Abstract
It is proposed that most papillary thyroid cancers originate in infancy and childhood, based on the early rise in sporadic thyroid carcinoma incidence, the pattern of radiation-induced risk (highest in those exposed as infants), and the high prevalence of sporadic papillary thyroid cancers in children and adolescents (ultrasound screening after the Fukushima accident). The early origin can be linked to the growth pattern of follicular cells, with a high mitotic rate in infancy falling to very low replacement levels in adult life. The cell of origin of thyroid cancers, the differentiated follicular cell, has a limited growth potential. Unlike cancers originating in stem cells, loss of the usually tight link between differentiation and replicative senescence is required for immortalisation. It is suggested that this loss distinguishes larger clinically significant papillary thyroid cancers from micro-papillary thyroid cancers of little clinical significance. Papillary carcinogenesis can then be divided into 3 stages: (1) initiation, the first mutation in the carcinogenic cascade, for radiation-induced papillary thyroid cancers usually a RET rearrangement, (2) progression, acquisition of the additional mutations needed for low-grade malignancy, and (3) escape, further mutations giving immortality and a higher net growth rate. Most papillary thyroid cancers will not have achieved full immortality by adulthood, and remain as so-called micro-carcinomas with a very low growth rate. The use of the term ‘cancer’ to describe micro-papillary thyroid cancers in older patients encourages overtreatment and alarms patients. Invasive papillary thyroid tumours show a spectrum of malignancy, which at its lowest poses no threat to life. The treatment protocols and nomenclature for small papillary carcinomas need to be reconsidered in the light of the new evidence available, the continuing discovery of smaller lesions, and the model of thyroid carcinogenesis proposed.
Key Words: Thyroid cancer pathogenesis, Thyroid growth, Chernobyl, Fukushima, Papillary microcarcinoma
Introduction
Malignant tumours are considered to arise from the sequential accumulation within one cell of mutations in genes controlling growth and invasion. As most mutations occur during cell division, a link between the growth of a tissue and the pattern of occurrence of cancers would be expected. The thyroid is particularly unusual both in its growth pattern and in the behaviour of its cancers. To explore the relationship between thyroid growth and thyroid cancer, evidence from several areas needs to be considered: the age-specific incidence of thyroid malignancy, the age-related sensitivity to radiation-induced thyroid cancer, the molecular findings in malignancy, the cell of origin of thyroid carcinomas, and the growth pattern of the gland. Together these suggest that the initial mutations in the cascade that results in the development of papillary thyroid cancer commonly occur in infancy or early childhood and that those cancers that have not achieved full immortality before adulthood continue with a slow growth rate and a very low chance of progression. This conclusion has implications for the treatment of thyroid cancer in adults.
Incidence of Thyroid Cancer
The pattern of the age-specific incidence of thyroid cancer differs from most other cancers. Many types of carcinoma are rare in childhood and early adult life, and increase exponentially in frequency in older people. A few, usually cancers of immature cells such as retinoblastomas, are virtually confined to the first few years of life. Thyroid cancer shows neither of these patterns: incidence increases during adolescence and early adult life. In the USA the rise continues to about age 45 with little subsequent change until it declines in those over 75. The incidence at age 30-39 is about the same as the incidence in those over 80. The great majority of thyroid carcinomas are papillary in type. This analysis is primarily concerned with papillary carcinoma, although the main conclusions on age of origin will apply also to follicular carcinoma, the other differentiated cancer derived from the follicular cell. Medullary carcinoma, derived from C cells, is not considered.
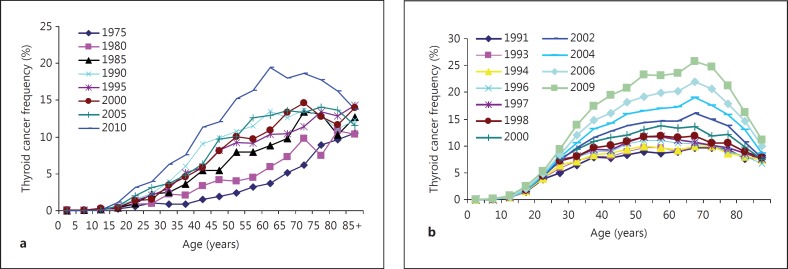
The incidence of thyroid carcinoma has risen steadily for many years; in the USA there has been an approximately threefold rise over the last four decades, almost entirely due to papillary carcinoma [1]. Similar increases have been recorded in other developed countries, including Japan (fig. 1). The increase has been attributed largely to the increasing use of ultrasound scans and other imaging techniques, although environmental factors may also be important [2]. It was found many years ago that small papillary carcinomas were a common finding at autopsy, reaching a frequency of 36% in one study [3], suggesting that there is a large reservoir of small papillary carcinomas that do not present clinically during life, but can be uncovered by ultrasound or other screening techniques.
