Abstract
Currently, metastatic pancreatic cancer is associated with disappointing survival outcomes. This is largely due to a rapid progression of the disease and a precipitous deterioration in the health of affected individuals, especially elderly patients who are often unable to tolerate chemotherapy. The aim of this study was to evaluate the efficacy and safety of adoptive immunotherapy using cytokine-induced killer cells (CIK) as a first-line treatment for metastatic pancreatic cancer. Between December 2010 and June 2012 eight patients were enrolled in this study. All participants were elderly, suffering from metastatic pancreatic cancer, and unable to tolerate chemotherapy. All patients in this study received R-CIK therapy only as a first-line treatment. In the eight patients, 1 had complete response (CR), 5 had stable disease (SD) and 2 had progression disease (PD). Therefore, the overall response rate (ORR) was 12.5% (1/8) and the disease control rate (DCR) was 75.0% (6/8 patients). The 1-year survival rate was 37.5%, and the median overall survival time (mOS) was 13.04 months (95% CI: 5.9-20.2). The results indicated that no significant positive or negative predictive factors were identified by univariate analysis. The main adverse effect of R-CIK was fever and the side effect rate was 25.0% (2/8). Adoptive immunotherapy using R-CIK cells showed comparable OS to survival data seen in previous trials assessing conventional chemotherapies in elderly patients and the adverse effect is less pronounced.
Keywords: cytokine-induced killer cells, elderly pancreatic adenocarcinoma, immunotherapy
Introduction
Pancreatic adenocarcinoma is one of the most aggressive malignant cancers known, and the associated mortality rates have remained largely unchanged despite active research into therapies that have been successful against other malignant neoplasia [1]. Despite improvements in imaging and surgery, long-term survival remains poor with a median survival of one year for patients with resected disease, 8-10 months for those with locally advanced, unresectable disease and less than 6 months for patients with metastatic disease [2, 3]. This poor prognosis is attributed mainly to the high incidence of metastasis at the time of presentation, the aggressive course of disease and limited efficacy of available systemic treatments [4]. At present, chemotherapy is still the primary therapeutic option for metastatic pancreatic adenocarcinoma. While some studies have yielded improvements in ORR and progression free survival time (PFS), benefits in OS have been limited. Additionally, the mOS of elderly patients that received chemotherapy was only 5.9-7.3 months [5]. Therefore, the need to explore a new treatment to prolong the mOS of elderly patients is of the utmost importance.
Over the past decade, adoptive immunotherapy has gained more and more attention as a treatment option for many kinds of malignant carcinoma, including metastatic pancreatic adenocarcinoma [6–10]. Recently, CIK therapy has become the most widely used immunotherapeutic method, largely due to the rapid proliferation of this cell type in vitro as well as its strong anti-tumor activity against a broad spectrum of solid tumors. It is well known that the effect of CIK therapy depends on the quality and quantity of the cultured cells. Recently, studies have reported that use of RetroNectin can improve the conglutination, extension, differentiation and proliferation of cells, resulting in more efficient stimulation of T cells [11–14]. In this study, we investigated the clinical effects and safety of RetroNectin treated CIK cells (called R-CIK) in the treatment of elderly patients with metastatic pancreatic carcinoma in the hopes of establishing a new and much needed treatment option.
Material and methods
Patients
Eight patients with metastatic pancreatic adenocarcinoma were enrolled in this study. Selected participants had a minimum age of 65 years and the median age was 71.3 years (with an age range of 65-79 years). The Eastern Cooperative Oncology Group (ECOG) scores were 2 or 3. All selected patients were unable to tolerate chemotherapy because of advanced age and poor performance. The eight patients’ characteristics are detailed in Table 1. The study was approved by the ethics committee at The Affiliated Cancer Hospital of Zhengzhou University, and approved consent forms were signed by all patients. The procedures were in accord with the Helsinki Declaration of 1975.
Table 1.
