mentally challenging color/word Stroop task as previously reported (Farrell et al. 2013). Visual stimuli were projected onto a screen that was visible to participants lying in the magnetic resonance imaging (MRI) scanner via a mirror mounted on the head coil. A sequence of colored words was presented in a random order. This contained both congruently (e.g., the word “red” written with red text) and incongruently colored letters (e.g., the word “red” written with green text). These were presented for a 2-min block followed by a 30-s rest interval, and the cycle was repeated three times during functional brain-scanning scans. The task was to count the number of nominated events (e.g., count the words “red” written with yellow letters) that occurred in each 2-min block. The tasks (counting congruent or incongruent events) and stimuli (color words and text colors) used during each blocks were varied within and across scanning runs. Participants used a button box to record their count by indicating their choice of answer from options displayed at the conclusion of the 2-min block. These responses were not recorded. However, the timing of the participants' actions were taken into account in the analysis of functional brain images to prevent their potential confounding influence (elaborated below).
Image Acquisition
Images were acquired with a Siemens 3-Tesla magnetic resonance imaging scanner (Trio system) with a 32-channel head coil at the Murdoch Children's Research Institute (Melbourne, Australia). High-resolution structural images of participants' brains were collected using a T1-weighted image sequence (192 × 0.9 mm sagittal slices, 256 × 256 matrix, in-slice resolution 0.8 mm × 0.8 mm, TR = 1,900 ms, TE = 2.59 ms, flip angle = 9°). Functional brain images sensitive to blood oxygen level-dependent (BOLD) contrast were also acquired for all participants (TR = 1,900 ms, TE = 35 ms, flip angle = 90°). The field of view of the functional images encompassed the brain hemispheres, cerebellum, and brain stem (30 slices of 4-mm thickness, in-slice resolution = 3.6 × 3.6 mm). Each functional scan lasted for 7 min and 55 s and included 250 sequential brain volumes (1.9 s per brain volume). Two of these functional scans were collected from each participant.
Analysis
Preprocessing.
Preparation and statistical analysis of functional brain images was performed with the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library [FMRIB, Oxford, UK, FSL version 4.1 (http://www.fmrib.ox.ac.uk/fsl/)]. Sequential functional brain images from single scanning runs were realigned to the middle image of the time series to correct for any head movement during the scanning run using MCFLIRT (Jenkinson et al. 2002). Images were spatially smoothed using a Gaussian kernel of 5-mm full width at half maximum. The time series of each scanning run was mean-based intensity normalized and high-pass filtered. Functional brain images from each participant were coregistered with a standard brain to facilitate amalgamation of statistical outcomes across participants. Transformations were performed with FMRIB's Linear Image Registration Tool (FLIRT) (Greve and Fischl 2009; Jenkinson et al. 2002; Jenkinson and Smith 2001).
Statistical analysis.
The first level of functional brain imaging analysis was performed on individual scanning runs using general linear modeling including local autocorrelation correction as instituted in the FMRI Expert Analysis Tool (FEAT), FMRIB's Improved Linear Model (FILM) (Smith et al. 2004; Woolrich et al. 2009). The regressor of interest for each scanning run analysis was derived from the recording of electrodermal events obtained contemporaneously with the functional brain images. The signals from the electrodermal recordings were initially downsampled to correspond with the acquisition time of functional brain images (one observation every 1.9 s). Timing adjustments were then made to the electrodermal regressor to take account of delays between activation of brain regions involved in sweating control and the occurrence of electrodermal events. Homma and colleagues (1998) recorded EEG, median sympathetic nerve activity, and sweating at the finger tip during performance of mental arithmetic, and using dipole source localization, concluded that activity in cortical regions occurred 5–5.5 s before sweating events (Homma et al. 1998). Consequently, the electrodermal regressors were translated backward in time by an interval corresponding to the acquisition of three brain volumes (5.7 s). The mental task involved instructions, blocks of visual stimuli, and button presses to indicate participants' responses. Regressors representing the mental task components were included in the modeling of BOLD signal changes to take account of these known sources of variance. Specifically, a regressor was used to indicate the onsets, durations, and offsets of periods during which participants were viewing images for the Stroop task, and another regressor was included in the analysis to indicate the timing of response cues and resulting button presses. The hemodynamic response measured with BOLD contrast occurs at a delay of 4–6 s after neural activation and so the electrodermal and task regressors were convolved with a model of the hemodynamic response function (Gamma function) and included temporal derivatives of the regressor to account for variations in the timing of events (Henson and Friston 2006).
Additional regressors were included in the general linear modeling of BOLD signal changes during scanning runs to take account of non-neural-related variability. Physiological processes contribute to BOLD signal noise including respiratory effects on local magnetic field properties and changes in the blood and cerebrospinal fluid associated with the cardiac cycle. This physiological noise does not usually confound fMRI analysis because it varies independently of many stimuli and tasks employed in functional brain imaging paradigms. However, sweating events are likely to correlate with other physiological processes, especially in the case of mental stress (Fechir et al. 2008). To reduce any confounding effects of physiological noise, regressors from three regions of interest were used in the model to account for variance associated with the cardiac and respiratory cycles. The regions of interest were identified in the white matter, ventricles, and circulation for each scanning run, according to procedures previously described (Farrell et al. 2012), and signals from those regions were extracted and included as the physiological noise regressors. The influence of head movement on BOLD signal intensity was also taken into account by including the six motion parameters (three translations and three rotations) into the modeling of signal changes.
