Abstract
Currently, the metabolic syndrome (MS) is occurring at growing rates worldwide, raising extensive concerns on the mechanisms and therapeutic interventions for this disorder. Herein, we described a novel method of establishing MS model in rodents. Male Institute of Cancer Research (ICR) mice were fed with high-fat-high-fructose (HFHF) diet or normal chow (NC) respectively for 12 weeks. Metabolic phenotypes were assessed by glucose tolerance test, insulin tolerance test and hyperinsulinemic-euglycemic clamp. Blood pressure was measured by a tail-cuff system. At the end of the experiment, mice were sacrificed, and blood and tissues were harvested for subsequent analysis. Serum insulin levels were measured by ELISA, and lipid profiles were determined biochemically. The HFHF diet-fed ICR mice exhibited obvious characteristics of the components of MS, including obvious obesity, severe insulin resistance, hyperinsulinemia, dislipidemia, significant hypertension and hyperuricemia. Our data suggest that HFHF diet-fed ICR mice may be a robust and efficient animal model that could well mimic the basic pathogenesis of human MS.
Keywords: animal model, high-fat-high-fructose, Institute of Cancer Research mice, insulin resistance, metabolic syndrome
Introduction
With the prevalence of the western life style, the metabolic syndrome (MS) is occurring at growing rates worldwide, leading to frightful consequences to human’s health [1, 27]. According to the International Diabetes Federation (IDF) definition, this syndrome is usually defined as a cluster of metabolic abnormalities, including abdominal obesity, insulin resistance, and dyslipidemia, hypertension, fatty liver disease and type 2 diabetes mellitus [10]. Despite its high prevalence, the pathogenesis of MS is not completely understood [21]. A better understanding of the molecular causes of MS will provide further evidences for developing novel preventative and therapeutic strategies.
In fact, there have emerged multiple types of animal models that commonly used to study the pathophysiology and mechanism of MS. Firstly, the ob/ob [9] and db/db mice [3], which were commonly used in the study of obesity, insulin resistance, diabetes, dyslipidemia and fatty liver, exhibit the main features of MS happened in human. Moreover, the apoE deficient mice [16], the Goto-Kakisaki (GK) rats [28], Zucker rats [13], OLEFT rats [29], were also most widely used monogenetically mutant rodent models for diabetes and MS. Besides, there are recently increasing use of diet-induced MS models. Previous studies have shown that the high-fat or high-fructose diet in combination with alloxan or low dose streptozotocin (STZ) are effective methods to establish type 2 diabetes or insulin resistance in animals [26, 30]. Although these models showed the typical characteristics of MS, they are either based on monogenic mutation or beta cell destruction, thus of little clinical relevance. Therefore, they are in fact, not most suitable models for studying the molecular mechanisms underlying these basic defects.
Recently, there are emerging evidences showing that a combination use of HFHF diet tends to predispose to insulin resistance and deteriorate the metabolic disorders in animals [12, 25, 34]. It has been shown that HFHF feeding successfully establishes MS model in rats, as demonstrated by increased abdominal fat deposition, impaired glucose tolerance, dyslipidemia, hyperinsulinemia, and increased systolic blood pressure [6, 22]. It was also reported that HFHF diet impairs hepatic glucose uptake and disturbs the suppressive effect of insulin on lipolysis in partially pancreatectomized dogs, indicating that a consumption of HFHF diet leads to deleterious metabolic consequences [5]. However, there is still no researches elucidate whether this HFHF feeding protocol also works effectively in mice.
Moreover, there are recently a growing number of researches performed in ICR mice aimed to establish the diabetes or MS model [4, 8, 23, 24, 38]. In fact, although the C57BL6J mice are most widely used in metabolic researches, there are existing evidences showing that ICR mice are potentially a more suitable animal for studying the MS. It was reported that goldthioglucose induced diabetic changes were more severe in ICR mice than C57BL mice [19]. Moreover, in comparision with fat-fed C57BL/6J mice, fat-fed ICR seems to be more sensitive to diabetogenic effects of STZ, suggessting that ICR might be more suitable for establishing MS than C57BL/6J mice [17]. In addition, it was shown that combination use of high-fat diet with nicotinamide and STZ in ICR mice induces significant insulin resistance, hyperlipidemia, impaired insulin secretion, glucose intolerance, and obesity. Yet, high-fructose feeding alone exerts no significant effect on ICR mice [31]. This study indicated that the high-fat diet-fed ICR mice with partly destructed beta cells demonstrated key features of MS. However, whether the combination use of high-fat and high-fructose diet establishes MS in ICR mice has not been clarified.
