Abstract
Background
Paediatric respiratory tract infections are among the most common reasons for preschool and school absences and visits to physicians. The disease mainly involves the upper respiratory tract and is associated with fever, cough, sore throat, and running nose. Children with recurrent respiratory infections (RRI), which are defined as more than six serious diseases a year, are a difficult diagnostic challenge. The aim of this study was to assess immunological deviations in laboratory tests performed in children with RRI.
Material and methods
In the retrospective study 25 children suffering from recurrent respiratory tract infection, aged 4.1 ±2.3 years, 13 boys and 12 girls, were involved. For all children chemiluminescence of granulocytes and immunophenotyping of lymphocytes from peripheral blood were examined. An immunophenotype of peripheral blood lymphocytes involved evaluation of T cell, B cells, and NK cells, examined with flow cytometry.
Results
Eleven of the studied children had decreased chemiluminescent response to stimulants, normal response was found for nine children, and five children had an increased result of the test. Five of the 25 children had decreased B cells number, and five had decreased number of T cells including decrease of CD4, as well as CD8 positive cells. Children with decreased chemiluminescence had more frequent neutropaenia than children with normal or increased chemiluminescent response, p < 0.05 (exact Fisher test).
Conclusions
Recurrent respiratory tract infection could be associated with improper neutrophils response to pathogens, and immunological examination should be performed to find the reason for the increased number of infections in a year.
Keywords: chemiluminescence, immunological disturbances, immunophenotyping, recurrent respiratory tract infection
Introduction
Frequent respiratory tract infections in children are among the most common causes of preschool and school absences and visits to physicians. The disease mainly involves the upper respiratory tract and is associated with fever, cough, sore throat, and rhinitis. More than six serious diseases a year are defined as recurrent respiratory tract infections (RRI). Epidemiologists estimate that ca. 15% of children suffer from RRI, which could be related to several factors that can act alone or together [1]. Among the predisposing factors immune system deficiencies can be considered as well as anatomic and functional alteration in the respiratory tract, air pollution exposure, or poor social conditions. During the development of infections many different immunologic disturbances can occur, hence they are a difficult diagnostic challenge. Nevertheless, early and accurate diagnosis is essential to ensure that the appropriate treatment is given and to minimise irreversible changes [2].
Immunodeficiency may be due to defects of B-cells, T-cells, NK cells, complement system, or phagocytes [3]. In light of the current knowledge about the complexity of the immune system a wide range of laboratory tests can be performed to identify such disorders.
Chemiluminescence of granulocytes is a method for the estimation of their overall activity as measured by the production of reactive oxygen species. The chemiluminescence test is recommended by the World Health Organisation to assess non-specific immunity [4]. Due to various stimuli granulocytes produce free oxygen radicals, which react with luminol, resulting in light emission (chemiluminescence). It could be inducted by chemotactic agonist (fLMP – Formyl-Methionyl-Leucyl-Phenylalanine), antibodies, and complement Fc-fragment receptor (opsonized zymosan – OZ) or in a non-receptor way (PMA – Phorbol 12-myristate 13-acetate) [5, 6]. It is a test performed to evaluate the ability of granulocytes to produce reactive oxygen species (ROS), which are responsible for the bactericidal properties of phagocytes. Dysfunction of granulocytes can be connected with their decreased level or impaired function of phagocytosis [7]. The disruption of only one of the functions of these cells can lead to severe recurrent infections, including RRI [8].
Another point worth mentioning is the fact that if there is suspicion of an immunodeficiency, one of the first tests prescribed is blood lymphocyte immunophenotyping [9–11]. Peripheral blood T and B lymphocyte count is used especially for the diagnosis of primary immune deficiency (PID).