A parameter estimate was calculated for the fit of each regressor to the observed BOLD signal for each voxel in the space of functional brain images resulting in a series of statistical parametric maps for each scanning run. Parameter estimates for the fits of regressors of interest (electrodermal responses) were carried forward to higher levels of analysis that first averaged responses across scanning runs for individual participants using fixed effects, and then subsequently calculated average responses among group participants (Thermogenic Group, Psychogenic Group) and between groups (Thermogenic greater than or lesser than Psychogenic) using mixed effects [FMRIB's Local Analysis of Mixed Effects (FLAME)] (Beckmann et al. 2003). Regions of activation were considered statistically significant when the constituent voxels had values exceeding z = 2.3, and a cluster-corrected threshold of P < 0.05 to take account of the spatial smoothness of the images and the effects of multiple comparisons on inferences of significance (Worsley et al. 1992).
Brain hemodynamic responses associated with sweating were characterized with region of interest (ROI) analyses. This was done to confirm that the timing of events was compatible with the expected response profiles, to compare outcomes with previous reports from the study cohort, and to characterize the nature of any between-group differences. ROIs were selected from the midbrain and medulla according to previously described procedures (Farrell et al. 2013). Clusters of between-group activation were also defined as ROI. BOLD signals extracted from ROI were compiled in one of two ways. The first method was used to assess the temporal profile of sweating-related signal change. BOLD signals were averaged across the voxels within ROI for each time point in individual scanning runs after motion correction, high-pass filtering, and spatial smoothing. Sections of time corresponding with sweating events were identified for scanning runs by calculating the mean and standard deviation of electrodermal signals and choosing peaks with values greater than the sum of the mean and 1 SD. Time points 28.5 s before and after each peak were extracted from ROI time series and averaged. The average BOLD signals during sweating events of individual scanning runs were expressed as a percentage of the average of the first three time points (28.5 to 24.7 s before the peak). Percentage signal changes of all scanning runs were averaged to produce the grand mean of BOLD signal changes during sweating events. The second method was used to visualize the relative size of sweating-related signal changes between the groups. The Featquery tool was used to estimate percentage signal changes across sweating events for each scanning run, and the outcomes were averaged across groups.
RESULTS
Skin Temperature and Sweating Event Frequency
Passive heating for thermogenic sweating was associated with an increased skin temperature [36.2 ± 1.3 (SD)°C] compared with the nonheated state during psychogenic sweating [33.2 ± 1.2°C, t(20) = 5.7, P < 0.001]. Sweating events occurred with similar frequency during functional brain scans for the two experimental procedures [Thermogenic = 7.7 ± 1.7 per scan, Psychogenic = 7.5 ± 2.0 (SD) per scan, t(20) = 0.2, NS]. Sweating activity patterns were similar to those reported previously (Farrell et al. 2013).
Sweating Event-Related Activation
Sweating events were associated with activation in widely distributed regions of the brain for both thermogenic and psychogenic stimuli (Fig. 1, Tables 1 and 2). Extensive cingulate activations were principally in the dorsal midcingulate cortex with psychogenic sweating events, whereas thermogenic sweating was notable for clusters of event-related activation in the posterior and pregenual cingulate cortexes. Mesial activation was also seen in the precuneus for both stimulus types, while only thermogenic sweating was associated with activation in the anterior hypothalamus/preoptic area (AH/PO). Sweating-related activation was seen in the insula with both stimuli, being predominantly anterior in both hemispheres with psychogenic sweating events, and mid (right) and posterior (left) for thermogenic sweating events. Right prefrontal cortex activation was noted with both types of sweating event (Middle Frontal Gyrus, BA10). Other regions activated with both types of sweating event were the bilateral thalami, lentiform nuclei, and cerebellum.