Eight patients’ characteristics
| Characteristics | |
|---|---|
| Age (year) > 70 ≤ 70 |
median 71.3 years (65-79 years) 4 4 |
| Sex male female |
3 5 |
| ECOG (scores) = 2 = 3 |
6 2 |
| CA-199 U/ml > 1000 ≤ 1000 |
2 6 |
| Cycles of CIK ≥ 6 < 6 |
3 5 |
| Once surgery yes no |
3 5 |
| ALT (U/l) > ULN ≤ ULN |
3 5 |
| AST (U/l) > ULN ≤ ULN |
2 6 |
| γ-GGT (U/l) > ULN ≤ ULN |
2 6 |
| Bilirubin (µmol/l) > ULN ≤ ULN |
3 5 |
| Albumin (g/l) > LLN ≤ LLN |
6 2 |
| PLT (g/l) > LLN 6 ≤ LLN |
7 1 |
| HGB (g/l) > LLN 6 ≤ LLN |
4 4 |
| Calcium (mmol/l) > ULN ≤ ULN |
3 5 |
ECOG – Eastern Cooperative Oncology Group; CA-199 – carbohydrateantigen-199; ALT – alanine transaminase; AST – aspartate transaminase; γ-GGT – γ glutamyl transpeptidase; PLT – platelet; HGB – hemoglobin; ULN – upper limit of normal; LLN – lower limit of normal
Study design and response
In this study, all patients received R-CIK therapy only as a first-line treatment. When disease progression became evident, patients continued to receive R-CIK therapy combined with best supportive care or best supportive care only. During treatment, patients received regular follow-up and were reviewed to evaluate the changes in their condition. The follow-up deadline was August 1, 2013. Response to therapy was evaluated using RECIST 1.1 criteria [15]. According to the criteria, clinical effects were divided into CR, partial response (PR), SD, and PD. When the disease met the criteria for PD, subsequent therapies were applied. OS was calculated from the first R-CIK treatment to death or to the time of re-censoring at the last instance of contact with the patient alive. Evaluation of adverse reactions to the experimental treatment regime was performed using the National Cancer Institute Common Toxicity Criteria 3.0 (NCI-CTC3.0), and adverse reactions were divided into grades 1-4 [16].
Preparation of R-CIK
The method used for R-CIK cell preparation in our study was slightly different from methods described in previous literature [7]. Peripheral blood mononuclear cells (PBMCs) were collected from 50 ml samples of the patient's peripheral blood, coated in with RetroNectin (10 µg/ml) and anti-CD3 antibody (5 µg/ml) for 24 h and then cultured in GT-T551 medium, which contains recombinant human interleukin-2 (rhIL-2, 1000 U/ml), interferon-γ (IFN-γ, 1000 U/ml) and 5% inactive autogeneic plasma. Cells were cultured at 37°C with 5% CO2 for 4 days. Then, the cells were cultured in the fresh IL-2 and 2% inactive autogeneic plasma-containing medium for 5 days. At day 10, R-CIK were harvested and analyzed for phenotype. All products were free of bacterial, mycoplasma, and fungal contamination. By LAL sensitivity review, endotoxin levels of the final preparation were less than 5 EU.
Phenotypic assessment of R-CIK
R-CIK (1 × 106) and PBMC (1 × 106) were harvested and double stained with 10 µl fluorescein isothiocyanate (FITC) CD3 and phycoerythrin (PE) CD4, CD8 and CD56. Samples were incubated at 4°C for 30 min, then washed twice with PBS and re-suspended in 500 ml PBS. Fluorescence was detected by FACS Calibur flow cytometer (BD, USA) and data on 10,000 cells were acquired and analyzed. Propidium Iodide and Annexin V-FITC (BD, USA) measured viability and apoptosis.
Cytotoxicity assessment of R-CIK
The anti-tumor activity of our cultured R-CIK cells was tested in an overnight cytotoxicity assay at the end of cell expansion with K-562 (chronic myeloid leukemia) cell lines used as targets. The cytotoxic activity of cells was investigated in a lactate dehydrogenase (LDH) release assay (CytoTox 96, Promega). This non-radioactive assay is a colorimetric alternative to 51Cr release assay and quantitatively measures LDH that is released upon cell lysis in the same way that 51Cr is released. Every experiment, at each effector cell concentration, was performed in a triplicate set of wells and the mean value was calculated.