Approximately 50-60% of all identified PIDs are caused by defects in antibody production, whereas T-cell disorders account for 9% of primary immunodeficiency diseases [12]. T cells are essential for cell-mediated immunity that is critical to the control of intracellular pathogens, viruses, and opportunistic infections. CD4+ T-cell activation of phagocytes enables them to clear intracellular pathogens, fungi, and protozoa, while CD8+ T cells are essential to control viral infections [13]. T-cell defects may result in serious and frequent infections of the respiratory system or skin. In addition T-cells are important to the normal functioning of B-cells. Consequently most T-cell disorders lead to a combined T-cell and B-cell disorder.
Unexplained lymphocytopaenia is often ignored, but it may be an important clue suggesting immunodeficiency, and should prompt investigation of the underlying cause [14]. Furthermore, pan-lymphocytopaenia implies Severe Combined Immune Deficiency (SCID), but selective deficiencies in one or other subset (e.g. selective CD4 T-cell deficiency) can be masked within a normal total lymphocyte count [15]. That is why immunophenotyping is useful in SCID diagnosis.
The lymphocytic subpopulations can be detected and quantified by flow cytometry, using monoclonal antibodies bound with fluorochromes.
The aim of this study was to assess immunological deviations in laboratory tests – neutrophil chemiluminescence and peripheral blood lymphocyte immunophenotyping – performed in children with RRI.
Material and methods
In the retrospective study 25 children suffering from recurrent respiratory tract infection, aged 4.1 ±2.3 years, 13 boys and 12 girls, were involved. For all children chemiluminescence of granulocytes and immunophenotyping of lymphocytes from peripheral blood were examined.
Patients with RRI had at least six serious diseases in a year. The diagnosis was based on clinical evaluation and the patient's history.
Chemiluminescence
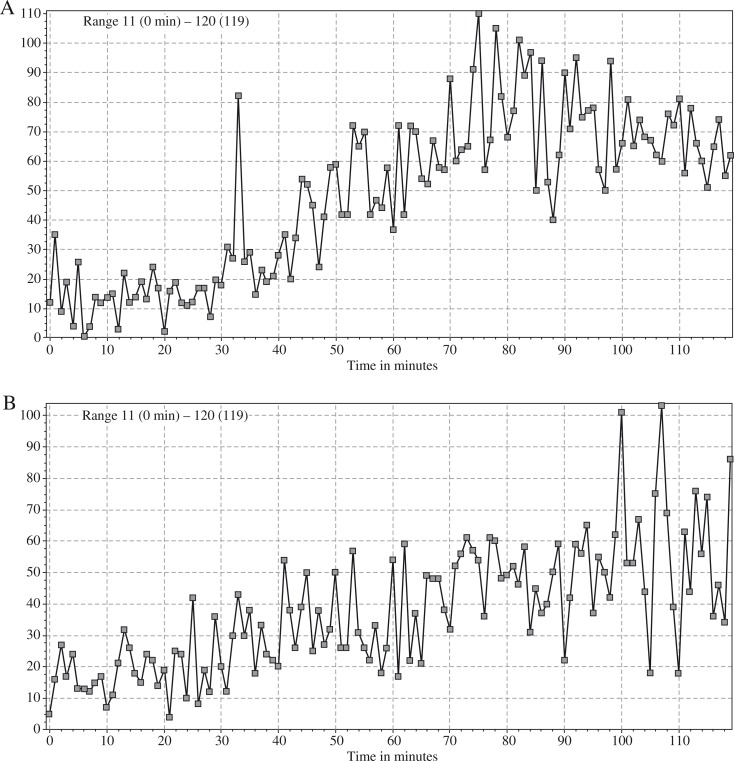
Neutrophil luminol-dependent chemiluminescence was assessed after stimulation with 0.9% NaCl (saline), fMLP (10–3 M), OZ (20 μg/ml), and PMA (1 μg/ml). Briefly, 200 μl of Hanks medium and 50 μl of peripheral blood collected in a tube containing sodium heparin was added to each of four wells of a 12-well reaction plate. Then 20 μl of appropriate reagent (saline, fMLP, OZ, or PMA) was added to the blood. Next, 200 μl of luminol in a concentration of 10–5 M was added to each well. After five minutes preincubation the plate was placed in a Fluorostar Omega (BMG, LabTech) for 120 minutes. The dynamics of chemiluminescence was measured with a luminometer. The maximal luminescence signal reported between 61-120 minutes of the test for each stimulus was chosen for the interpretation of the test result. Final results were shown as a chemiluminescence index (spontaneous vs. stimulated) and compared with values obtained in a healthy reference group of children of corresponding age. An example of a chemiluminescent assay result is presented in Fig. 1.