In the present study, we aimed to explore the possibility of establishing MS model in ICR mice with combined HFHF feeding, so as to establish a novel mice model for the mechanistic and therapeutic studies of MS.
Materials and Methods
Reagents
Unless otherwise stated, all reagents were purchased from Sigma-aldrich.
Animals
Male ICR mice aged 8 weeks were purchased from Shanghai Laboratory Animal Center, Chinese academy of science (CAS). All experimental procedures were approved by the ethics committees for laboratory animals at Shanghai Jiao Tong University School of Medicine. Mice were fed in a standard animal room with 12/12 dark/light cycles. After an adaptive feeding for 1 week, animals were randomly divided into two groups: normal chow group (NC); high-fructose high-fat diet group (HFHF), with 12 mice in each group. Subsequently, animals were fed with NC or HFHF diet respectively for 12 weeks (pivotal test have been performed for setting the appropriate feeding periods in before starting this study). For the NC, 10% of calories were in the form of fat, 20% in the form of protein, and 70% in the form of carbohydrates. The HFHF diet is consisted of 49.0% of calories from fat, 15.4% of calories from protein, and 35.6% of calories from carbohydrate (Shanghai R&S Biotechnology Co., Ltd.).
Glucose tolerance test and insulin tolerance test
For the glucose tolerance test, mice were fasted for 12 h, and then injected intraperitoneally with glucose (1 g/kg) to measure glucose tolerance. Blood glucose was measured using blood samples taken from cut tail tips at baseline and at 30, 60, 90, and 120 min after the injection of glucose. For insulin tolerance tests (ITT), mice were intraperitoneally injected with insulin (0.25 U/kg) in 0.9% NaCl after fasted for 6 h. A tail vein blood sample was taken immediately prior to and 30, 60, 90, and 120 min after the injection for determination of blood glucose. The results were expressed as percentage of basal glucose.
Hyperinsulinemic-euglycemic clamp (HEC)
The HEC study was performed as previously described [35]. In brief, male adult ICR mice were anesthetized intraperitoneally with pentobarbital sodium. A cannula was inserted into the right internal jugular vein, and mice were allowed to recover for 5 days. The cannula was flushed daily with a 0.9% NaCl solution containing 100 IU/ml heparin. For the HEC study, animals were fasted for 15 h. On the morning of the experiments, catheters were flushed with heparinized saline. After a 90-min equilibration period, mice were infused with 4 mIU/kg/min insulin and 25% glucose through a three-way connector. Blood glucose level was measured every 10 min from the tail tip, and glucose infusion rate (GIR) was adjusted to maintain blood glucose at approximately 6.7 mM. The steady state was maintained for at least 30 min, and insulin sensitivity was assessed by glucose infusion rate during the steady state as previously described.
ELISA examination
Serum was harvested from ICR mice at the end of the experiment and stored at −80°C. Insulin levels were measured with a mouse ELISA kit according to the manufacturer’s instructions (Mercodia, Sweden). Adponectin, lepetin and TNF-αlevels were measured with a mouse ELISA kit according to the manufacturer’s instructions (R&D, US).
Blood pressure and heart rate measurement
Systolic blood pressure (SBP) and heart rate (HR) were measured at the end of research with a non-invasive tail-cuff system (Blood Pressure Analysis System BP- 98AW monitor, Softron Co., Ltd., Tokyo, Japan). In brief, mice were placed in a clear plastic tube with nose cone and tail hole pieces secured at either end. Mice were allowed to warm on a platform with a temperature of 35 for 10–15 min prior to the experiment. Thereafter, measurements were taken on each mouse. All measurements were performed between 06:00 and 12:00 h each day.