Statistical methods
SPSS 17.0 was used to perform the statistical analysis. Data on phenotypic analysis of R-CIK cells and cytotoxicity were analyzed by independent sample t-test. For survival time analysis, the Kaplan-Meier method was used and prognostic factors of survival time analysis were used for the log-rank test and multivariable analysis. For all data, p < 0.05 was considered to indicate a statistically significant difference.
Results
Phenotypic analysis of R-CIK
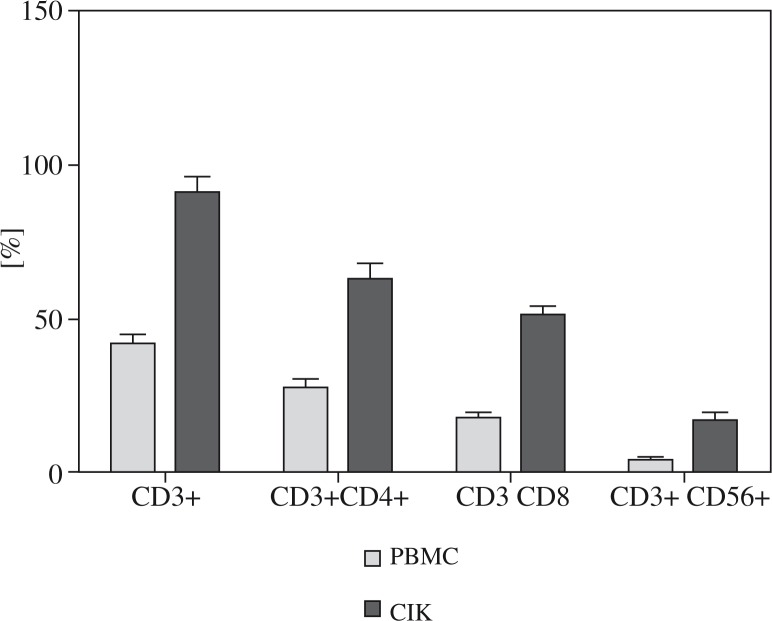
Phenotypic analysis of R-CIK in the patients before culture and after 10 days of culture showed that percentages of CD3+, CD3 + /CD4+, CD3 + /CD8+ and CD3 + / CD56+, increased from 41.89 ±3.19%, 27.92 ±2.14%, 17.96 ±1.79% and 4.09 ±0.62% to 91.32 ±4.98%, 63.03 ±4.99%, 51.22 ±2.98% and 17.03 ±2.78% respectively, with p values < 0.05. The details are shown in Fig. 1.
Fig. 1.
Phenotype of PBMC and CIK
Cytotoxicity of R-CIK in vitro
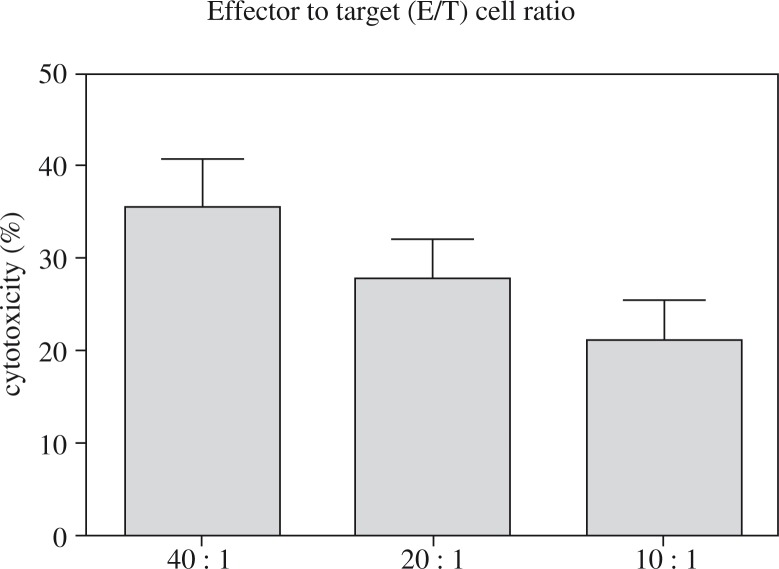
In our center, we regularly detected the cytotoxicity of R-CIK in vitro. A sample of each R-CIK expansion obtained from a total of eight patients was tested for cytotoxicity against K-562, an NK-sensitive leukemia target cell line. All experiments were performed in triplicate and the mean value for each patient is represented by a single dot. At an effector to target (E/T) cell ratio of 40: 1, 20: 1 and 10: 1, the median levels of cytotoxicity were 35.45 ±5.28%, 27.75 ±4.33% and 21.20 ±4.32%, respectively. The details are shown in Fig. 2.