Fig. 1.
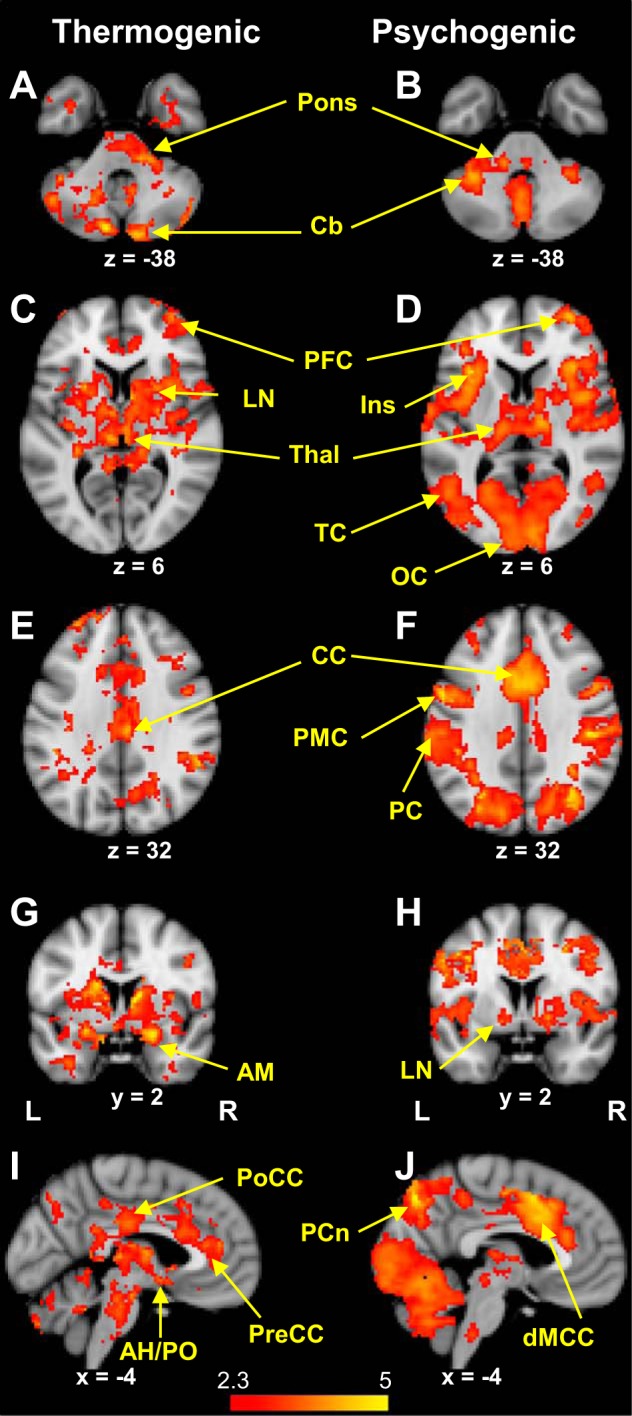
A: thermogenic sweating event-related activation occurred bilaterally in the cerebellum (CB) (lobule Crus II) and pons. B: pontine and cerebellar activation was apparent in association with psychogenic sweating events, the latter occurring bilaterally in the declive (lobule VI). C: bilateral thalamic (Thal) and lentiform (LN) activation occurred during thermogenic sweating events, as well as activation in the right prefrontal cortex (PFC). D: right prefrontal activation was also a feature of psychogenic sweating events, along with bilateral insula (Ins), temporal (TC), and occipital (OC) cortexes. E: cingulate cortex (CC) activation occurred during thermogenic sweating events. F: in addition to activation in the cingulate cortex, psychogenic sweating events were associated with activation in the premotor (PMC) and parietal cortexes (PC). G: thermogenic sweating event-related activation was seen bilaterally in the amygdalae (AM). H: amygdala activation was not seen in association with psychogenic sweating events, but did occur bilaterally in lentiform nuclei (LN). I: mesial activation during thermogenic sweating events occurred in clusters incorporating the posterior cingulate cortex (PoCC), dorsal midcingulate cortex (dMCC), and pregenual cingulate cortex (PreCC). Activation was also seen in the anterior hypothalamus/preoptic area (AH/PO). J: the dorsal midcingulate cortex was activated during psychogenic sweating events, as was the precuneus (PCn).
Table 1.
Clusters of significant activation during psychogenic sweating
| Region | BA | Side | MNI Coordinates |
Z Score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Supplementary motor area | 6 | R | 2 | −12 | 52 | 4.73 |
| Cingulate cortex | 24 | R | 6 | 8 | 42 | 4.77 |
| 32 | L | −2 | 12 | 40 | 4.71 | |
| Middle frontal gyrus | 10 | R | 34 | 58 | 6 | 4.33 |
| Precentral gyrus | 6 | R | 46 | −2 | 50 | 4.57 |
| 4 | L | −38 | −10 | 50 | 4.47 | |
| Premotor cortex | 6 | R | 50 | 6 | 34 | 3.99 |
| 6 | L | −52 | 4 | 36 | 4.77 | |
| Inferior parietal lobule | 40 | R | 50 | −26 | 38 | 4.65 |
| 40 | L | −62 | −34 | 40 | 4.48 | |
| Superior parietal lobule | 7 | R | 38 | −66 | 40 | 3.77 |
| 7 | L | −28 | −68 | 34 | 4.71 | |
| Precuneus | 7 | L | −4 | −74 | 46 | 4.97 |
| Middle temporal gyrus | 37 | L | −52 | −64 | 2 | 4.46 |
| Lingual gyrus | 18 | R | 8 | −90 | 8 | 3.89 |
| Cuneus | 18 | L | −8 | −96 | 10 | 3.63 |
| Middle occipital gyrus | 19 | R | 56 | −62 | −6 | 3.83 |
| 19 | L | −48 | −66 | −10 | 4.60 | |
| Fusiform gyrus | 19 | R | 34 | −68 | −16 | 4.46 |
| 19 | L | −48 | −64 | −14 | 4.47 | |
| Insula | 13 | R | 36 | 22 | 6 | 4.22 |
| 13 | L | −34 | 20 | 6 | 4.92 | |
| Thalamus | R | 14 | −14 | 6 | 4.18 | |
| L | −14 | −18 | 8 | 3.63 | ||
| Globus pallidus | R | 18 | 0 | −4 | 3.69 | |
| L | −16 | −2 | −6 | 3.45 | ||
| Cerebellum, declive (VI) | R | 36 | −68 | −18 | 4.57 | |
| L | −24 | −62 | −22 | 4.32 | ||
| R | 38 | −46 | −30 | 4.47 | ||
| L | −28 | −52 | −28 | 4.19 | ||
| Midbrain | R | 10 | −18 | −6 | 3.60 | |
| L | −12 | −18 | −6 | 3.19 | ||
| Pons | −12 | −36 | −38 | 3.73 | ||
| Medulla | 0 | −34 | −48 | 3.18 | ||
Shown are coordinates of the voxel with the highest Z-score in each cluster of activation, and records the value of that Z-score. The Brodmann Areas (BA) of peak voxels are listed for clusters located in cortical regions. The coordinates correspond to the Montreal Neuroscience Institute (MNI) standard brain template: x-values are mm left (−) or right (+) of the anterior commissure; y-values denote mm anterior (+) or posterior (−) to the anterior commissure; z gives mm above (+) or below (−) the anterior commissure.