Metabolic parameters and lipid profile
Mice body weight was measured at the end of the experimental. Serum was harvested from cut tail and centrifuged immediately at 13,000 g. The supernanant was removed and stored at −80°C. Measurements of plasma glucose, total cholesterol, high density lipoprotein-cholesterol (HDL-C), and low density lipoprotein-cholesterol (LDL-C), triglycerides, free fatty acids (FFA), uric acid (UA) were performed using a Beckman Coulter Synchron CX5 Delta chemistry analyzer (Beckman Coulter, Inc., Diagnostic Division, Brea, CA, USA).
Statistical analysis
Values are expressed as means ± SEM. All groups of animals were studied in parallel. Comparisons between different groups were performed by Student’s t-test for unpaired samples. The level of significance was P<0.05.
Results
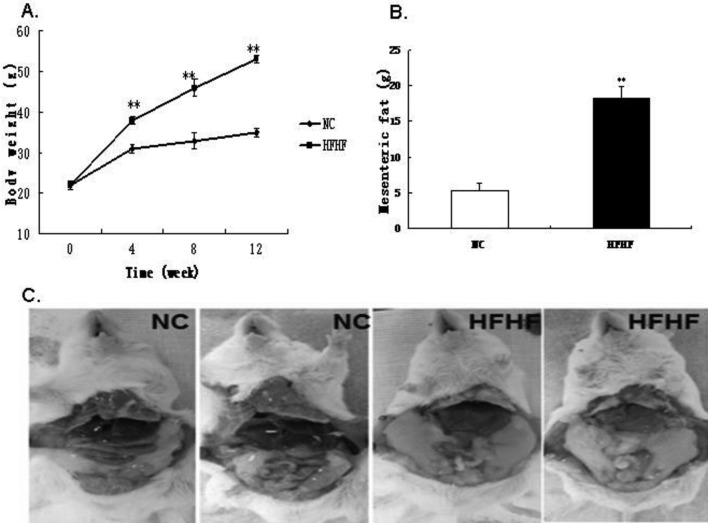
The HFHF diet increases body weight and abdominal adipose tissue mass
We first observed the effect of HFHF diet feeding on body weight. It was shown that, at the end of the experiment, the HFHF fed ICR mice were much heavier than the NC fed mice (P<0.05). Interestingly, this enhancement could be largely attributed to the increase of body adipose tissue mass. As is shown in Fig. 1, the abdominal adipose tissue content was markedly augmented in HFHF diet- fed mice than in NC mice.
Fig. 1.
HFHF diet feeding induces obesity and abdominal adipose tissue depots in ICR mice. A. Fasting body weight of ICR mice during the experimental period. B. Weight of fat mass after 12 weeks feeding. C. Representative mice of NC and HFHF group. Results were expressed as means ± SEM, n=12, **P<0.01 vs. NC group.
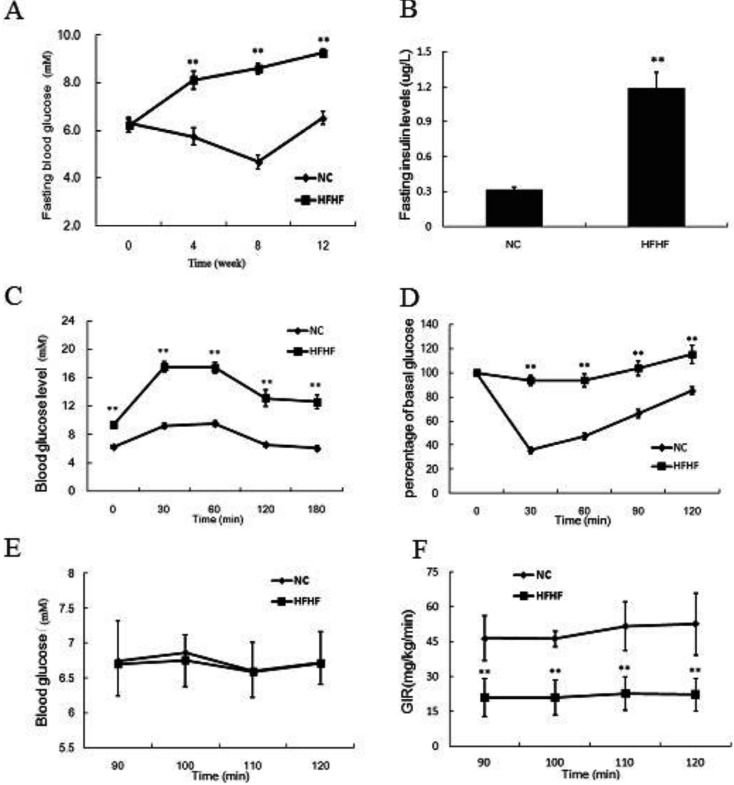
The HFHF diet impairs glucose tolerance and whole body insulin sensitivity
We measured the fasting blood glucose and insulin levels at the end of the experimental. As is shown in Fig. 2, the fasting blood glucose and insulin levels were significantly increased in the HFHF diet-fed mice relative to NC mice (P<0.05). Furthermore, we determined the glucose tolerance and insulin tolerance of two groups of mice. It was demonstrated that the HFHF diet-fed mice exhibited significantly impaired glucose tolerance and insulin tolerance as compared with NC mice (P<0.05). Moreover, in the HEC study, the glucose infusion rate was also much lower in the HFHF diet group, indicating decreased whole body insulin sensitivity.