Fig. 1.
Kinetics of neutrophil chemiluminescence measured by luminol fluorescence evaluations after stimulation with fMLP A) and PMA B). Reaction time – 120 minutes, maximal fluorescence was used to describe chemiluminescent reactivity of neutrophils
Immunophenotyping
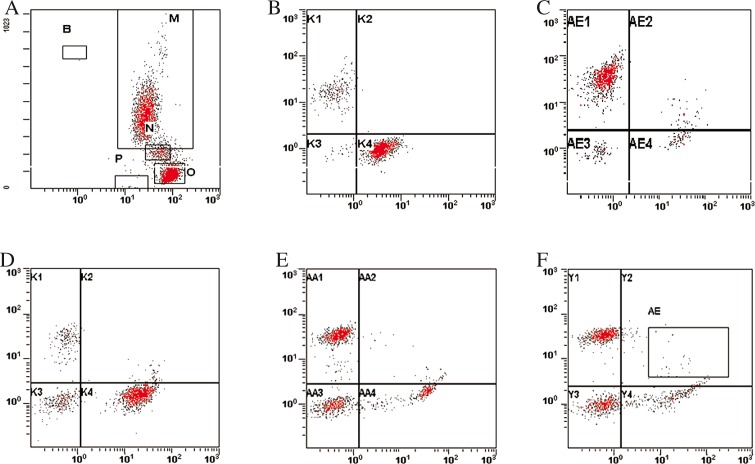
Peripheral blood samples collected in tubes containing K2EDTA were processed for flow cytometry by a stainlyse method using OptiLyse C Non-wash Lysing Solution (Beckman Coulter). Each case was assessed with antibodies directed against the following antigens: CD2, CD3, CD4, CD5, CD8, CD19, CD20, CD16_56, HLA-DR, and CD45 (all from Beckman Coulter). Monoclonal antibodies were conjugated with PE, FITC, or PC5. Stainings were performed in three-colour combinations using CD45-PC5 in each tube. Isotype-matched controls were performed for every analysis for evaluation of possible nonspecific staining and autofluorescence. Briefly, to each cytometric tube 50 μL of peripheral blood and 15 μl of specific monoclonal antibodies were added. After 20 minutes of incubation in the dark, at room temperature, erythrocytes were lysed with lysing solution for 15 minutes and then incubated with saline for 15 minutes in the dark, at room temperature. Acquisition and analysis of flow cytometry data were performed using a Cytomics FC500 (Beckman Coulter). An example of lymphocyte immunophenotyping analysis is presented in Fig. 2.
Fig. 2.
Immunophenotyping of peripheral blood lymphocyte subpopulations. A) SS versus Cd45log. B) Dot-plot of CD19 versus CD2. C) Dot plot of CD5 versus CD20. D) Dot plot of CD 16/56 versus CD3. E) Dot plot CD 4 versus CD8. F) Dot plot CD4 versus HLA-DR
Statistical analysis
The Fisher exact test was performed to assess if there are non-random associations between:
chemiluminescent response and changes in lymphocytes subpopulations,
chemiluminescent response and more frequent neutropaenia.
A probability of less than or equal to 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism software.
Results
In the studied group different changes were found. Most children presented impairment in neutrophil chemiluminescent activity. Changes in lymphocyte subpopulations were observed in less than 50% of enrolled children. Table 1 presents the numbers of children for whom appropriate results were obtained.
Table 1.