Table 2.
Clusters of significant activation during thermogenic sweating
| Region | BA | Side | MNI Coordinates |
Z Score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Cingulate cortex | 32 | R | 4 | 38 | 12 | 4.04 |
| 32 | L | −2 | 40 | 14 | 4.33 | |
| 23 | R | 6 | −22 | 30 | 4.25 | |
| 23 | L | −2 | −12 | 34 | 3.72 | |
| Middle frontal gyrus | 10 | R | 34 | 58 | 8 | 4.09 |
| Superior frontal gyrus | 9 | L | −28 | 48 | 28 | 4.21 |
| Premotor cortex | 44 | R | 48 | 4 | 38 | 4.00 |
| Inferior parietal lobule | 40 | R | 54 | −44 | 32 | 4.42 |
| Precuneus | 7 | R | 4 | −70 | 44 | 3.83 |
| Insula | 13 | R | 44 | 6 | −2 | 3.65 |
| 13 | L | −34 | −18 | 4 | 3.30 | |
| Amygdala | R | 22 | 2 | −16 | 4.76 | |
| L | −14 | −18 | −10 | 3.83 | ||
| Caudate | R | 12 | −2 | 12 | 5.51 | |
| L | −10 | −2 | 12 | 4.63 | ||
| Putamen | R | 24 | 14 | −4 | 4.48 | |
| L | −26 | −2 | −6 | 4.22 | ||
| Thalamus | R | 18 | −24 | 10 | 3.81 | |
| L | −8 | −22 | 8 | 4.70 | ||
| Hypothalamus | −2 | 0 | −8 | 3.43 | ||
| Cerebellum, crus II | R | 12 | −86 | −38 | 4.28 | |
| L | −10 | −82 | −38 | 4.88 | ||
| Midbrain | R | 10 | −24 | −10 | 3.70 | |
| L | −14 | −18 | −10 | 3.83 | ||
| 0 | −20 | −22 | 4.13 | |||
| Pons | R | 2 | −22 | −30 | 4.10 | |
| Medulla | −6 | −36 | −48 | 3.62 | ||
See notes for Table 1 for explanations.
Thermogenic and psychogenic sweating event-related activation was seen in the midbrain, pons, and medulla. In agreement with previous findings (Farrell et al. 2013), the location of midbrain activations was similar for the two types of sweating and was mainly seen in the dorsal part of the region (Fig. 2),. Overlaps of sweating activations for thermogenic and psychogenic stimuli were also apparent in the rostral medulla, being located symmetrically lateral (Fig. 2). This again agrees with previous findings (Farrell et al. 2013).
Fig. 2.
A: a logical map representing voxels in the brain stem that were activated during thermogenic sweating events (red), psychogenic sweating events (blue), or both (green). B: event-related activation was commonly seen for thermogenic and psychogenic sweating in the dorsal midbrain bilaterally (yellow arrows). C: BOLD signals extracted from commonly activated voxels in the left dorsal midbrain show increases at the time of sweating events in both the thermogenic and psychogenic experimental runs. D: the right dorsal midbrain also showed BOLD signal increases at the time of both thermogenic and psychogenic sweating events. E: commonly activated voxels were seen in the rostral medulla at symmetrical, lateral locations (yellow arrows). Both the left (F) and right (G) commonly activated voxels in the rostral medulla showed BOLD signal increases during both types of sweating event.
Differential Activation by Psychogenic and Thermogenic Sweating Events
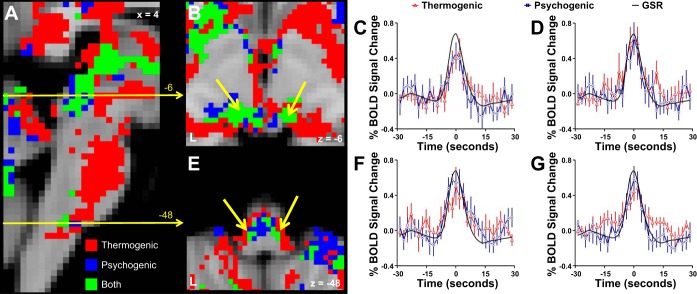
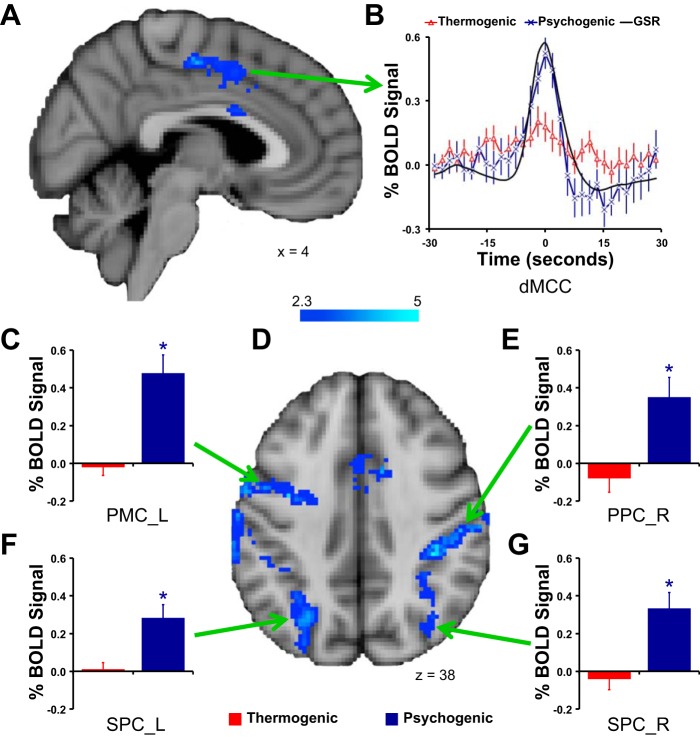
Contrasts between the two types of sweating event-related activation revealed sites of significantly greater activation with psychogenic than with thermogenic sweating events, but not the reverse (Fig. 3, Table 3). Regions showing increased psychogenic vs. thermogenic sweating activation included the dorsal midcingulate cortex, premotor regions, parietal associative cortex, occipital cortex, and cerebellum. Examinations of time series of BOLD signal changes and mean levels of sweating activations suggested that these regions were exclusively activated with psychogenic sweating events, with little or no activation related to thermogenic sweating events (Fig. 3).