Fig. 2.
HFHF diet feeding deteriorates glucose tolerance and insulin sensitivity in ICR mice. A. Fasting blood glucose of ICR mice during the experimental period. B. Fasting insulin levels at the end of the experimental. C. Intraperitoneal glucose tolerance test of ICR mice at the end of the experimental. D. Insulin tolerance test of ICR mice at the end of the experimental. E. Blood glucose levels of ICR mice during the steady state of the HEC test. F. Glucose infusion rate of ICR mice during the steady state of the HEC test. Results were expressed as means ± SEM, n=12, **P<0.01 vs. NC group.
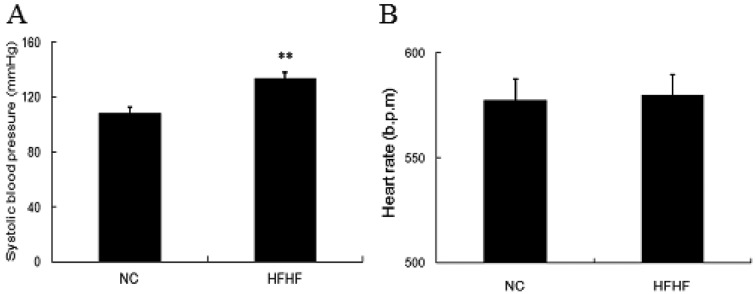
The HFHF diet elevates blood pressure
Previous studies have indicated that high-fructose diet feeding enhances blood pressure in several types of animals [33, 34]. Thus, in our study, we also determined the changes of blood pressure in mice. It was shown that, the HFHF diet mice demonstrated remarkably elevated systolic pressure as compared with the NC mice (Fig. 3), while no changes on heart rate was observed, indicating deleterious effect of HFHF diet on cardiovascular system.
Fig. 3.
HFHF diet feeding elevates systolic blood pressure in ICR mice. Mice were fed with NC or HFHF diet for 12 weeks. At the end of the feeding, mice blood pressure was measured by the tail-cuff method. A. Mice systolic blood pressure. B. Mice heart rate. Results were expressed as means ± SEM, n=12, **P<0.01 vs. NC group.
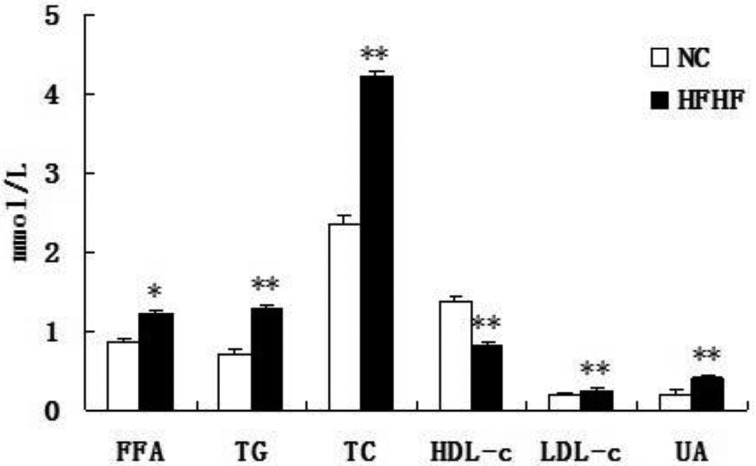
The HFHF diet disturbs lipids and uric acid metabolism
We also analyzed the changes of serum lipids profile and uric acid levels, which have recently been included in the components of MS [11]. It was shown that, the FFA, LDL-C, TG and TC were markedly increased in HFHF mice (P<0.05 or P<0.01), while HDL was decreased in the sample of serum (P<0.01), indicating the disturbance of lipids metabolism (Fig. 4). In addition, the uric acid in the serum was also increased in HFHF group.