The number of children for whom decreased, increased, or normal ranges of the studied parameters were observed
| Decreased values | Values in reference range | Increased values | |
|---|---|---|---|
| Chemiluminescence | 11 | 9 | 5 |
| T cells number | 7 | 18 | 0 |
| CD4+ T cell number | 6 | 14 | 5 |
| CD8+ T cell number | 4 | 17 | 4 |
| CD19+ B cell number | 5 | 20 | 0 |
| NK cell number | 0 | 21 | 4 |
There was no significant association between chemiluminescent response of neutrophils and changes in lymphocyte subpopulations (exact Fisher test) (Table 2 and Table 3)
Table 2.
An exact Fisher test for the association between the number of CD8+ cells in peripheral blood and chemiluminescence index, p > 0.05
| Decreased CD8+ cells number | Increased CD8+ cells number | |
|---|---|---|
| Low chemiluminescence index | 2 | 2 |
| High chemiluminescence index | 2 | 2 |
Table 3.
An exact Fisher test for the association between the number of CD4+ cells in peripheral blood and chemiluminescence index, p > 0.05
| Decreased CD4+ cells number | Increase of CD4+ cells number | |
|---|---|---|
| Low chemiluminescence index | 5 | 3 |
| High chemiluminescence index | 1 | 2 |
Children with decreased chemiluminescence had more frequent neutropaenia than children with normal or increased chemiluminescent response, p < 0.05 (exact Fisher test) (Table 4).
Table 4.
An exact Fisher test for the association between the number of neutrophils in peripheral blood and chemiluminescence index, p = 0.033654. The result is significant at p < 0.05
| Decreased neutrophils number | Normal neutrophils number | |
|---|---|---|
| Low chemiluminescence index | 7 | 4 |
| High chemiluminescence index | 0 | 5 |
Discussion
In the present study the immunological deviations in children with RRI were evaluated. The most frequent abnormalities were connected with improper absolute count of CD4- and CD8-positive cells, B lymphocytes, and neutrophils. Improper neutrophil response to stimulation in chemiluminescence assay was also noticed.
RRI could be associated with improper neutrophil response to pathogens. Granulocytes play a key role in inducting the nonspecific immune response and protecting the organism against pathogenic bacteria. The first step in the diagnosis of such disorders should be an absolute neutrophil count and morphological analysis of neutrophils. Disorders that can manifest with RRI are leukocyte adhesion deficiency, chronic granulomatous disease, glucose- 6-phosphate dehydrogenase deficiency, myeloperoxidase deficiency, and hyper IgE syndrome [16, 17].
The chemiluminescence assay examines the ability of granulocytes to produce ROS, which regulates the immune response and helps in the destruction of pathogens. Improper immune response may be a result of decreased values of ROS and may manifest as RRI. Neutrophils can occur in a resting, pre-activated, activated, and exhausted physiological stage [18]. The latter stage may result in reduced response to stimulation in chemiluminescence assay. Functional exhaustion was observed in patients suffering from RRI [19–21]. Many studies confirm that recurrent infections can be promoted by reduced values of neutrophils [19–24]. On the other hand, suppressed chemiluminescence can be influenced by other codominant diseases such as rheumatoid arthritis [25] or diabetes [26]. Our study shows that almost 50% of RRI children have decreased neutrophil activity, measured by chemiluminescence. In a corresponding study including 90 RRI subjects, decreased neutrophil action measured by phagocytosis assay (FAG) were found in 15.5% of children. Their results are in line with ours because no association was found between defects in lymphocyte subpopulation pathologies and function of neutrophils [27]. Dan et al. found that children with RRI obtained lower values for FAG activity and lower numbers of CD4, CD8, CD19, and NK cells than healthy children. We compared the mentioned results to reference values for age and gender and found no significant variance between normal ranges and results from enrolled subjects [27].