Fig. 3.
Brain regions showing differential activation when thermogenic and psychogenic sweating event-related activations were contrasted. No region was more strongly activated with thermogenic sweating events: sites more strongly activated with psychogenic sweating events are shown here by shades of blue in A and D. B shows the BOLD signal time courses extracted from the dorsal midcingulate cortex (dMCC) region shown in A. Bar graphs in C–G show the mean ± SE BOLD signal changes with thermogenic and psychogenic sweating events in the regional clusters indicated: left premotor cortex (PMC_L) (C); right posterior parietal cortex (PPC_R) (E); left superior parietal cortex (SPC_L) (F); right superior parietal cortex (SPC_R) (G).
Table 3.
Psychogenic greater than thermogenic sweating activation
| X | y | z | MNI Coordinates |
Z Score | ||
|---|---|---|---|---|---|---|
| Region | BA | Side | ||||
| Cingulate cortex | 24 | R | 2 | 6 | 46 | 3.14 |
| 24 | L | −4 | 6 | 48 | 3.57 | |
| Supplementary motor cortex | 6 | R | 8 | −10 | 52 | 4.64 |
| Premotor cortex | 6 | L | −36 | −4 | 38 | 4.68 |
| Posterior parietal cortex | 40 | R | 38 | −34 | 38 | 5.38 |
| Superior parietal cortex | 7 | R | 34 | −66 | 32 | 4.10 |
| 7 | L | −28 | −68 | 36 | 4.17 | |
| Precuneus | 7 | L | −8 | −76 | 56 | 4.09 |
| Middle occipital gyrus | 19 | L | −46 | −64 | −8 | 3.41 |
| Cerebellum | L | −24 | −60 | −8 | 3.41 | |
See notes for Table 1 for explanations.
Several brain regions showed substantial clusters of activation that were prominent only with thermogenic sweating, but statistical comparison fell short of proving that this was greater than with psychogenic sweating events. Such regions included the lentiform nuclei (P = 0.1), amygdalae (P = 0.1), and AH/PO (P = 0.09).
DISCUSSION
This study has provided the first direct description of the network of forebrain regions associated with thermogenic sweating events. It also confirmed the findings of previous studies on the cerebral activations associated with psychogenic sweating events (Craig et al. 2000; Fredrikson et al. 1998; Nagai et al. 2004; Patterson et al. 2002; Williams et al. 2000) and skin sympathetic nerve activity (SSNA), which includes both sweating and vasomotor events (James et al. 2013). Besides confirming our previous finding of common brain stem regions activated with both thermogenic and psychogenic sweating events (Farrell et al. 2013), we now find such common regions in the cerebral hemispheres. Those regions include parts of the cingulate, insular, and premotor cortexes, thalamus, lentiform nuclei and cerebellum. But the current study also revealed important differences. Psychogenic sweating events were associated with significantly greater activation than thermogenic sweating events in the supplementary motor area, premotor cortex, parietal cortex, parts of the cerebellum and dorsal midcingulate cortex. Thus, as would be predicted, the two types of sweating event were linked to distinct patterns of brain activation.
Both these types of sweating event occur episodically and synchronously across the entire body surface (Hagbarth et al. 1972; van Beaumont et al. 1966) following sweat gland priming (Machado-Moreira and Taylor 2012a,b). The phasic sweating events that informed the analysis used in this study are driven by bursts of sympathetic sudomotor activity (Bini et al. 1980b; Hagbarth et al. 1972; Ogawa and Bullard 1972). The measure used in this study, episodic increases in skin conductance, measures the timing and amplitude of sweating events. Although measured here at the fingers, the timing and amplitude of these conductance changes accurately and linearly reflect bursts of sudomotor nerve activity, which are highly correlated across skin regions (Bini et al. 1980a). We are therefore confident that the brain activation patterns we have identified here apply not only to finger sweating but to sweating events across the body. The sudomotor bursts driving the sweating events are likely to be driven by the output of rostral-lateral medullary nuclei, whose event-related activation was identified in this and an earlier study (Farrell et al. 2013), and whose location is homologous with the sympathetic premotor nuclei for sweating identified in the cat medulla (Shafton and McAllen 2013).
Resistance of the skin is principally due to the stratum corneum (Lawler et al. 1960), and skin conductance increases only when fluid-filled sweat ducts pierce that resistive layer. This makes skin conductance measurements insensitive to any change in skin blood flow. In line with this, atropine abolishes sweating events but leaves skin vasomotor responses intact, while bretylium does the reverse (Lader and Montagu 1962). Even though vasomotor nerve volleys may be activated at the same time as sudomotor nerve volleys (Bini et al. 1980a), they should have had no influence on the electrodermal measurements that we used to identify activated brain regions.