Fig. 4.
HFHF diet feeding disturbs lipids and uric acid metabolism in ICR mice. Mice were fed with NC or HFHF diet for 12 weeks. Serum was harvested at the end of the experimental and parameters were determined biochemically. Results were expressed as means ± SEM, n=12, *P<0.05, **P<0.01 vs. NC group.
The effect of HFHF diet on adipokine levels
We also analyzed the changes of plasma adipokine levels, which have recently been demonstrated strongly association with MS. It was shown that, the leptin, and TNF-α were markedly increased in HFHF mice (P<0.05), while adiponectin was decreased in the sample of plasma (P<0.01) (Table 1).
Table 1. Adipokine levels (pg/ml) in 12 week-old NC and HFHF ICR mice.
| NC | HFHF | |
|---|---|---|
| Adiponectin | 9,325 ± 447 | 6,168 ± 439* |
| Leptin | 6,694 ± 2157 | 16,294 ± 4,832* |
| TNF-α | 8.46 ± 0.32 | 11.95 ± 0.61* |
*P<0.05 vs. NC group.
Discussion
With the global prevalence of the western life style, MS is now occurring at epidemic rates and severely threatens human’s health [1, 10, 27]. Although various existing rodent models have been fundamental to advance our knowledge of the basis of obesity, type 2 diabetes, hypertension, and dyslipidemia, we are still in lack of knowledge of MS as a complex disorder [21]. The widespread occurrence of MS urges us to better our understanding of molecular basis and progression of this syndrome, which require reliable and clinically relevant experimental models that develop all major aspects of MS.
In the present study, we successfully established MS model in ICR mice by 12 weeks’ HFHF diet feeding. This model demonstrates most characteristics of the components of human MS. In addition, no chemical destruction of pancreatic beta cell was introduced.
As we known, the western style diet is the root cause and driving forces for the epidemic of type 2 diabetes and MS in western countries [18]. Accordingly, the high calorie feeding has been increasingly used to establish the type 2 diabetes or MS models. Previously, diets high in saturated fats have been shown to induce weight gain, insulin resistance, and hyperlipidemia and hepatic steatosis in humans and animals [2, 37]. A commonly used and relevant model is the high-fat diet fed C57BL/6J mouse that well resembles the MS happened in human [36]. Furthermore, due to a high intake of sucrose and high fructose, the dietary fructose consumption has increased significantly in the western countries [15]. Therefore, the high-fructose feeding has also recently been used as another efficient method to establish MS. The high-fructose fed rats often exhibit hypertension, insulin resistance, impaired glucose tolerance, dislipidemia and obesity et al. [14, 32, 33].
Although high-fat or high-fructose diet induces most of the components of human MS in rodents, they do not resemble the diet causing MS and the relevant complications, as the human diet is much more complex than high-fat or high-fructose diet alone. Interestingly, however, there is an emerging evidence indicating that the combination use of both gradients would has a synergic effect in inducing MS [25].
In our study, we fed ICR mice with a combination of high-fat and high-fructose diet for 12 weeks so as to most closely resemble the pathogenesis of MS happened in human. It was demonstrated that, in comparison with other diabetes and obesity models, this model exhibited nearly all aspects of disorder of MS, including increase in body weight, abdominal fat deposition, along with impaired glucose tolerance, dyslipidemia, hyperinsulinemia, hypertension and hyperuricemia. Thus, we showed in our study that the HFHF diet feeding is a reliable and effective method of establishing MS model in rodents. In fact, the HFHF diet induced MS model has been newly reported in Wistar rats and dogs [5, 12, 22, 25, 34], while no relevant study was performed in mice. Our study testified this protocol in mice for the first time and provided confirming evidences for using this protocol in MS researches.
