Abstract
Pseudomonas syringae is a Gram-negative bacterium that infects multiple plant species by manipulating cellular processes via injection of type three secreted effectors (T3SEs) into host cells. Nucleotide-binding leucine-rich repeat (NLR) resistance (R) proteins recognize specific T3SEs and trigger a robust immune response, called effector-triggered immunity (ETI), which limits pathogen proliferation and is often associated with localized programmed cell death, known as the hypersensitive response (HR). In this study, we examine the influence of elevated temperature on two ETI outputs: HR and pathogen virulence suppression. We found that in the Arabidopsis thaliana accession Col-0, elevated temperatures suppress the HR, but have minimal influence on ETI-associated P. syringae virulence suppression, thereby uncoupling these two ETI responses. We also identify accessions of Arabidopsis that exhibit impaired P. syringae virulence suppression at elevated temperature, highlighting the natural variation that exists in coping with biotic and abiotic stresses. These results not only reinforce the influence of abiotic factors on plant immunity but also emphasize the importance of carefully documented environmental conditions in studies of plant immunity.
Keywords: Arabidopsis thaliana, Pseudomonas syringae, effector-triggered immunity, hypersensitive response, elevated temperature, abiotic stress, programmed cell death, disease resistance
Introduction
Gram negative bacterial pathogens, such as Pseudomonas syringae, use a type III secretion system (T3SS) to inject type III secreted effectors (T3SEs) into host cells where they target cellular components to disrupt host immunity (Lewis et al., 2009; Deslandes and Rivas, 2012). In response, plants have evolved intracellular nucleotide-binding domain leucine-rich repeat (NLR) proteins to directly or indirectly recognize effectors and initiate effector-triggered immunity (ETI; Jones and Dangl, 2006; Dodds and Rathjen, 2010). ETI is often, but not always, associated with a localized programmed cell death (PCD) termed the hypersensitive response (HR; Jones and Dangl, 2006).
Temperature has been shown to modulate outputs of plant ETI in several plant systems. For example, the tobacco N (necrosis) gene encodes an NLR that confers resistance to tobacco mosaic virus (TMV) and a rapid HR after infection at a permissive temperature (21°) (Whitham et al., 1994). However, shifting plants to an elevated temperature (28°) prevents the development of N-mediated-HR (Samuel, 1931; Whitham et al., 1994). Similarly, the HR responses induced by the T3SEs AvrRpt2, AvrRpm1, and AvrRps4 in Arabidopsis thaliana (hereafter Arabidopsis) are inhibited at elevated temperature (28°C) (Goel et al., 2008; Freeman and Beattie, 2009; Wang et al., 2009; Cheng et al., 2013). Furthermore, constitutive expression of the NLR RPS4 results in temperature-conditioned autoimmunity that is suppressed at 28°C (Heidrich et al., 2013). Although these examples demonstrate a strong influence of temperature on the ETI-associated HR, limited information is available about how ETI-associated virulence suppression is affected by temperature.
In our studies of the ETI response activated by the T3SE HopZ1a in Arabidopsis, we have observed that at elevated temperatures (28–30°C) the HR induced by this T3SE was suppressed, however, the reduction of bacterial growth associated with this ETI response remained intact. As such, two facets of the ETI-response, HR and virulence suppression can be differentially affected, and uncoupled, by elevated temperature conditions.
Materials and Methods
Plant Materials, Growth Conditions, Stress Conditions
Arabidopsis plants were grown in 12 h of light (130–150 microeinsteins m–2 s–1) and 12 h of darkness at 21–22°C and 50–65% humidity in Sunshine Mix 1 soil supplemented with 20:20:20 fertilizer at 1g/L. Except for accession spray assays, all assays were performed in the Col-0 background.
Prior to assays, plants were subjected to temperature priming for 24 h to avoid developmental differences. For elevated temperature priming, plants were incubated in 24 h of light (130–150 microeinsteins m–2 s–1) at 28–30°C and 15–50% humidity. Conversely, for room temperature control condition, plants were primed in 24 h of ambient light intensity, ambient room temperature (21–24°C), and ambient relative humidity (15–50%). Experiments were all performed at ambient temperature, and plants were placed in respective conditions (elevated or room temperature) for the duration of the experiment.