Fig. 1.
The large increase in incidence of thyroid cancer over the last few decades is illustrated by cancer registry data from Japan and the USA. The reasons for the differing patterns of age-related incidence are not known but might be related to stable dietary iodide differences. a Japan from 1975 to 2010 (data from the National Cancer Center Japan [14]). b USA (SEER) from 1991 to 2009.
Statistics on the age and sex incidence of the different major types of cancer are based on the age at which the tumour is detected; the age at which individual cancers start and the time between their origin and clinical presentation is not known. This information is relevant to the investigation of possible causative factors and to the understanding of the mechanisms of carcinogenesis. Attempts have been made for some tumours to develop models to estimate cancer latency [4], but the exposure of a large population to significant amounts of a carcinogen such as radiation allows cancer latency and factors influencing sensitivity to carcinogenesis to be observed more directly.
Radiation and Thyroid Cancer
There have been many studies of thyroid cancer incidence after radiation exposure starting from the 1950s, mostly on a small scale until the long-term effects of radiation from the atomic bombs in Japan were investigated. About 100,000 of those who survived the exposure to whole body radiation have been studied for many years. The nuclear power plant accident at Chernobyl led to the exposure of many millions of the general public to radioisotopes in fallout, and to the exposure of hundreds of thousands of emergency workers, many of whom received significant doses of whole body radiation. After Chernobyl exposure to internal radiation from iodine 131, a major component of fallout, gave a radiation dose to the thyroid that was hundreds of times greater than the dose to other organs. In contrast, exposure to external radiation from atomic bombs gave similar doses to all tissues, with severe illness or death above a dose of a few Gy. After Chernobyl, thyroid doses of tens of Gy have been reported in those exposed to fallout.
The striking initial observation in those exposed to high levels of fallout from Chernobyl was the dramatic increase in the incidence of thyroid cancer in young children, starting only 4 years after the accident [5,6]. As the increase continued, the peak age at which the tumours presented rose; analysis of the age at exposure of the cases showed a major change in sensitivity to carcinogenesis, with the youngest at exposure carrying the highest risk [7,8]. This pattern of age at exposure-related sensitivity is still present 20 years after the accident. There is no confirmed evidence of any increase in those who were adults at exposure, although a small increase cannot be excluded. One of the reasons that the early reports of a thyroid carcinoma increase after Chernobyl were doubted was that radioiodines were at that time thought to have low or absent carcinogenic activity in man. The evidence for that view was based on studies of the use of iodine 131 in thyrotoxicosis [9]; those studied were almost entirely adults. In retrospect, these findings should not have been applied to children, but the lack of effect in adults is consistent with the very low or absent thyroid cancer risk for those exposed as adults to Chernobyl fallout. Some of the age-related sensitivity after Chernobyl may be related to differences in thyroid dose: children have a higher uptake of iodine and also drink relatively more milk than adults. Additionally, individual thyroid doses are difficult to ascertain accurately in children, particularly in a country with variable iodine deficiency. However, similar age-at-exposure sensitivity changes have been observed after external radiation, where radiation dose is more easily determined. One pooled study found that those exposed at ages 0-5 had a fivefold greater risk/Gy of developing thyroid cancer than those exposed at ages 10-15, while adults had a very low or undetectable risk [10]. Studies of those exposed to atomic bomb radiation found a 70% drop in excess absolute risk per decade increase in age at exposure [11].
The findings that for both external and internal radiation the youngest at exposure are at the highest risk of thyroid cancer while adults have little or no risk needs explanation; it also raises the question whether sporadic thyroid cancers also start in infancy or childhood.
Evidence from Fukushima
The importance of imaging techniques in determining the recorded incidence of thyroid cancer and the problems posed by the increasing frequency of detection of small thyroid carcinomas are brought into sharp focus by the results of screening the population exposed to fallout from the 2011 Fukushima nuclear power plant accident. Because of the increased incidence of thyroid cancer in those exposed as children to Chernobyl fallout, a Fukushima Prefecture thyroid ultrasound programme was set up to screen everyone under the age of 19 at the time of the accident [12]. After 3 years a lot more thyroid cancers than expected were found. Among about 300,000 individuals, 110 cases of suspected cancer were identified by fine needle aspiration, and 86 of the 87 suspected cases so far operated have been confirmed as thyroid cancer [13]. Sixty-two of the cases with proven cancer occurred in about 51,000 adolescents aged 15-19, a prevalence of about 120/100,000; the expected Japanese incidence at this age is 0.8/100,000 [14].