RRI should also be a warning sign of primary immunodeficiency. It is a characteristic symptom of common variable immunodeficiency (CVID) – a disease with a heterogeneous phenotype [28]. Many studies state that the number of B cells is greatly reduced in patients suffering from CVID [29–32]. A disturbance in B lymphocytes may result in reduction of immunoglobulin concentration. The measurement of B cell numbers by flow cytometry and examination of serum immunoglobulin concentration are the gold standard in CVID diagnosis [31, 33, 34]. Defects in B cells can be found at different levels during maturation to immunoglobulin-secreting plasmablasts. What is more, the capacity of memory B cells to differentiate into antibody secreting cells is diminished. Both pathologies occur with great heterogeneity [28]. However, some test modalities exist, which may evaluate for B lymphocyte signalling defects and problems with immunoglobulin synthesis [11]. Siebert et al. studied B cell numbers in a group of children with recurrent lower respiratory infection, comparing them with healthy individuals in the same age-ranged group. They found a significant increase of immunoglobulin synthesised cells numbers in RRI children; however, the median values of B cells in both groups – RRI and healthy – were still in the reference ranges for age and gender [35]. In contrast, we did not find any significant disturbances in B cell numbers; only 25% of enrolled children had decreased CD19 positive cell numbers, and there were no subjects with increased B cells.
RRI are not only connected with abnormalities of neutrophils and B cells. A defective or reduced number of T cells can lead to frequent infections, which can include opportunistic pathogens, which are more challenging to treat [13]. Severe combined immune deficits present a real diagnostic challenge. The most severe of these is SCID, which exhibits reduced T cell and B cell numbers or function [36].
Immunophenotyping is a useful tool in the diagnosis of combined immunodeficiency. It helps in the diagnosis of different abnormalities associated with various clinical phenotypes of B/T/NK cells, especially in SCID [16]. In addition, this technology for cell enumeration can help in the diagnosis of rare immune defects such as Wiskott – Aldrich Syndrome, DiGeorge Syndrom, or Major Histocompability Complex Class II Deficiency [16]. The lymphocyte subpopulation counts should be compared to age adjusted values [37].
Over a ten-year period (2003-2012) the Immunology Quality Assessment (IQA) Program was performed with the goal of assessing the proficiency in CD3+ 4+ and CD3+ 8+ lymphocyte subset immunophenotyping. An overall improvement of performance with greater precision and accuracy was observed. What is more, the absolute count of cells is more reliable than percentage values of variables, and this parameter should be the basis for possible diagnosis [38].
It should be pointed that immunity reaches its greatest efficacy during the fifth or sixth years of age [2]. Hence, many children with RRI do not have immunodeficiency. The cause of RRI may be the childhood itself. However RRI should be a warning sign for a physician. It is essential to recognise or exclude disorders connected with immunodeficiency. An unrecognised primary immunodeficiency may cause end-organ damage, such as hearing injury. Early immunological assessment allows the introduction of effective treatment – in B cell deficiency it would be immunoglobulin replacement; in T cell or granulocytes defects it would be antibiotic or antiviral prophylaxis. The wide availability of flow cytometry analysis of peripheral blood lymphocytes and tests for neutrophil activity should be of interest to paediatricians, and knowledge about such tests interpretation should be especially common among primary care physicians [39].
The authors declare no conflict of interest.