In addition, most of the previous protocol for diet-induced diabetes or MS model in animals introduced the use of STZ to destroy the beta cells. And low dose-STZ in combination with high-fat diet induction of diabetes model has been used in recent studies [20, 30]. However, according to the 2011 Banting lecture, the initial causative factor of insulin resistance is the hyperinsulinemia, and beta cell deficiency is the consequence, not the cause of diabetes [7]. In this sense, to destroy the beta cell chemically or physically would fail to objectively reflect the procedures undergoing in the development of MS. In our study, we did not use STZ, thus, without impairment of the beta cells. From this point of view, our model more closely resembles the pathophysiology that happened in human MS.
Another advantage of our protocol is the mice we have chosen in our study. Previous studies have indicated that, compared with commonly used C57BL/6J mice, ICR mice are more susceptible to induce diabetes, making it a more optional model for establishing MS [17, 19]. Indeed, in our study, after 12 weeks’ HFHF diet feeding, the ICR mice developed obvious features of MS. The differences between HFHF group and NC group were dramatically significant. To be noted, the main purpose of our study was to testify the possibility of establishing a novel MS model with HFHF diet feeding in ICR mice. Hence, we have not made adequate direct comparisons between our model and other existed models, and these may be done in our future work. Last but not least, the ICR mouse is relative cheaper than the most widely used C57BL/6J mouse, making it more available in our daily research.
In conclusion, we report for the first time that the HFHF diet-fed ICR mice exhibit most of the components of MS, including central obesity, severe insulin resistance, hyperinsulinemia, significant lipid disorder, significant hypertension and hyperuricemia. This method more closely mimics the pathogenesis happened in human MS, and would be an effective and reliable tool for both mechanistic and treatment studies of MS.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the Shanghai Science and Technology Commission (10411956600, 14ZR1427400), National Natural Science Foundation of China (81000332, 81300667, 81370953, 81370935), Shanghai Health System Outstanding Young Talents Training Program (XYQ2013098), Chinesse Society of Endocrinology.
References
- 1.Borch-Johnsen K.2007. The metabolic syndrome in a global perspective. The public health impact—secondary publication. Dan. Med. Bull. 54: 157–159. [PubMed] [Google Scholar]
- 2.Buettner R., Parhofer K.G., Woenckhaus M., Wrede C.E., Kunz-Schughart L.A., Schölmerich J., Bollheimer L.C.2006. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 36: 485–501. doi: 10.1677/jme.1.01909 [DOI] [PubMed] [Google Scholar]
- 3.Chen H., Charlat O., Tartaglia L.A., Woolf E.A., Weng X., Ellis S.J., Lakey N.D., Culpepper J., Moore K.J., Breitbart R.E., Duyk G.M., Tepper R.I., Morgenstern J.P.1996. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495. doi: 10.1016/S0092-8674(00)81294-5 [DOI] [PubMed] [Google Scholar]
- 4.Choi S.I., Lee H.R., Goo J.S., Kim J.E., Nam S.H., Hwang I.S., Lee Y.J., Prak S.H., Lee H.S., Lee J.S., Jang I.S., Son H.J., Hwang D.Y.2011. Effects of steaming time and frequency for manufactured red liriope platyphylla on the insulin secretion ability and insulin receptor signaling pathway. Lab. Anim. Res. 27: 117–126. doi: 10.5625/lar.2011.27.2.