Pseudomonas syringae Macroscopic HR, Ion Leakage, and in planta Bacterial Growth Assays
Pseudomonas syringae pv. tomato DC3000 (PtoDC3000) carried empty vector (pUCP20), HopZ1a (Ma et al., 2006) or AvrRpt2 (Mudgett and Staskawicz, 1999). For macroscopic HR assays, P. syringae was resuspended to an optical density at 600 nm (OD600) of 0.1 (5 × 107 CFU/mL) and syringe-infiltrated into the right side of leaves of 5 week-old wild type Col-0 Arabidopsis. Macroscopic HR phenotypes were scored between 12 and 16 hpi. HR phenotypes were scored as “strong” (paper-like dryness and grayish in color) or “weak” (wilting but not dry and retaining green color).
For ion leakage assays, P. syringae was resuspended and diluted to a concentration of OD600 = 0.04 (2 × 107 CFU/mL), and syringe-infiltrated into four leaves per plant of 4.5–5 week-old wild type Col-0 Arabidopsis. After infiltration, four leaf disks (1.5 cm2) per plant (one disk per leaf) were harvested, washed in sterile double distilled H2O (ddH2O) for 30 min on a bench-top shaker at 250 rpm, and transferred to 6 mL of sterile ddH2O. Readings were obtained with an Orion 3 Star benchtop conductivity meter (Thermo Fisher Scientific Inc., Fort Collins, CO, USA).
For in planta bacterial growth assays, P. syringae was resuspended and diluted to obtain a concentration of OD600 = 0.0002 (1 × 105 CFU/mL), and infiltrated into four leaves per plant of 3.5–4 week-old wild type Col-0 Arabidopsis. On Day 0, four disks (1 cm2) per plant (one disk per leaf) were harvested from 2 to 3 plants approximately 1 h after infiltration, ground in 1 mL sterile 10 mM MgCl2, and plated on KB with rifampicin (50 μg/mL) and cycloheximide (50 μg/mL) for colony quantification. On Day 3, four disks (1 cm2) per plant (one disk per leaf) were harvested from 10 plants, ground in 1 mL sterile 10 mM MgCl2, and plated on KB with rifampicin (50 μg/mL) and cycloheximide (50 μg/mL) for colony quantification.
For disease resistance spray assays, P. syringae was resuspended and diluted to obtain a concentration of OD600 = 0.4 (2 × 108 CFU/mL) or OD600 = 0.8 (4 × 108 CFU/mL). Silwet L-77 was added to 0.04% (v/v). Plants were sprayed on Day 0 and Day 3 using a Prevall sprayer, and domed immediately. Dome was removed on Day 4 after second spray, and plants were monitored for disease symptoms up to 7–10 days.
Results
Elevated Temperature Suppresses ETI-associated Hypersensitive Responses
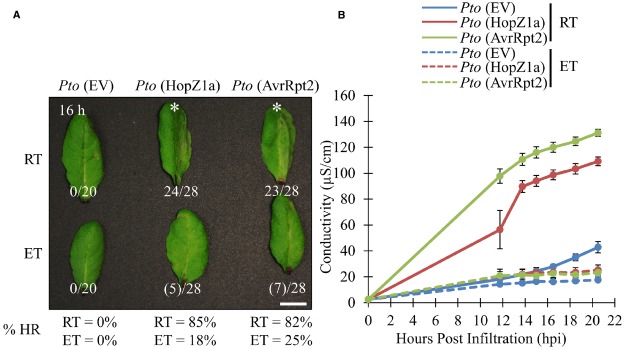
We examined two T3SEs delivered by P. syringae pv. tomato (PtoDC3000), HopZ1a and AvrRpt2, which elicit ETI, and associated HR in Arabidopsis accession Col-0 (Bent et al., 1994; Mindrinos et al., 1994; Lewis et al., 2008). We examined the ability of both effectors to induce ETI-associated HR under either ambient (21–24°C) or elevated temperature conditions (30°C). Both groups of plants were grown under identical growth conditions for 3 to 5 weeks, and subject to experimental temperatures 24 h prior to infiltration (see Materials and Methods). Delivery of AvrRpt2 or HopZ1a from the virulent PtoDC3000 triggers a strong macroscopic HR between 12 and 16 h post infiltration (hpi) at ambient temperature conditions, as expected (Figure 1A). At elevated temperature, both the PtoDC3000 (AvrRpt2) and PtoDC3000 (HopZ1a)-induced HR was suppressed, with no leaves showing a strong HR between 12 and 16 hpi, and only ∼20% of leaves showing a weak HR-like collapse (see Materials and Methods; Figure 1A).
FIGURE 1.