References
- 1.Kilic SS. Recent Advances in Pediatrics. New Delhi: Jaypee Brothers Medical Publishers; 2004. Recurrent respiratory tract infection; pp. 1–18. [Google Scholar]
- 2.Jenesak M, Ciljakova M, Rennerova Z, et al. Recurrent Respiratory Infections in Children – Definition, Diagnostic Approach, Treatment and Prevention. In: Martin-Loeches I, editor. Bronchitis. InTech; 2011. pp. 119–148. [Google Scholar]
- 3.Stokłosa T. Niedobory odporności. In: Gołąb J, Jakóbisiak M, Lasek W, Stokłosa T, editors. Immunologia. Warsaw: PWN; 2007. pp. 398–426. [Google Scholar]
- 4.Raport of WHO Scientific Group. Primary immunodeficiency diseases. Clin Exp Immunol. 1997;109:26–28. [PubMed] [Google Scholar]
- 5.Kowalska M, Kowalska H, Zawadzka-Głos L, et al. Dysfunction of peripheral blood granulocyte oxidative metabolism in children with upper recurrent respiratory tract infections. Int J Pediatr Othorhinolaryngol. 2003;67:365–371. doi: 10.1016/s0165-5876(02)00402-0. [DOI] [PubMed] [Google Scholar]
- 6.Rybczyńska J, Wąsik M. Chemiluminescencja – emisja światła w fagocytach. Zasada I przydatność kliniczna. Pediatr Pol. 2001;76:283–287. [Google Scholar]
- 7.Sasson Y, Zeltser D, Rogowski O, et al. Neutrophilia of infection/inflammation: are we really dealing with “inflamed” leukocytes? J Med. 1998;29:217–229. [PubMed] [Google Scholar]
- 8.Ottonello L, Dapino P, Pastorino G, et al. Neutrophil dysfunction and increased susceptibility to infection. Eur J Clin Inv. 1995;25:687–692. doi: 10.1111/j.1365-2362.1995.tb01987.x. [DOI] [PubMed] [Google Scholar]
- 9.de Vries E. Patient-centred screening for primary immunodeficiency: a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012;167:108–119. doi: 10.1111/j.1365-2249.2011.04461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Notarangelo LD, Fischer A, Geha RS, et al. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124:1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125:297–305. doi: 10.1016/j.jaci.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gathmann B, Grimbacher B, Beauté J, et al. The European Internet-based patient and research database for primary immunodeficiencies: results 2006-2008. Clin Exp Immunol. 2009;157:3–11. doi: 10.1111/j.1365-2249.2009.03954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folds JD, Schmitz JL. Clinical and laboratory assessment of immunity. J Allergy Clin Immunol. 2003;111:702–711. doi: 10.1067/mai.2003.122. [DOI] [PubMed] [Google Scholar]
- 14.Krishna MT, Tarrant JL, Cheadle EA. An audit of lymphopenia in infants under 3 months of age. Arch Dis Child. 2008;93:90–91. doi: 10.1136/adc.2007.123497. [DOI] [PubMed] [Google Scholar]
- 15.Bright PD, Rooney N, Virgo PF, et al. Laboratory clues to immunodeficiency; missed chances for early diagnosis? J Clin Pathol. 2015;68:1–5. doi: 10.1136/jclinpath-2014-202618. [DOI] [PubMed] [Google Scholar]
- 16.Locke BA, Trivikram D, Verbsky JW. Laboratory Diagnosis of Primary Immunodeficiencies. Clin Rev Allergy Immunol. 2014;46:154–168. doi: 10.1007/s12016-014-8412-4. [DOI] [PubMed] [Google Scholar]
- 17.Grimbacher B, Holland SM, Gallin JI, et al. Hyper IgE syndrome with recurrent respiratory tract infectious – an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 18.Zgilczyński JM, Kwasnowska E, Selmaszyńska T, et al. Functional states of neutrophils as suggested by whole blood chemiluminescence. Acta Biochim Pol. 1988;35:331–342. [PubMed] [Google Scholar]
- 19.Lewandowicz-Uszyńska A, Jankowski A. Chemiluminescence of neutrophils stimulated by opsonized Zymosan in children with asthma bronchiale and pneumonia. Proc SPIE. 2004;5566:165–170. [Google Scholar]
- 20.Lewandowicz-Uszyńska A, Jankowski A. Application of neutrophils chemiluminescence test in the different diagnosis of asthma and recurrent respiratory tract infectious the remission period in children. Proc SPIE. 2001;91:4515. [Google Scholar]