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coate K.C., Kraft G., Lautz M., Smith M., Neal D.W., Cherrington A.D.2011. A high-fat, high-fructose diet accelerates nutrient absorption and impairs net hepatic glucose uptake in response to a mixed meal in partially pancreatectomized dogs. J. Nutr. 141: 1643–1651. doi: 10.3945/jn.111.145359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coate K.C., Scott M., Farmer B., Moore M.C., Smith M., Roop J., Neal D.W., Williams P., Cherrington A.D.2010. Chronic consumption of a high-fat/high-fructose diet renders the liver incapable of net hepatic glucose uptake. Am. J. Physiol. Endocrinol. Metab. 299: E887–E898. doi: 10.1152/ajpendo.00372.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corkey B.E.2012. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 61: 4–13. doi: 10.2337/db11-1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekel Y., Glucksam Y., Elron-Gross I., Margalit R.2009. Insights into modeling streptozotocin-induced diabetes in ICR mice. Lab. Anim. (NY) 38: 55–60. doi: 10.1038/laban0209-55 [DOI] [PubMed] [Google Scholar]
- 9.Dubuc P.U.1976. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism 25: 1567–1574. doi: 10.1016/0026-0495(76)90109-8 [DOI] [PubMed] [Google Scholar]
- 10.Ford E.S.2005. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 28: 2745–2749. [DOI] [PubMed] [Google Scholar]
- 11.Ford E.S., Li C., Cook S., Choi H.K.2007. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 115: 2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627 [DOI] [PubMed] [Google Scholar]
- 12.Kohli R., Kirby M., Xanthakos S.A., Softic S., Feldstein A.E., Saxena V., Tang P.H., Miles L., Miles M.V., Balistreri W.F., Woods S.C., Seeley R.J.2010. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 52: 934–944. doi: 10.1002/hep.23797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz T.W., Morris R.C., Pershadsingh H.A.1989. The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension 13: 896–901. doi: 10.1161/01.HYP.13.6.896 [DOI] [PubMed] [Google Scholar]
- 14.Lê K.A., Tappy L.2006. Metabolic effects of fructose. Curr. Opin. Clin. Nutr. Metab. Care 9: 469–475. doi: 10.1097/01.mco.0000232910.61612.4d [DOI] [PubMed] [Google Scholar]
- 15.Lim J.S., Mietus-Snyder M., Valente A., Schwarz J.M., Lustig R.H.2010. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 7: 251–264. doi: 10.1038/nrgastro.2010.41 [DOI] [PubMed] [Google Scholar]
- 16.Lloyd D.J., McCormick J., Helmering J., Kim K.W., Wang M., Fordstrom P., Kaufman S.A., Lindberg R.A., Véniant M.M.2008. Generation and characterization of two novel mouse models exhibiting the phenotypes of the metabolic syndrome: Apob48-/-Lepob/ob mice devoid of ApoE or Ldlr. Am. J. Physiol. Endocrinol. Metab. 294: E496–E505. doi: 10.1152/ajpendo.00509.2007 [DOI] [PubMed] [Google Scholar]
- 17.Luo J., Quan J., Tsai J., Hobensack C.K., Sullivan C., Hector R., Reaven G.M.1998. Nongenetic mouse models of non-insulin-dependent diabetes mellitus. Metabolism 47: 663–668. doi: 10.1016/S0026-0495(98)90027-0 [DOI] [PubMed] [Google Scholar]
- 18.Massiera F., Barbry P., Guesnet P., Joly A., Luquet S., Moreilhon-Brest C., Mohsen-Kanson T., Amri E.Z., Ailhaud G.2010. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J. Lipid Res. 51: 2352–2361. doi: 10.1194/jlr.M006866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuo T., Shino A.1972. Induction of diabetic alterations by goldthioglucose-obesity in KK,ICR and C57BL mice. Diabetologia 8: 391–397. doi: 10.1007/BF01212165 [DOI] [PubMed] [Google Scholar]
- 20.Ménard S.L., Croteau E., Sarrhini O., Gélinas R., Brassard P., Ouellet R., Bentourkia M., van Lier J.E., Des Rosiers C., Lecomte R., Carpentier A.C.2010. Abnormal in vivo myocardial energy substrate uptake in diet-induced type 2 diabetic cardiomyopathy in rats. Am. J. Physiol. Endocrinol. Metab. 298: E1049–1057. [DOI] [PubMed] [Google Scholar]
- 21.Olufadi R., Byrne C.D.2008. Clinical and laboratory diagnosis of the metabolic syndrome. J. Clin. Pathol. 61: 697–706. doi: 10.1136/jcp.2007.048363 [DOI] [PubMed] [Google Scholar]