As have others, we found widespread forebrain regions activated in association with psychogenic sweating events (Craig et al. 2000; Fredrikson et al. 1998; Nagai et al. 2004; Patterson et al. 2002; Williams et al. 2000). It is likely that many of these represent other arousal-related cerebral events that coactivate with sweating (e.g., cognitive, premotor and other autonomic processes). Prevailing views on the interaction between autonomic responses and cognition posit a link between arousal state and the behavioral significance of stimuli (Critchley et al. 2013). Research using similar mental stresses, (i.e., Stroop task), and additional measures of autonomic responses would suggest that performance errors trigger a change in bodily state, especially when there is a conscious awareness of the error (Critchley et al. 2005; Hajcak et al. 2003; Nieuwenhuis et al. 2001). This shift in autonomic responses is conceptualized as a somatic marker that provides cognitive feedback to decrease the probability of future errors.
The dorsal midcingulate cortex has been identified as the common cortical area associated with autonomic activation, including sweating, in response to both physical and mental effort (Critchley et al. 2000). In line with that view, the present study confirmed the expectation that this region would be strongly activated with psychogenic sweating events. Strikingly, however, this was a site of strong functional contrast: it was not activated by thermogenic sweating. If the dorsal midcingulate represents a key cortical site controlling several “stress arousal-related” sympathetic outflows (Craig 2009; Critchley et al. 2000; Critchley et al. 2003), the source of thermogenic sweating events needs to be sought elsewhere.
Methodologically, finding such a contrast is reassuring, because it tells us clearly that different brain mechanisms drive the two types of sweating event. Therefore, the thermogenic sweating events were not simply mini-arousals that happened to occur during whole body heating. On the other hand, we cannot exclude the possibility that some such arousals did occur, even though the heated condition was generally relaxing and subjects were left undisturbed. If so, that might explain some of the forebrain sites that were activated with sweating events from both protocols.
While no site was identified that showed significantly greater activation with thermogenic than psychogenic sweating events, this could be a false-negative conclusion due to noisy data. In this context, the AH/PO area merits mention. This brain region is known from animal studies to play a key role in thermoregulatory processes (Nakamura 2011) and, when locally heated, it can drive sweating (Beaton et al. 1941). A discrete cluster there was activated with thermogenic sweating events, but not psychogenic sweating events (Fig. 1). We have reported elsewhere that this region showed enhanced functional connectivity with other brain regions in humans during whole body heating compared with during the normothermic state (Farrell et al. 2014). Whether the AH/PO area in humans is truly a source of thermogenic but not psychogenic sweating events must await determination by more detailed study.
We still do not know exactly where either type of sweating event originates. Presumably in each case there is a network of neurons whose synchronous activity acts as the source of the burst. These in turn may be influenced by such factors as brain temperature, or state of arousal. Other attempts to localize the source of psychogenic sweating events have included the following. Event-related fMRI studies have identified activation in prefrontal, cingulate, parietal, motor, insular and occipital cortex, and hippocampus, thalamus, and cerebellum to psychogenic sweating events (Craig et al. 2000; Fredrikson et al. 1998; Nagai et al. 2004; Patterson et al. 2002; Williams et al. 2000), and additionally activation in the cingulate, superior frontal, precentral, and occipital cortexes to the mean sweating level by skin resistance (Fan et al. 2012; Nagai et al. 2004; Zhang et al. 2014). James and colleagues sought cerebral signals to variations in skin sympathetic nerve activity (SSNA) (James et al. 2013). The SSNA signal includes vasomotor as well as sudomotor nerve activity (Bini et al. 1980b; Macefield and Wallin 1996), although burst activity associated with these two autonomic responses can be synchronized (Bini et al. 1980a), in which case the associated regional brain activation would be the same. In the case of psychogenic sweating events a related EEG signal has been studied, and its sources located in two subjects to the inferior frontal gyrus, amygdala, and hippocampus (Homma et al. 1998). No one has previously attempted to localize the origins of thermogenic sweating events.
Limitations
As with all fMRI studies, the results obtained here are correlative only. This means that the brain regions we identified as activated with sweating events could be due to other processes that may occur consistently at the same time. This may apply, for example, to cutaneous vasoconstrictor traffic that is coactivated with sudomotor bursts during psychogenic stimuli. It should not apply to thermogenic sweating events. This distinction may explain some of the differences we observe between the brain regions activated with psychogenic vs. thermogenic sweating events.
Second, the study involved two groups of participants. A within-subject design would have been ideal, but a lag between study times prevented us recruiting all the same participants twice.
Our experiment was designed to give a clear distinction between thermogenic and psychogenic stimuli, but we cannot eliminate the possibility that some psychogenic sweating events also occurred during the thermal stimulus runs and contributed to the fMRI findings. Against this, the heating protocol we used was mild, and likely to have been relaxing. Moreover, vasoconstrictor sympathetic nerve bursts were not found to occur in resting heated subjects (Bini et al. 1980b; Macefield and Wallin 1996).
Finally, to measure responses through the whole brain we traded spatial for temporal resolution. The size of voxels used could have prevented us from resolving small distinct clusters from a larger mass of activation, and could have made it more difficult to detect local differences between psychogenic and thermogenic sweating-related activations.