ETI-associated hypersensitive response and ion leakage are suppressed by elevated temperature in Arabidopsis accession Col-0. For ambient room temperature (RT) or elevated temperature (ET) conditions, plants were subject to 21–24°C and 30°C, respectively, for 24 h prior to infiltration. (A) Arabidopsis half-leaves were infiltrated with PtoDC3000 (Pto) expressing empty vector (EV), HopZ1a or AvrRpt2 at OD600 = 0.1 (5 × 107 CFU/mL). Hypersensitive response (HR) phenotypes were scored between 10 and 20 h post infiltration (hpi). Fractions indicate number of leaves displaying HR phenotype out of total number of leaves infiltrated. This is also reflected in percentages at the bottom of the figure. Numbers in brackets indicate weak HR phenotypes; asterisks (*) indicate strong HR phenotypes on the images presented. Macroscopic HR assays were performed three times with similar results. (RT = 23.7°C, 47% relative humidity (RH); ET = 30.0°C, 48% RH) (B) Arabidopsis leaves were infiltrated with PtoDC3000 (Pto) expressing empty vector (EV), HopZ1a or AvrRpt2 at OD600 = 0.04 (2 × 107 CFU/mL). Four leaf disks per plant (one disk per leaf, total leaf tissue 1.5 cm2) were transferred to 6 mL of sterile ddH2O and conductivity readings were taken between 10 and 20 hpi (see Materials and Methods). Ion leakage assays were conducted three times with similar results. (RT = 24.6°C, 51% RH; ET = 30.0°C, 50% RH).
To quantify the suppressive effects of elevated temperature on ETI-associated HR, we quantified ion leakage as a proxy for HR. As expected, both PtoDC3000 (AvrRpt2) and PtoDC3000 (HopZ1a) induced HR-associated ion leakage in ambient temperature conditions by 12 hpi relative to the PtoDC3000 empty vector (EV) control (Figure 1B). However, under elevated temperature conditions the HR-associated ion leakage for both PtoDC3000 (HopZ1a) and PtoDC3000 (AvrRpt2)-induced HR was suppressed, and not significantly different from the PtoDC3000 (EV) control (Figure 1B).
ETI-associated Virulence Suppression is not Inhibited by Elevated Temperatures
We have demonstrated that the ETI-associated HR induced by PtoDC3000 (HopZ1a) and PtoDC3000 (AvrRpt2) is suppressed in elevated temperature (30°C) conditions, corroborating previously published data examining the influence of elevated temperature on HR (Figure 1; Freeman and Beattie, 2009; Cheng et al., 2013). In order to examine whether ETI-associated virulence suppression was also impaired by elevated temperature incubation, we conducted in planta bacterial growth assays (Figure 2A). As expected, the ETI triggered by both T3SEs decreased bacterial growth by ∼1.5 log CFU/cm2 at ambient temperature relative to the EV negative control (Figure 2A). Plants incubated at elevated temperature (30°C) exhibited increased bacterial growth of the EV control (∼1.5 log) relative to ambient temperature. Nevertheless, the ETI-associated virulence suppression induced by both T3SEs was still observed under elevated temperature conditions (Figure 2A). In fact, the magnitude of ETI-associated PtoDC3000 growth reduction was an order of magnitude greater for both T3SEs at elevated temperatures (∼2.5 log CFU/cm2) than at ambient temperature (∼1.5 log CFU/cm2; Figure 2A).
FIGURE 2.
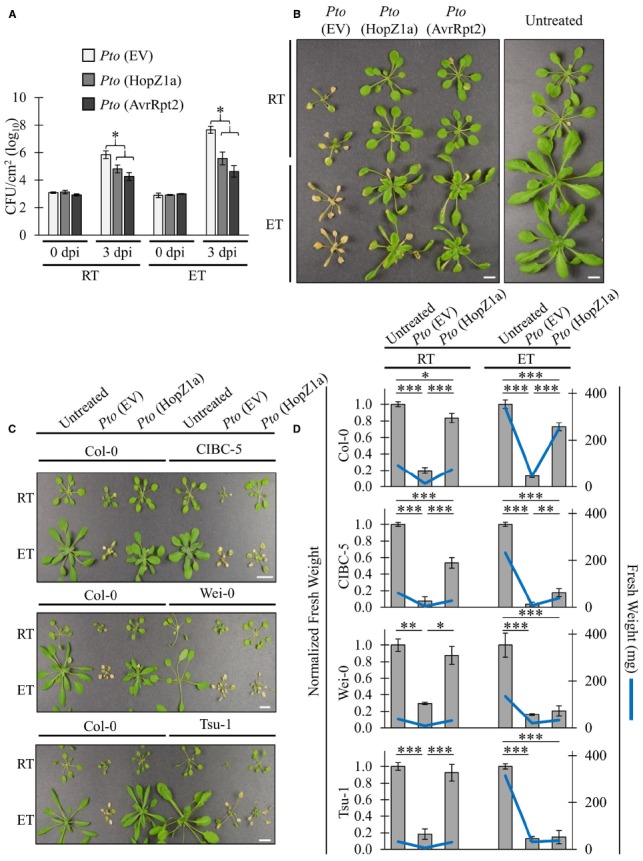