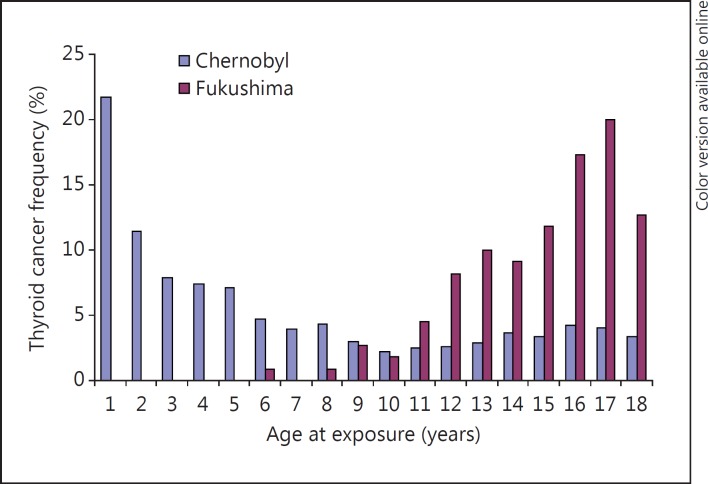
The scale of the apparent increase has led to considerable concern in the exposed population [15]. Nodules of less than 5 mm were not subject to further investigation, so the true thyroid cancer prevalence can be assumed to be much greater. However, the evidence from age at exposure and latency suggests that the large number of thyroid cancers so far detected is not due to radiation from the accident. After Chernobyl the risk was greatest in those who were infants at the time of the accident, falling rapidly with increasing age [8]. None of the Fukushima cases so far were infants at the time of the accident, the youngest was aged 6, and the majority were adolescents [13] (fig. 2).
Fig. 2.
Thyroid cancer frequency (percentage distribution) by age at exposure to fallout from Chernobyl and in the first 3 years after Fukushima. The Chernobyl distribution shows the high risk related to a young age at exposure to radiation with a steep fall with increasing age. The Fukushima distribution lacks any increased risk in the youngest, and the increasing incidence with age is similar to the normal increase, suggesting there has been no radiation-related increase in the first 3 years after the accident. The slight rise in those aged over 10 after Chernobyl probably also represents the normal incidence.
The screening programme after Fukushima divided the prefecture into three areas, screened in successive years. The first year's screening found a similar incidence and tumour size as other areas in subsequent years. Even with ultrasound it would seem biologically almost impossible for cells with the initial radiation-induced mutation to acquire the additional changes needed to give a cancer and to reach a detectable size within 1 year after the accident. The amount of radioiodine released from Fukushima is reported to be approximately one seventh of that released from Chernobyl [16]. That UNSCEAR report has been criticised for using the lower of the estimates available [17], but the higher estimate is still less than a third of that from Chernobyl, and most of the activity released from the Fukushima accident was blown out to sea. Few early direct thyroid measurements were made in the immediate aftermath of the tsunami; using reconstructed doses the maximum absorbed dose to a child's thyroid from Fukushima fallout has been estimated as 66 mGy compared to 5,000 mGy after Chernobyl [16]. A low level of dietary stable iodine increases the risk of radiation-induced thyroid cancer [18]; dietary iodide was low in the areas around Chernobyl, but is high in Japan. Each of these separate pieces of evidence suggests that the high prevalence of thyroid carcinoma found in the first 3 years was not related to the accident.
In the absence of any other known cause of a massive apparent increase in thyroid cancer incidence, the current findings must represent the normal situation, uncovered by highly sensitive ultrasound. Some of the tumours found might, in the absence of screening, have presented clinically within a few years, others with a slower initial growth rate might have presented in older individuals, and some might have regressed or never have presented clinically. The extent to which resection of screen-detected tumours reduces the later incidence of clinically significant cancers should become apparent on the planned follow-up, as will the age-related progression of detectable nodules less than 5 mm in diameter. Although there is no evidence of a radiation-related increase in thyroid cancer in the first 3 years after the accident, it is likely that one will occur. The level of exposure combined with the high dietary stable iodine suggests that it will be on a much smaller scale and with a longer latent period than after Chernobyl. One forecast suggests it could be 6% of the normal incidence [19].