Fig. 2.
CIK cell cytotoxicity for K-562 cell line
Viability and apoptosis of R-CIK in vitro
At day 10, we regularly detected the viability and apoptosis of R-CIK in vitro. The results indicated that the viability rate of R-CIKs was more than 95%, the early apoptotic rate was 7.65 ±1.32% and the late apoptotic rate was 0.69 ±0.27%.
Treatment outcomes
In the eight patients, 1 had CR, 5 had SD and 2 had PD. Therefore, the ORR was 12.5% (1/8) and the DCR was 75.0% (6/8 patients). The 1-year survival rate was 37.5% and the mOS was 13.04 months (95% CI: 5.9-20.2). All patient survival times and status are shown in Table 2.
Table 2.
Eight patients’ survival time and status
| No. | Sex | Age (y) | ECOG | Cycles of CIK | Response | OS (months) |
|---|---|---|---|---|---|---|
| 1 | Male | 66 | 2 | 3 | SD | 5.3 |
| 2 | Male | 65 | 3 | 3 | PD | 5.5 |
| 3 | Female | 67 | 3 | 3 | PD | 5.57 |
| 4 | Female | 76 | 2 | 6 | SD | 7.3 |
| 5 | Female | 75 | 2 | 3 | SD | 9.07 |
| 6 | Female | 79 | 2 | 4 | SD | 23 |
| 7 | Female | 77 | 2 | 30 | CR | 33.53 |
| 8 | Male | 65 | 2 | 12 | SD | 15.07 |
Prognosis factors of R-CIK treatment
In this study, in order to explore the prognosis factors of R-CIK treatment, we used univariate analysis, however, the results indicated that no significant positive or negative predictive factors were identified (Table 3).
Table 3.
Univariate analyses
| OS | χ2 | P-value | |
|---|---|---|---|
| Sex male female |
8.623 15.694 |
1.683 | 0.195 |
| Cycles of CIK ≥ 6 < 6 |
18.633 9.688 |
1.458 | 0.227 |
| Age (years) > 70 ≤ 70 |
18.225 7.860 |
2.969 | 0.085 |
| ECOG (scores) = 2 = 3 |
15.545 5.535 |
3.174 | 0.075 |
| CA-199 (U/ml) > 1000 ≤ 1000 |
6.400 15.257 |
1.745 | 0.187 |
| Once surgery yes no |
20.610 8.502 |
1.774 | 0.183 |
| ALT (U/l) > ULN ≤ ULN |
9.334 19.223 |
2.034 | 0.154 |
| AST (U/l) > ULN ≤ ULN |
10.918 19.415 |
0.560 | 0.454 |
| γ-GGT (U/l) > ULN ≤ ULN |
8.803 17.283 |
0.980 | 0.119 |
| Bilirubin (µmol/l) > ULN ≤ ULN |
8.448 20.700 |
2.427 | 0.322 |
| Albumin (g/l) > LLN ≤ LLN |
20.415 10.585 |
1.208 | 0.272 |
| PLT (/l) > LLN ≤ LLN |
15.070 12.753 |
0.050 | 0.822 |
| HGB (g/l) > LLN ≤ LLN |
13.868 12.218 |
0.268 | 0.604 |
| Calcium (mmol/l) > ULN ≤ ULN |
11.588 15.467 |
0.380 | 0.538 |
ECOG – Eastern Cooperative Oncology Group; CA-199 – carbohydrate antigen-199; ALT – alanine transaminase; AST – aspartate transaminase; γ-GGT – g glutamyl transpeptidase; PLT – platelet; HGB – hemoglobin; ULN – upper limit of normal; LLN – lower limit of normal
Adverse events
In this study, eight patients received R-CIK therapy. None of the enrolled patients failed to complete a full course of immunotherapy or were ruled out because of side effects. No severe adverse effects (grade 3 or grade 4) were associated with R-CIK therapy. The primary side effect of immunotherapy (grade 1 or 2) was fever. The side effect rate was 25.0% (2/8). No other side effects emerged.