Perspectives
This study has been the first successful foray into the functional imaging of thermogenic sweating activation in the hemispheres of the human brain. The network of brain regions activated during thermogenic sweating events shares some common regions that are activated also during psychogenic sweating events, but regional differences are also apparent. Notably, neural activation in the dorsal midcingulate cortex was linked to psychogenic more than thermogenic sweating events. This difference is likely to reflect greater levels of arousal during psychogenic sweating. Previous studies have linked this region with sympathetic activation (including sweating) during stress and arousal. Our findings support that view. But the sources of thermogenic sweating events are clearly different and may ultimately be found in subcortical structures such as the AH/PO area. Taken together with previous findings, this study suggests that distinct supratentorial mechanisms generate psychogenic and thermogenic sweating events, but these converge on common brain stem pathways en route to the sweat glands.
GRANTS
This study was supported by the National Health and Medical Research Council of the Commonwealth Government of Australia (Project Grant 509089) and the Victorian government through the Operational Infrastructure Scheme. R. M. McAllen received a Principal Research Fellowship (566667) from the National Health and Medical Research Council, and M. J. Farrell was supported in part by the Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation and the G. Harold and Leila Y. Mathers Charitable Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.F., N.A.S.T., and R.M.M. conception and design of research; M.J.F. and D.T. performed experiments; M.J.F. and D.T. analyzed data; M.J.F., D.T., N.A.S.T., and R.M.M. interpreted results of experiments; M.J.F. prepared figures; M.J.F. and R.M.M. drafted manuscript; M.J.F., N.A.S.T., and R.M.M. edited and revised manuscript; M.J.F., D.T., N.A.S.T., and R.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the technical expertise provided by M. Kean of the Children's Magnetic Resonance Imaging Centre (Melbourne, Australia).
REFERENCES
- Beaton LE, McKinley WA, Berry CM, Ranson SW. Localization of cerebral center activating heat-loss mechanisms in monkeys. J Neurophysiol 4: 478–485, 1941. [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage 20: 1052–1063, 2003. [DOI] [PubMed] [Google Scholar]
- Bini G, Hagbarth KE, Hynninen P, Wallin BG. Regional similarities and differences in thermoregulatory vaso- and sudomotor tone. J Physiol 306: 553–565, 1980a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol 306: 537–552, 1980b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers TM, Keele CA. The nervous and chemical control of sweating. Br J Dermatol 64: 43–54, 1952. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70, 2009. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci 3: 184–190, 2000. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523: 259–270, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Eccles J, Garfinkel SN. Interaction between cognition, emotion, and the autonomic nervous system. Handbook Clin Neurol 117: 59–77, 2013. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126: 2139–2152, 2003. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage 27: 885–895, 2005. [DOI] [PubMed] [Google Scholar]
- Darrow CW. Neural mechanisms controlling the palmar galvanic skin reflex and palmar sweating. Arch Neurol Psychiat 37: 641–663, 1937. [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972. [DOI] [PubMed] [Google Scholar]
- Egan GF, Johnson J, Farrell M, McAllen R, Zamarripa F, McKinley MJ, Lancaster J, Denton D, Fox PT. Cortical, thalamic, and hypothalamic responses to cooling and warming the skin in awake humans: a positron-emission tomography study. Proc Natl Acad Sci USA 102: 5262–5267, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Xu P, Van Dam NT, Eilam-Stock T, Gu X, Luo YJ, Hof PR. Spontaneous brain activity relates to autonomic arousal. J Neurosci 32: 11176–11186, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Cole LJ, Chiapoco D, Egan GF, Mazzone SB. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. Neuroimage 61: 1324–1335, 2012. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Trevaks D, McAllen RM. Preoptic activation and connectivity during thermal sweating in humans. Temperature 1: 135–141, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Trevaks D, Taylor NAS, McAllen RM. Brain stem representation of thermal and psychogenic sweating in humans. Am J Physiol Regul Integr Comp Physiol 304: R810–R817, 2013. [DOI] [PubMed] [Google Scholar]
- Fechir M, Klega A, Buchholz HG, Pfeifer N, Balon S, Schlereth T, Geber C, Breimhorst M, Maihofner C, Birklein F, Schreckenberger M. Cortical control of thermoregulatory sympathetic activation. Eur J Neurosci 31: 2101–2111, 2010. [DOI] [PubMed] [Google Scholar]
- Fechir M, Schlereth T, Purat T, Kritzmann S, Geber C, Eberle T, Gamer M, Birklein F. Patterns of sympathetic responses induced by different stress tasks. O pen Neurol J 2: 25–31, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson M, Furmark T, Olsson MT, Fischer H, Andersson J, Langstrom B. Functional neuroanatomical correlates of electrodermal activity: a positron emission tomographic study. Psychophysiology 35: 179–185, 1998. [PubMed] [Google Scholar]
- Gilbert SS, van den Heuvel CJ, Ferguson SA, Dawson D. Thermoregulation as a sleep signalling system. Sleep Med Rev 8: 81–93, 2004. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48: 63–72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand 84: 164–176, 1972. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology 40: 895–903, 2003. [DOI] [PubMed] [Google Scholar]