ETI-associated virulence suppression is not inhibited by elevated temperatures. For ambient room temperature (RT) or elevated temperature (ET) conditions, plants were subject to 21–24°C and 30°C, respectively, for 24 h prior to infiltration. (A) Arabidopsis leaves were infiltrated with PtoDC3000 (Pto) expressing empty vector (EV), HopZ1a, or AvrRpt2 at OD600 = 0.0002 (1 × 105 CFU/mL). Bacterial counts were determined 1 h post-infiltration (0 dpi) and 3 days post-infiltration (3 dpi). Two-tailed homoscedastic t-tests were performed to test for significant differences. Treatments were compared to empty vector in either the ambient room temperature (RT) or elevated temperature (ET) condition and significant differences are indicated by an asterisk (*P < 0.01). Error bars indicate the standard deviation from the mean of 2 samples (0 dpi) and 10 samples (3 dpi). Bacterial growth assays for HopZ1a and AvrRpt2 were conducted four times with similar results. (RT = 24.6–25.4°C, 16–26% RH; ET = 30.0°C, 25–26% RH) (B) Arabidopsis plants were spray-inoculated with PtoDC3000 (Pto) expressing empty vector (EV), HopZ1a or AvrRpt2 at OD600 = 0.4 (2 × 108 CFU/mL). Photos shown were taken 10 days post-spraying (dps). Assay was conducted four times with similar results. Scale bar indicates 1 cm. (RT = 22.8–25°C, 48–53% RH; ET = 29.7–30.0°C, 35–50% RH) (C) Arabidopsis accessions Col-0, CIBC-5, Wei-0 and Tsu-1 were spray inoculated with PtoDC3000 (Pto) expressing empty vector (EV) or HopZ1a at OD600 = 0.8 (4 × 108 CFU/mL). Independent Col-0 control plants were grown on same flat as each indicated accession. Photos shown were taken 10 dps. Assay was conducted four times with similar results. Scale bar indicates 1cm. (RT = 22.8–23.7°C, 48–51% RH; ET = 30.0°C, 41–51% RH) (D) Normalized and absolute fresh weight measurements of Arabidopsis accessions Col-0, CIBC-5, Wei-0 and Tsu-1 for untreated plants, and plants treated with Pto (EV) or HopZ1a at 10 dps as in panel (C). Normalized fresh weight values were calculated relative to the respective untreated controls for each temperature and accession assay to account for the greater general growth observed under ET conditions. Absolute fresh weight in milligrams (mg) is shown on the secondary Y-axis. Data are combined from multiple trials. Error bars indicate standard errors. Two-tailed homoscedastic t-tests were performed to test significance and asterisk indicate P-values (0.05 > * > 0.01 > ** > 0.001 > ***).
We performed disease resistance spray assays in order to qualitatively visualize the immune response at elevated temperatures (Figure 2B). Plants pre-incubated in ambient or elevated temperature were sprayed at a high bacterial density and disease symptoms were monitored. At ambient temperature, disease symptoms and chlorosis of plants sprayed with PtoDC3000 (EV) became visible at approximately 2 to 3 days post infection (dpi). At 5 dpi, disease-associated chlorosis became more severe, and plant growth was stunted relative to healthy plants. In contrast, plants sprayed with PtoDC3000 (HopZ1a) or PtoDC3000 (AvrRpt2) remained relatively healthy, and plant growth resembled uninfected plants (Figure 2B). Similarly, at elevated temperature, plants sprayed with PtoDC3000 (HopZ1a) or PtoDC3000 (AvrRpt2) remained relatively symptom-free relative to plants infected with PtoDC3000 (EV; Figure 2B). This observation combined with the quantification of in planta bacterial growth indicated that ETI-associated virulence suppression remained intact under elevated temperature conditions that suppressed ETI-associated HR.