Discussion
Metastatic pancreatic cancer represents a considerable challenge to the clinician, particularly when working with an elderly patient population. Elderly patients have a higher rate of concomitant disease, which may lead to contraindications for chemotherapy. Additionally, they are more susceptible to the adverse side effects of chemotherapy. For these reasons, both patients and physicians may hesitate to undertake chemotherapy with cytotoxic drugs for elderly patients. Furthermore, the literature reveals that even the most current chemotherapy achieve mOS of only 5.9-10.4 months in elderly pancreatic cancer patients, a modest gain by any standard [5, 17].
In recent years, adoptive immunotherapy with CIK cells has attracted more and more attention from clinicians. CIK therapy is a realistic new option in the field of cancer immunotherapy, showing strong anti-tumor activity and improving the overall survival time of cancer patients when used alone or combined with other conventional therapies. For example, Wang et al. reported that a regimen of S-1 plus CIK therapy was well tolerated in a second-line setting in patients with gemcitabine-refractory and advanced pancreatic cancer [18]. Chung et al. reported that adoptive immunotherapy using CIK cells showed comparable PFS and OS to survival data of previous trials that assessed conventional chemotherapies while maintaining tolerability and showing encouraging results in terms of patient quality of life (QOL) in gemcitabine-refractory advanced pancreatic cancer [7]. These reports indicate that CIK immunotherapy has potential benefits in advanced pancreatic cancer patients.
As we all know, the efficacy of CIK therapy depends on the quality and quantity of CIK. In our CIK preparation, our method was slightly different from previous literatures [7]. We added RetroNectin (RN) in the culture of CIK preparation (called R-CIK), which could improve the conglutination, extension, differentiation and proliferation of cells [19]. Therefore, through this method we could obtain R-CIK cells with stronger anti-tumor activity and transfer them to patients. In our previous work, one enrolled patient, a 77-year-old female, underwent resection of the pancreatic body and tail as well as the spleen. Analysis of the pancreatic mass revealed poorly differentiated adenocarcinoma, including squamous cell carcinoma differentiation, with positive bilateral margins. One month later, imaging examination revealed that a low-density nodule had emerged at the resection margin, and carbohydrate antigen-199 (CA-199) level had risen to 1,000 U/ml (reference range, 0-35 U/ml). This patient had been reported in our previous work, and was notable for a PFS of more than 19 months [6]. When the patient's disease resumed progression, we continued to apply R-CIK therapy combined with best supportive care. The patient then experienced slow progression of disease which ultimately resulted in an OS of 33.53 months. In our current study, we have continued to apply R-CIK therapy to treat elderly patients with metastatic pancreatic cancer. Encouragingly, in the eight patients, 1 had CR, 5 had SD and 2 had PD. Therefore, the ORR was 12.5% (1/8) and the DCR was 75.0% (6/8 patients). The 1-year survival rate was 37.5% and the mOS was 13.04 months (95% CI: 5.9-20.2). In predictive factor analyses, no significant positive or negative factors were found, indicating that the effects of R-CIK treatment cannot be predicted based on preliminary laboratory tests or imaging reports. And our results indicated that no severe side effects emerged, all the patients could tolerate the side effect. Compared with previous trials in elderly patients, the mOS was obviously prolonged. Although, this study only enrolled eight patients, the patients clearly benefited from R-CIK therapy. This is certainly a cause to conduct further research in the treatment of elderly patients with metastatic pancreatic cancer using R-CIK therapy or R-CIK therapy combined with mono-chemotherapy.
In conclusion, adoptive immunotherapy using R-CIK showed comparable OS to data from previous trials assessing the use of conventional chemotherapy in elderly patients with similar diseases. Because of the poorly tolerated side effects of these conventional therapies, it appears that R-CIK immunotherapy has the potential to fulfill a much needed role in the clinical treatment of this deadly and difficult to treat illness.