- Henson RN, Friston KJ. Convolution models for fMRI. In: Statistical Parametric Mapping: The Analysis of Functional Brain Images, edited by Friston K, Ashburner J, Kiebel S, Nichols TE, Penny W. London: Elsevier, 2006, p. 178–192. [Google Scholar]
- Homma S, Nakajima Y, Toma S, Ito T, Shibata T. Intracerebral source localization of mental process-related potentials elicited prior to mental sweating response in humans. Neurosci Lett 247: 25–28, 1998. [DOI] [PubMed] [Google Scholar]
- Iwase S, Ikeda T, Kitazawa H, Hakusui S, Sugenoya J, Mano T. Altered response in cutaneous sympathetic outflow to mental and thermal stimuli in primary palmoplantar hyperhidrosis. J Autonom Nerv Syst 64: 65–73, 1997. [DOI] [PubMed] [Google Scholar]
- James C, Henderson L, Macefield VG. Real-time imaging of brain areas involved in the generation of spontaneous skin sympathetic nerve activity at rest. Neuroimage 74: 188–194, 2013. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Analysis 5: 143–156, 2001. [DOI] [PubMed] [Google Scholar]
- Kunimoto M, Kirno K, Elam M, Wallin BG. Neuroeffector characteristics of sweat glands in the human hand activated by regular neural stimuli. J Physiol 442: 391–411, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno Y. Human Perspiration. Springfield, IL: Thomas, 1956. [Google Scholar]
- Lader MH, Montagu JD. The psycho-galvanic reflex: a pharmacological study of the peripheral mechanism. J Neurol Neurosurg Psychiatry 25: 126–133, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler JC, Davis MJ, Griffith EC. Electrical characteristics of the skin. The impedance of the surface sheath and deep tissues. J Invest Dermatol 34: 301–308, 1960. [DOI] [PubMed] [Google Scholar]
- List CF, Peet MM. Sweat secretion in man. I. Sweating responses in normal persons. Arch Neurol Psychiat 38: 1228–1237, 1938. [Google Scholar]
- Macefield VG, Wallin BG. The discharge behaviour of single sympathetic neurones supplying human sweat glands. J Autonom Nerv Syst 61: 277–286, 1996. [DOI] [PubMed] [Google Scholar]
- Machado-Moreira CA, McLennan PL, Lillioja S, van Dijk W, Caldwell JN, Taylor NAS. The cholinergic blockade of both thermally and non-thermally induced human eccrine sweating. Exp Physiol 97: 930–942, 2012. [DOI] [PubMed] [Google Scholar]
- Machado-Moreira CA, Taylor NAS. Psychological sweating from glabrous and nonglabrous skin surfaces under thermoneutral conditions. Psychophysiology 49: 369–374, 2012a. [DOI] [PubMed] [Google Scholar]
- Machado-Moreira CA, Taylor NAS. Sudomotor responses from glabrous and non-glabrous skin during cognitive and painful stimulations following passive heating. Acta Physiol 204: 571–581, 2012b. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage 22: 243–251, 2004. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol 301: R1207–R1228, 2011. [DOI] [PubMed] [Google Scholar]
- Nakazato Y, Tamura N, Ohkuma A, Yoshimaru K, Shimazu K. Idiopathic pure sudomotor failure: anhidrosis due to deficits in cholinergic transmission. Neurology 63: 1476–1480, 2004. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology 38: 752–760, 2001. [PubMed] [Google Scholar]
- Noppen M, Sevens C, Vincken WG. Effects of non-pharmacological sympathetic sudomotor denervation on sweating in humans with essential palmar hyperhidrosis. Clin Biochem 30: 171–175, 1997. [DOI] [PubMed] [Google Scholar]
- Nunneley SA, Martin CC, Slauson JW, Hearon CM, Nickerson LD, Mason PA. Changes in regional cerebral metabolism during systemic hyperthermia in humans. J Appl Physiol (1985) 92: 846–851, 2002. [DOI] [PubMed] [Google Scholar]
- Ogawa T. Thermal influence on palmar sweating and mental influence on generalized sweating in man. Jpn J Physiol 25: 525–536, 1975. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Bullard RW. Characteristics of subthreshold sudomotor neural impulses. J Appl Physiol 33: 300–305, 1972. [DOI] [PubMed] [Google Scholar]
- Patterson JC 2nd, Ungerleider LG, Bandettini PA. Task-independent functional brain activity correlation with skin conductance changes: an fMRI study. Neuroimage 17: 1797–1806, 2002. [DOI] [PubMed] [Google Scholar]
- Qian S, Jiang Q, Liu K, Li B, Li M, Li L, Yang X, Yang Z, Sun G. Effects of short-term environmental hyperthermia on patterns of cerebral blood flow. Physiol Behav 128: 99–107, 2014. [DOI] [PubMed] [Google Scholar]
- Robertshaw D. Neuroendocrine control of sweat glands. J Invest Dermatol 69: 121–129, 1977. [DOI] [PubMed] [Google Scholar]
- Shafton AD, McAllen RM. Location of cat brain stem neurons that drive sweating. Am J Physiol Regul Integr Comp Physiol 304: R804–R809, 2013. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, Suppl 1: S208–S219, 2004. [DOI] [PubMed] [Google Scholar]
- van Beaumont W, Bullard RW, Banjeree MR. Observations on human sweating by resistance hygrometry. Dermatol Dig 1966: 75–87, 1966. [Google Scholar]
- Williams LM, Brammer MJ, Skerrett D, Lagopolous J, Rennie C, Kozek K, Olivieri G, Peduto T, Gordon E. The neural correlates of orienting: an integration of fMRI and skin conductance orienting. Neuroreport 11: 3011–3015, 2000. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage 45: S173–S186, 2009. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918, 1992. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Ide JS, Luo X, Farr OM, Li CS. Ventromedial prefrontal cortex and the regulation of physiological arousal. Social Cognitive Affect Neurosci 9: 900–908, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]