Arabidopsis Accessions Exhibit Varying Temperature Sensitivity
We demonstrated that ETI-associated virulence suppression remains intact under elevated temperature conditions that otherwise suppress ETI-associated HR in the Arabidopsis Col-0 accession. In order to examine whether local adaptation to varying climates can influence disease resistance under elevated temperature conditions, we tested Arabidopsis accessions isolated from varying geographic climate regions for loss of PtoDC3000 (HopZ1a)-triggered immunity at elevated temperature. Although most accessions showed a Col-0 like retention of ETI at elevated temperature (data not shown), we identified three accessions CIBC-5, Wei-0 and Tsu-1, that displayed a loss of ETI-associated virulence suppression at elevated temperature, despite showing a normal (Col-0 like) response at ambient temperature (Figure 2C). Unlike Col-0, CIBC-5, Wei-0 and Tsu-1, developed a chlorotic, disease phenotype when sprayed with PtoDC3000 (HopZ1a) in elevated temperature, similar to that of PtoDC3000 (EV; Figure 2C).
As we were conducting our spray assays we noted a noticeable size difference in resistant (green) versus susceptible (chlorotic) plants. Therefore, in order to quantify the loss of ETI-associated virulence suppression, we measured the fresh weight (both absolute and normalized) of plants at 10 days post-infection (Figure 2D). We normalized the fresh weight to assess the magnitude of differences between treatment in light of the overall increased growth associated with elevated temperatures. This normalization was done based on the weight of the untreated plant for each accession-by-temperature comparison, and was done independently for each trial. At both ambient and elevated temperature, Col-0 plants sprayed with PtoDC3000 (HopZ1a) were of a significantly higher fresh weight than plants sprayed with PtoDC3000 (EV). CIBC-5, Wei-0 and Tsu-1 plants sprayed with PtoDC3000 (HopZ1a) also had higher fresh weight relative to PtoDC3000 (EV) at ambient temperature, but this difference was reduced (CIBC-5) or lost (Wei-0 and Tsu-1) at elevated temperature (Figure 2D). Overall, these data demonstrate that the ETI response of Arabidopsis accessions is differentially affected by temperature.
Discussion
Our study corroborates previous findings that temperature is a direct modulator of plant immunity. However, we find that different outputs of immunity are differentially sensitive to elevated temperature. A previous study has proposed that lower temperatures favor the activation of ETI, whereas ETI is compromised at elevated temperatures (Cheng et al., 2013). In light of our results, we would add that different facets of ETI are differentially influenced by temperature, with HR being more sensitive than ETI-associated virulence suppression. In addition, our results support the hypothesis that HR cell death is dispensable for resistance to PtoDC3000.
In order to minimize plant developmental differences between experiments conducted at elevated temperature versus ambient temperature, plants were grown for 3–5 weeks under our standard plant growth conditions, and then acclimated to elevated temperature conditions for 24 h prior to infection assays. Relative humidity was controlled to be within a moderate to low range (15–50%) in order to avoid the additive suppressive effects of high humidity since, like elevated temperature, high relative humidity has also been demonstrated to suppress ETI responses (Samuel, 1931; Jambunathan et al., 2001; Yoshioka et al., 2001; Lorrain et al., 2003; Zhou et al., 2004; Noutoshi et al., 2005; Moeder and Yoshioka, 2008; Freeman and Beattie, 2009; Mosher et al., 2010; Beattie, 2011; Hangyang et al., 2015). Corroborating this data, we have found HopZ1a and AvrRpt2 HR to be delayed under high relative humidity conditions (data not shown). It should be noted that relative humidity was significantly influenced by seasonal variation (i.e., significantly lower in the winter months than in summer months) which contributed to the relatively large humidity range of our experiments despite our control efforts. We nevertheless aimed to maintain humidity below 50% in all cases and to have comparable relative humidity across ambient and elevated temperature treatments (see Figure Legends for specific values).
It is important to note that the suppressed HR at elevated temperature is not due to inhibition of the type III secretion system since P. syringae type III effector expression assays are routinely conducted at 28–30°C. Additionally, the in planta growth rate of virulent PtoDC3000 actually increased at elevated temperature, corroborating previously published data (Freeman and Beattie, 2009; Wang et al., 2009; Cheng et al., 2013).
Two different assays to monitor the ETI-associated HR yielded slightly differing results. Macroscopic HR was significantly weakened in our experiments, but not entirely inhibited (Figure 1A). On the other hand, HR-associated ion leakage was entirely inhibited for both T3SEs tested resulting in measurements similar to those of the EV control (Figure 1B). This is likely influenced by the fact that for ion leakage assays, infiltrated leaf disks are placed in water (i.e., 100% humidity) immediately post-infiltration in order to monitor changes in water conductance. As such, the ion leakage assays are conducted in both an elevated temperature and high humidity environment. We believe that the combined suppressive effects of elevated temperature and humidity result in a complete loss of HR ion leakage associated, whereas macroscopic HR is delayed but still observed since these assays are conducted at ambient relative humidity (Samuel, 1931; Jambunathan et al., 2001; Yoshioka et al., 2001; Lorrain et al., 2003; Zhou et al., 2004; Noutoshi et al., 2005; Moeder and Yoshioka, 2008; Freeman and Beattie, 2009; Mosher et al., 2010; Beattie, 2011; Hangyang et al., 2015).