The early age at which the incidence of sporadic thyroid carcinoma rises and the generally slow tumour growth shows that for many clinically significant sporadic thyroid cancers, the first mutations leading to malignancy occur in infancy or childhood. Chernobyl and other radiation studies show that the chance of a first mutation progressing to a clinically significant cancer is greatest if it occurs in the earliest years of life. The evidence from Fukushima shows that many more thyroid carcinomas than were previously realised must originate in early life. Autopsy studies found little or no change in frequency of micro-papillary thyroid cancers between young and older adults [20,21]. It is therefore suggested that both sporadic and radiation-induced cancer share the same age-related pattern of acquisition of potentially carcinogenic mutations, with for most cancers the first mutation in the carcinogenic cascade occurring in the first few years of life.
Molecular Findings in Sporadic and Radiation-Induced Thyroid Cancer
The great majority of sporadic papillary thyroid carcinomas have been shown to have mutations in one of two major oncogenes, RET or BRAF [22]. The range of driver mutations has been expanded in a recent comprehensive study of the genomic landscape in several hundred papillary carcinomas [23]. The RET gene in follicular cells is activated by rearrangement, the BRAF gene almost always by point mutation. Nearly all the thyroid cancers that have been attributed to external or internal radiation have been papillary in type; where the oncogenes involved have been studied rearrangements in RET rather than point mutations in BRAF have been linked to radiation carcinogenesis [24,25]. Interestingly, a rare finding in short latency papillary carcinomas in children exposed to Chernobyl fallout is activation of BRAF by rearrangement [26].
The fact that radiation is particularly effective in causing double-strand breaks in DNA and that double-strand breaks are necessary precursors of rearrangements provides an explanation for the importance of this type of mutation in radiation carcinogenesis [8]. It also suggests that the rearrangement is the initial step in the carcinogenic cascade leading to papillary carcinoma. Expression of an introduced rearranged RET gene in animal thyroids led to the development of papillary carcinomas [27], but only in some of the cells possessing the gene, supporting the view that rearranged RET alone is not carcinogenic and that additional events are needed. These additional events are assumed to be mutations, recognising that other changes heritable at the cellular level, for example epimutations or somatic copy number alterations, are likely to be relevant.
Many different rearrangements of the RET gene have been associated with papillary carcinomas; in each, one of the breakpoints leads to a growth stimulus through activation of the RET tyrosine kinase. In at least some rearrangements there is evidence that the other breakpoint may cause loss of function of genes associated with tumour suppression or DNA repair, and it has been suggested that this may be a common feature of oncogenic rearrangements [28]. Carcinogenesis is thought to be caused by the sequential acquisition of mutations; where a single event such as a rearrangement involves two of the limited number of steps in the carcinogenic cascade, the latent period would be expected to be shortened. This could contribute to the earlier age of onset associated with RET-positive compared to BRAF point mutation-positive sporadic papillary carcinomas.
The post-Chernobyl papillary carcinomas with a BRAF rearrangement were associated with a very short latency, again compatible with a carcinogenic contribution from the other breakpoint – in this case involving AKAP9. This gene has been linked to many functions; it is mutated in some oral squamous carcinomas and is also overexpressed in metastatic melanoma [29,30]. The nature of the subsequent events needed for a cell bearing an RET rearrangement or a BRAF point mutation to give rise to a papillary carcinoma is not known, although a variety of mutations have been described. The risk of developing papillary carcinoma must depend not only on the risk of a cell acquiring a RET or other relevant initial mutation, but also on the risk of that cell acquiring the further changes needed for carcinogenesis.
Cell of Origin of Thyroid Cancer
It is important to determine whether normal extra-uterine thyroid growth derives from stem cells or differentiated cells, and whether thyroid neoplasms originate in stem or differentiated cells. Several groups have identified cells derived from follicular epithelium showing some of the properties of stem cells, and leading to the assumption that stem cells are present in adult thyroid and are the cell of origin of thyroid cancers. These studies cannot be regarded as definitive as they have depended on in vitro manipulation of follicular cells, and lineage studies are essential for identification of active stem cells [31]. In addition, it has been shown that differentiated follicular cells can be manipulated to give rise to multilineage progenitor cells [32]. No lineage studies showing that extra-uterine follicular cell growth originates in thyroid stem cells have been published, and follicles lack the clonal structure that would be expected if they were maintained by stem cells [33]. Tissues maintained by stem cells such as skin or intestines show morphological changes of differentiation with proliferative zones, as do their well-differentiated tumours. Normal follicular cells show uniform high levels of differentiation and lack proliferative zones; most thyroid cancers also show uniform high levels of differentiation and lack proliferative zones. Undifferentiated carcinomas do not arise directly from the normal gland but from pre-existing differentiated cancers. It is therefore concluded that thyroid growth in extra-uterine life is not maintained by stem cells and that thyroid tumours originate in differentiated follicular cells.