Conclusions
Adoptive immunotherapy using R-CIK cells showed comparable OS to survival data seen in previous trials assessing conventional chemotherapies in elderly patients and the adverse effect is less pronounced.
We are grateful for the collaboration received from the participating college and its staff. We are also grateful to Dr. Weiquan Lu from the department of cancer prevention, Henan Cancer hospital, China, for statistical analysis.
The authors declare no conflict of interest.
References
- 1.StatBite. U.S. Pancreatic cancer rates. J Natl Cancer Inst. 2010;102:1822. doi: 10.1093/jnci/djq517. [DOI] [PubMed] [Google Scholar]
- 2.Wolff RA. Chemotherapy for pancreatic cancer: From metastatic disease to adjuvant therapy. Cancer J. 2007;13:175–184. doi: 10.1097/PPO.0b013e318074e6c3. [DOI] [PubMed] [Google Scholar]
- 3.Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: Evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 5.Kougioumtzopoulou AS, Syrigos KN, Saif MW. Elderly patients with pancreatic cancer. JOP. 2014;15:322–325. doi: 10.6092/1590-8577/2682. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Xu LP, DI Zhao L, et al. Cytokine-induced killer cell therapy for advanced pancreatic adenocarcinoma: A case report and review of the literature. Oncol Lett. 2013;5:1427–1429. doi: 10.3892/ol.2013.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung MJ, Park JY, Bang S, et al. Phase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63:939–946. doi: 10.1007/s00262-014-1566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Zhang W, Qi X, et al. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res. 2012;18:1751–1759. doi: 10.1158/1078-0432.CCR-11-2442. [DOI] [PubMed] [Google Scholar]
- 9.Pan K, Li YQ, Wang W, et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol. 2013;20:4305–4311. doi: 10.1245/s10434-013-3144-x. [DOI] [PubMed] [Google Scholar]
- 10.Sangiolo D, Mesiano G, Gammaitoni L, et al. Activity of cytokine-induced killer cells against bone and soft tissue sarcoma. Oncoimmunology. 2014;3:e28269. doi: 10.4161/onci.28269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H, Zhang Y, Wang Q, et al. Therapeutic safety and effects of adjuvant autologous RetroNectin activated killer cell immunotherapy for patients with primary hepatocellular carcinoma after radiofrequency ablation. Cancer Biol Ther. 2010;9:903–907. doi: 10.4161/cbt.9.11.11697. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Lee YS, Kim HS, et al. Retronectin enhances lentivirus-mediated gene delivery into hematopoietic progenitor cells. Biologicals. 2009;37:203–209. doi: 10.1016/j.biologicals.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Lamers CH, van Elzakker P, van Steenbergen SC, et al. Retronectin-assisted retroviral transduction of primary human T lymphocytes under good manufacturing practice conditions: Tissue culture bag critically determines cell yield. Cytotherapy. 2008;10:406–416. doi: 10.1080/14653240801982961. [DOI] [PubMed] [Google Scholar]
- 14.Yu SS, Nukaya I, Enoki T, et al. In vivo persistence of genetically modified T cells generated ex vivo using the fibronectin CH296 stimulation method. Cancer Gene Ther. 2008;15:508–516. doi: 10.1038/cgt.2008.21. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe H, Okada M, Kaji Y, et al. [New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1)] Gan To Kagaku Ryoho. 2009;36:2495–2501. [PubMed] [Google Scholar]
- 16.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi Y, Higuchi H, Izumiya M, et al. Gemcitabine as first-line chemotherapy in elderly patients with unresectable pancreatic carcinoma. J Gastroenterol. 2010;45:1146–1154. doi: 10.1007/s00535-010-0258-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Shi SB, Qi JL, et al. S-1 plus CIK as second-line treatment for advanced pancreatic cancer. Med Oncol. 2013;30:747. doi: 10.1007/s12032-013-0747-9. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Zhang Y, Liu Y, et al. Association of myeloid-derived suppressor cells and efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma patients. J Immunother. 2014;37:43–50. doi: 10.1097/CJI.0000000000000005. [DOI] [PubMed] [Google Scholar]