Our results indicate that elevated temperatures can uncouple the HR response from ETI-associated virulence suppression. This is reminiscent of ETI examples that occur without apparent PCD. The NLRs TAO1 and RPS6 activate ETI in response to the T3SEs AvrB and HopA1 without manifestation of an HR (Shimizu et al., 2003; Gassmann, 2005; Eitas et al., 2008; Naito et al., 2008; Kim et al., 2009). The Arabidopsis NLR gene pair RPS4 and RRS1 confer resistance to PtoDC3000 expressing AvrRps4, which is accompanied by PCD in Ws-0, but not in Col-0 background (Hinsch and Staskawicz, 1996; Gassmann, 2005; Heidrich et al., 2013). These examples corroborate our observations that HR-associated PCD can be uncoupled from virulence suppression and suggest that HR-associated cell death is not required for resistance against virulent P. syringae.
NLRs can be subdivided into two main categories based on their N-terminal domains; coiled coil (CC)-NLR and Toll/interleukin-1 receptor (TIR)-NLR. HopZ1a and AvrRpt2 are recognized by the CC-NLRs ZAR1 and RPS2, respectively (Bent et al., 1994; Mindrinos et al., 1994; Lewis et al., 2010). Most examples of temperature sensitive ETI-responses are mediated by TIR-NLRs suggesting that this NLR subgroup could potentially be more sensitive to elevated temperatures (Hua, 2014). However, our preliminary experiments indicate that AvrRps4-induced virulence suppression, mediated by the TIR-NLRs RPS4 and RRS1, is retained in our elevated temperature conditions (data not shown).
In order to test the influence of local adaptation on ETI-associated virulence suppression, various Arabidopsis accessions were tested using spray assays and we identified three accessions that displayed compromised ETI at elevated temperature. CIBC-5 was isolated from Ascot, Berkshire, United Kingdom (51.4084°N, 0.6707°W), Tsu-1 from Tsushima, Japan (34.202°N, 129.29°E) and Wei-0 from Weiningen, Switzerland (47.419°N, 8.4326°E). The Col-0 accession which retained ETI-virulence suppression at elevated temperature is most similar to the Gü-0 accession, originally isolated from Gückingen, Germany (50.391°N, 8.008°E) and propagated for research use in the mid-1900s in Columbia, Missouri, USA (38.952°N, 92.334°W) (Nordborg et al., 2005). The CIBC-5 and Wei-0 accessions were isolated from locations that would experience slightly cooler average temperatures (highest monthly summer average temperature = 23°and 24°, respectively) than Gü-0 (25°), which may explain the lower tolerance of their ETI responses. However, Tsu-1 (33°) was isolated from a location that experiences much higher temperatures than any of the other accession discussed here (worldweatheronline.com). Therefore, the differences observed in the temperature tolerance of their respective ETI responses is likely influenced by additional factors. Nevertheless, the ecotypic differences observed in ETI responses at elevated temperature indicate that temperature tolerance may be an adapted trait that should be genetically tractable.
In this study, we have demonstrated that the virulence suppression and HR outputs of ETI are differentially sensitive to elevated temperature. At elevated temperatures, HR is significantly delayed or inhibited depending on the assay method used, whereas immunity, measured as bacterial growth inhibition or disease symptom development, is relatively unaffected. These observations not only emphasize the importance of carefully documented plant growth conditions in studies of plant immunity, but also highlight the differential sensitivity of various immunity outputs to abiotic stresses.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank members of the Desveaux and Guttman labs for valuable input on the project; in particular Dr. Pauline W. Wang, Dr. Brenden Hurley, Dr. Jianfeng Zhang, Jessica Schembri, Timothy Lo, Christine Cao and Noushin Koulena for reagents, troubleshooting advice, and thoughtful discussion. This work was supported by Natural Sciences and Engineering Research Council of Canada Discovery Grants to DG and DD; a Canada Research Chair in Plant-Microbe Systems Biology (DD) or Comparative Genomics (DG); the Centre for the Analysis of Genome Evolution and Function (DD and DG).