- 21.Zaburska-Jabłońska K, Broniek A. Disturbances of polymorphonuceal neutrophil leukocyte chemiluminescence in patients with chronic or recurrent respiratory tract infectious. Pneumonol Alergol Pol. 1997;65:649–656. [PubMed] [Google Scholar]
- 22.Sikora JP. Chemiluminescent assesment of aerobic metabolism of peripheral blood polymorphonuclear leukocytes in children with increased susceptibility to respiratory tract infectious. Centr Eur J Immunol. 1996;21:33–38. [Google Scholar]
- 23.Takeushi S, Sawaki M, Mikasa K, et al. Determination of neutrophil function in respiratory infection by chemiluminescence. II: Changes in neutrophil's CL by chemotherapy against chronic lower tract infection. Kansenshogaku Zasshi. 1994;68:595–600. doi: 10.11150/kansenshogakuzasshi1970.68.595. [DOI] [PubMed] [Google Scholar]
- 24.Lewandowicz-Uszyńska A. The influence of selected stimulators on chemiluminescence of neutrophils in whole blood in children with bronchial asthma. Pol Merkuriusz Lek. 2003;28:393–396. [PubMed] [Google Scholar]
- 25.Crocker IP, Baker PN, Fletcher J. Neutrophil function in pregnancy and rheumatoid arthritis. Ann Rheum Dis. 2000;59:555–564. doi: 10.1136/ard.59.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaczmarek M, Lewandowicz-Uszyńska A, Iwanicka Z, et al. Whole blood neutrophil chemiluminescence in children with diabetes depending on their clinical condition. Centr Eur J Immunol. 2012;37:345–349. [Google Scholar]
- 27.Don M, Fasoli L, Gregorutti V, Pisa F, Valent F, Prodan M, Canciani M. Recurrent respiratory infections and phagocytosis in childhood. Pediatr Int. 2007;49:40–47. doi: 10.1111/j.1442-200X.2007.02296.x. [DOI] [PubMed] [Google Scholar]
- 28.Rosel A, Scheibenbogen C, Schliesser U, et al. Classification of common variable immunodeficiencies using flow cytometry and a memory B-cell functionality assay. J Allergy Clin Immunol. 2015;135:198–208. doi: 10.1016/j.jaci.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Park MA, Li JT, Hagan JB, et al. Common Variable Immunodeficiency: a new look at an old disease. Lancet. 2008;372:489–502. doi: 10.1016/S0140-6736(08)61199-X. [DOI] [PubMed] [Google Scholar]
- 30.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 32.Berglund LJ, Wong SW, Fulcher DA. B-cell maturation defects in common variable immunodeficiency and association with clinical features. Pathology. 2008;40:288–294. doi: 10.1080/00313020801911470. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham-Rundles C. The many faces of common variable immunodeficiency. Hematology Am Soc Hematol Educ Program. 2012;2012:301–305. doi: 10.1182/asheducation-2012.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warnatz K, Denz A, Drager R, et al. Severe defficiency of switched memory B cells (CD27(+)IgM(–)IgD(–) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogenous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 35.Siebert JN, L'huillier AG, Grillet S, et al. Memory B cell compartment constitution and susceptibility to recurrent lower respiratory tract infections in young children. J Leukoc Biol. 2013;93:951–962. doi: 10.1189/jlb.0312117. [DOI] [PubMed] [Google Scholar]
- 36.Geha RS, Notarangelo LD, Casanova JL, et al. Primary Immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Commitee. J Allergy Clin Immunol. 2007;120:776–794. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1997;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 38.Bainbridge J, Wilkening CL, Rountree W, et al. The Immunology Quality Assessment Proficiency Testing Program for CD3+4+ and CD3+8+ lymphocyte subsets: A ten year review via longitudinal mixed effects modeling. J Immunol Methods. 2014;409:82–90. doi: 10.1016/j.jim.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lear S, Condliffe A. Respiratory infection and primary immune deficiency – what does the general physician need to know? J R Coll Physicians Edinb. 2014;44:149–155. doi: 10.4997/JRCPE.2014.214. [DOI] [PubMed] [Google Scholar]