- 22.Panchal S.K., Poudyal H., Iyer A., Nazer R., Alam M.A., Diwan V., Kauter K., Sernia C., Campbell F., Ward L., Gobe G., Fenning A., Brown L.2011. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 57: 611–624. doi: 10.1097/FJC.0b013e3181feb90a [DOI] [PubMed] [Google Scholar]
- 23.Park S.H., Ko S.K., Choi J.G., Chung S.H.2006. Salicornia herbacea prevents high fat diet-induced hyperglycemia and hyperlipidemia in ICR mice. Arch. Pharm. Res. 29: 256–264. doi: 10.1007/BF02969402 [DOI] [PubMed] [Google Scholar]
- 24.Park S.H., Ko S.K., Chung S.H.2005. Euonymus alatus prevents the hyperglycemia and hyperlipidemia induced by high-fat diet in ICR mice. J. Ethnopharmacol. 102: 326–335. doi: 10.1016/j.jep.2005.06.041 [DOI] [PubMed] [Google Scholar]
- 25.Poudyal H., Panchal S., Brown L.2010. Comparison of purple carrot juice and β-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br. J. Nutr. 104: 1322–1332. doi: 10.1017/S0007114510002308 [DOI] [PubMed] [Google Scholar]
- 26.Reed M.J., Meszaros K., Entes L.J., Claypool M.D., Pinkett J.G., Gadbois T.M., Reaven G.M.2000. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49: 1390–1394. doi: 10.1053/meta.2000.17721 [DOI] [PubMed] [Google Scholar]
- 27.Schwarz P.E., Reimann M., Li J., Bergmann A., Licinio J., Wong M.L., Bornstein S.R.2007. The Metabolic Syndrome - a global challenge for prevention. Horm. Metab. Res. 39: 777–780. doi: 10.1055/s-2007-990312 [DOI] [PubMed] [Google Scholar]
- 28.Serradas P., Gangnerau M.N., Giroix M.H., Saulnier C., Portha B.1998. Impaired pancreatic beta cell function in the fetal GK rat. Impact of diabetic inheritance. J. Clin. Invest. 101: 899–904. doi: 10.1172/JCI368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shima K., Zhu M., Mizuno A.1999. Pathoetiology and prevention of NIDDM lessons from the OLETF rat. J. Med. Invest. 46: 121–129. [PubMed] [Google Scholar]
- 30.Srinivasan K., Viswanad B., Asrat L., Kaul C.L., Ramarao P.2005. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 52: 313–320. doi: 10.1016/j.phrs.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 31.Tahara A., Matsuyama-Yokono A., Shibasaki M.2011. Effects of antidiabetic drugs in high-fat diet and streptozotocin-nicotinamide-induced type 2 diabetic mice. Eur. J. Pharmacol. 655: 108–116. doi: 10.1016/j.ejphar.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 32.Tappy L., Lê K.A.2010. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 90: 23–46. doi: 10.1152/physrev.00019.2009 [DOI] [PubMed] [Google Scholar]
- 33.Tran L.T., Yuen V.G., McNeill J.H.2009. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 332: 145–159. doi: 10.1007/s11010-009-0184-4 [DOI] [PubMed] [Google Scholar]
- 34.Wada T., Kenmochi H., Miyashita Y., Sasaki M., Ojima M., Sasahara M., Koya D., Tsuneki H., Sasaoka T.2010. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology 151: 2040–2049. doi: 10.1210/en.2009-0869 [DOI] [PubMed] [Google Scholar]
- 35.Wang Z., Xue L., Guo C., Han B., Pan C., Zhao S., Song H., Ma Q.2012. Stevioside ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by downregulating the NF-κB pathway. Biochem. Biophys. Res. Commun. 417: 1280–1285. doi: 10.1016/j.bbrc.2011.12.130 [DOI] [PubMed] [Google Scholar]
- 36.Winzell M.S., Ahrén B.2004. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53: S215–S219. doi: 10.2337/diabetes.53.suppl_3.S215 [DOI] [PubMed] [Google Scholar]
- 37.Woods S.C., Seeley R.J., Rushing P.A., D’Alessio D., Tso P.2003. A controlled high-fat diet induces an obese syndrome in rats. J. Nutr. 133: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 38.Yun S.N., Ko S.K., Lee K.H., Chung S.H.2007. Vinegar-processed ginseng radix improves metabolic syndrome induced by a high fat diet in ICR mice. Arch. Pharm. Res. 30: 587–595. doi: 10.1007/BF02977653 [DOI] [PubMed] [Google Scholar]