References
- Beattie G. A. (2011). Water relations in the interaction of foliar bacterial pathogens with plants. Annu. Rev. Phytopathol. 49, 533–555. 10.1146/annurev-phyto-073009-114436 [DOI] [PubMed] [Google Scholar]
- Bent A. F., Kunkel B. N., Dahlbeck D., Brown K. L., Schmidt R., Giraudat J., et al. (1994). RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265, 1856–1860. 10.1126/science.8091210 [DOI] [PubMed] [Google Scholar]
- Cheng C., Gao X., Feng B., Sheen J., Shan L., He P. (2013). Plant immune response to pathogens differs with changing temperatures. Nat. Commun. 4, 2530. 10.1038/ncomms3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes L., Rivas S. (2012). Catch me if you can: bacterial effectors and plant targets. Trends Plant Sci. 17, 644–655. 10.1016/j.tplants.2012.06.011 [DOI] [PubMed] [Google Scholar]
- Dodds P. N., Rathjen J. P. (2010). Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11, 539–548. 10.1038/nrg2812 [DOI] [PubMed] [Google Scholar]
- Eitas T. K., Nimchuk Z. L., Dangl J. L. (2008). Arabidopsis TAO1 is a TIR-NB-LRR protein that contributes to disease resistance induced by the Pseudomonas syringae effector AvrB. Proc. Natl. Acad. Sci. U.S.A. 105, 6475–6480. 10.1073/pnas.0802157105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. C., Beattie G. A. (2009). Bacterial growth restriction during host resistant to Pseudomonas syringae is associated with leaf water loss and localized cessation of vascular activity in Arabidopsis. Mol. Plant Microbe Interact. 22, 857–867. 10.1094/MPMI-22-7-0857 [DOI] [PubMed] [Google Scholar]
- Gassmann W. (2005). Natural variation in the Arabidopsis response to the avirulence gene hopPsyA uncouples the hypersensitive response from disease resistance. Mol. Plant Microbe Interact. 18, 1054–1060. 10.1094/MPMI-18-1054 [DOI] [PubMed] [Google Scholar]
- Goel A. K., Lundberg D., Torres M. A., Matthews R., Akimoto-Tomiyama C., Farmer L., et al. (2008). The Pseudomonas syringae type III effector HopAM1 enhances virulence on water-stressed plants. Mol. Plant Microbe Interact. 21, 361–370. 10.1094/MPMI-21-3-0361 [DOI] [PubMed] [Google Scholar]
- Hangyang C., Yang S., Yan Y., Xiao Z., Cheng J., Qu J., et al. (2015). CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. J. Exp. Bot. 66, 3163–3174. 10.1093/jxb/erv125 [DOI] [PubMed] [Google Scholar]
- Heidrich K., Tsuda K., Blanvillain-Baufumé S., Wirthmueller L., Bautor J., Parker J. E. (2013). Arabidopsis TNL-WRKY domain receptor RRS1 contributes to temperature-conditioned RPS4 auto-immunity. Front. Plant Sci. 4: 403. 10.3389/fpls.2013.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsch M., Staskawicz B. (1996). Identification of a new Arabidopsis disease resistance locus, RPS4, and cloning of the corresponding avirulence gene, avrRps4, from Pseudomonas syringae pv. pisi. Mol. Plant Microbe Interact. 9, 55–61. 10.1094/MPMI-9-0055 [DOI] [PubMed] [Google Scholar]
- Hua J. (2014). “Temperature and plant immunity,” in Temperature and Plant Development, eds Franklin K. A., Wigge P. A. (Oxford: John Wiley & Sons, Inc; ), 163–180. [Google Scholar]
- Jambunathan N., Siani J. M., McNellis T. W. (2001). A humidity-sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance. Plant Cell 13, 2225–2240. 10.1105/tpc.13.10.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. D. G., Dangl J. L. (2006). The plant immune system. Nature 444, 323–329. 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Kwon S. I., Saha D., Anyanwu N. C., Gassmann W. (2009). Resistance to the Pseudomonas syringae effector HopA1 is governed by the TIR-NBS-LRR protein RPS6 and is enhanced by mutations in SRFR1. Plant Physiol. 150, 1723–1732. 10.1104/pp.109.139238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. D., Abada W., Ma W., Guttman D. S., Desveaux D. (2008). The HopZ family of Pseudomonas syringae type III effectors require myristoylation for virulence and avirulence functions in Arabidopsis. J. Bacteriol. 190, 2880–2891. 10.1128/JB.01702-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. D., Guttman D. S., Desveaux D. (2009). The targeting of plant cellular systems by injected type III effector proteins. Semin. Cell Dev. Biol. 20, 1055–1063. 10.1016/j.semcdb.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Lewis J. D., Wu R., Guttman D. S., Desveaux D. (2010). Allele-specific virulence attenuation of the Pseudomonas syringae HopZ1a type III effector via the Arabidopsis ZAR1 resistance protein. PLoS Genet. 6:e1000894. 10.1371/journal.pgen.1000894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S., Vailleau F., Balagué C., Roby D. (2003). Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci. 8, 263–271. 10.1016/S1360-1385(03)00108-0 [DOI] [PubMed] [Google Scholar]
- Ma W., Dong F. F. T., Stavrinides J., Guttman D. S. (2006). Type III effector diversification via both pathoadaptation and horizontal transfer in response to a coevolutionary arms race. PLoS Genet. 2:e209. 10.1371/journal.pgen.0020209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindrinos M., Katagiri F., Yu G. L., Ausubel F. M. (1994). The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78, 1089–1099. 10.1016/0092-8674(94)90282-8 [DOI] [PubMed] [Google Scholar]
- Moeder W., Yoshioka K. (2008). Lesion mimic mutants: a classical, yet still fundamental approach to study programmed cell death. Plant Signal. Behav. 3, 764–767. 10.4161/psb.3.10.6545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher S., Moeder W., Nishimura N., Jikumaru Y., Joo S., Urquhart W., et al. (2010). The lesion-mimic mutant cpr22 shows alterations in abscisic acid signaling and abscisic acid insensitivity in a salicylic acid-dependent manner. Plant Physiol. 152, 1901–1913. 10.1104/pp.109.152603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett M. B., Staskawicz B. J. (1999). Characterization of the Pseudomonas syringae pv. tomato AvrRpt2 protein: demonstration of secretion and processing during bacterial pathogenesis. Mol. Microbiol. 32, 927–941. 10.1046/j.1365-2958.1999.01403.x [DOI] [PubMed] [Google Scholar]
- Naito K., Taguchi F., Suzuki T., Inagaki Y., Toyoda K., Shiraishi T., et al. (2008). Amino acid sequence of bacterial microbe-associated molecular pattern flg22 is required for virulence. Mol. Plant Microbe Interact. 21, 1165–1174. 10.1094/MPMI-21-9-1165 [DOI] [PubMed] [Google Scholar]
- Nordborg M., Hu T. T., Ishino Y., Jhaveri J., Toomajian C., Zheng H., et al. (2005). The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 3:e196. 10.1371/journal.pbio.0030196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutoshi Y., Ito T., Seki M., Nakashita H., Yoshida S., Marco Y., et al. (2005). A single amino acid insertion in the WRKY domain of the Arabidopsis TIR-NBS-LRR-WRKY-type disease resistance protein SLH1 (sensitive to low humidity 1) causes activation of defense responses and hypersensitive cell death. Plant J. 23, 873–888. 10.1111/j.1365-313X.2005.02500.x [DOI] [PubMed] [Google Scholar]
- Samuel G. (1931). Some experiments on inoculating methods with plant viruses, and on local lesions. Ann. Appl. Biol. 18, 494–507. 10.1111/j.1744-7348.1931.tb02320.x [DOI] [Google Scholar]
- Shimizu R., Taguchi F., Marutani M., Mukaihara T., Inagaki Y., Toyoda K., et al. (2003). The DfliD mutant of Pseudomonas syringae pv. tabaci, which secretes flagellin monomers, induces a strong hypersensitive reaction (HR) in non-host tomato cells. Mol. Genet. Genomics 269, 21–30. 10.1007/s00438-003-0817-3 [DOI] [PubMed] [Google Scholar]
- Wang Y., Bao Z., Zhu Y., Hua J. (2009). Analysis of temperature modulation of plant defense against biotrophic microbes. Mol. Plant Microbe Interact. 22, 498–506. 10.1094/MPMI-22-5-0498 [DOI] [PubMed] [Google Scholar]
- Whitham S., Dinesh-Kumar S. P., Choi D., Hehl R., Corr C., Baker B. (1994). The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell 78, 1101–1115. 10.1016/0092-8674(94)90283-6 [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Kachroo P., Tsui F., Sharm S. B., Shah J., Klessig D. F. (2001). Environmentally sensitive, SA-dependent defense responses in the cpr22 mutant of Arabidopsis. Plant J. 26, 447–459. 10.1046/j.1365-313X.2001.2641039.x [DOI] [PubMed] [Google Scholar]
- Zhou F., Menke F. L. H., Yoshioka K., Moder W., Shirano Y., Klessig D. F. (2004). High humidity suppresses ssi4-mediated cell death and disease resistance upstream of MAP kinase activation, H2O2 production and defense gene expression. Plant J. 39, 920–932. 10.1111/j.1365-313X.2004.02180.x [DOI] [PubMed] [Google Scholar]