Thyroid Growth and the Origins of Thyroid Cancer
The thyroid weighs approximately 1 g at birth rising to 16-20 g by early adult life, with considerable variation depending on dietary iodine levels. The weight normally changes little during adult life. In keeping with this, the follicular cell mitotic activity in extra-uterine life is highest at birth, reducing as the gland grows to reach very low levels in adults [34]. Expansile growth ceases in late adolescence, the low mitotic activity in adults represents replacement growth. It has previously been suggested that the relationship between age at exposure to radiation and risk of developing thyroid cancer could be related to the growth pattern of the follicular epithelium, either to the mitotic frequency or to the number of mitoses remaining in the progeny of the initially mutated cell [34,35]. Both drop with increasing age, reflecting the growth pattern of the thyroid. While most mutations occur at cell division, this is not the case for rearrangements as these occur during interphase and are influenced by the proximity of the genes involved [36]. On this basis, the RET rearrangement would be expected to occur at any age and the age-dependent sensitivity would be related to the chance of occurrence of subsequent mutations in the clone of cells derived from the initiated cell. The majority of these are probably point mutations leading to changes such as loss of function in tumour suppressor or DNA repair genes, and are likely to be associated with cell division.
Thyroid growth from birth to maturity involves at least a 16-fold increase in weight and a similar increase in cell number as there is little change in cell density. As a minimum of 4 rounds of cell division will be involved, the daughter cells derived from a cell in an infant that acquired a potentially carcinogenic mutation such as a RET rearrangement would pass through at least 15 cell divisions and the mutation would be present in 16 cells by adulthood. A RET mutation occurring in a follicular cell in a child aged about 10 might pass through only one cell division and be present in only 2 cells in adult life. This could provide a simple explanation for the age-related sensitivity; the chance of acquiring the first mutation is related to the radiation dose, and the chance of acquiring the subsequent mutations is related to the number of cell divisions in the subsequent clone.
The situation is of course much more complex. A RET rearrangement or other initial mutation might increase the growth rate of the affected clone of cells, loss of function in genes at the upstream breakpoint might lessen DNA repair efficiency and therefore increase the chance of subsequent point mutations. The growth potential of normal follicular cells is limited [37], and the clonal size generated by a mutated cell with increased growth but not immortality would also be limited. The younger the age at which the first mutation occurs, the greater the number of subsequent cell divisions in the progeny of the affected cell; however, the older the age, the greater the number of cells at risk, but the fewer the number of divisions available for further mutations. Further evidence showing the critical importance of post-mutagen exposure growth comes from experimental studies showing that complete suppression of thyroid growth immediately after radiation exposure abolishes carcinogenesis [38]. While there are many unknown variables, it can be seen that the growth pattern of the thyroid can provide an explanation for the finding that oncogene mutations occurring in the early years of life are much more likely to progress to malignancy than similar mutations occurring in older individuals.
A Model of Thyroid Carcinogenesis
It is suggested that cancer of the thyroid differs from most other cancers because it is derived from a differentiated cell with a limited growth capacity rather than a presumed immortal stem cell. The evidence discussed above supports a model of thyroid carcinogenesis in which the great majority of papillary cancers develop before adult life and show a very variable latency resulting in clinical presentation throughout life. The development of malignancy can be divided into three stages (table 1). In this model the initial mutation, necessary but not sufficient for malignancy, may or may not be related to cell division, but is only likely to progress to malignancy if it occurs in childhood. Progression to low-grade malignancy, stage 2, is dependent on mutations taking place during thyroid growth, almost all of which occurs in childhood and adolescence. Escape from the growth limitation found in normal follicular cells, stage 3, allows a greater growth rate and an increased chance of further mutation. If this does not take place before adult life, the tumour is likely to remain as a small slowly growing stage 2 cancer with limited growth capacity, currently referred to as a papillary micro-carcinoma. The extremely rare development of an anaplastic carcinoma from a pre-existing more differentiated carcinoma could be regarded as a fourth stage, in which the additional mutations needed cause the tumour cell to lose all thyroid differentiation and growth limitation. Only a minority of cells that possess the initial mutation are likely to acquire the additional mutations needed for malignancy. Only a minority of the resulting well-differentiated stage 2 papillary carcinomas are likely to acquire the further mutations needed to give rise to more aggressive tumours. Unless further mutations occur or they reach their growth limit, the great majority of stage 2 cancers would not be expected to significantly increase their low growth rate throughout life. Some might reduce or even stop growth in the way that normal cells reduce their growth rate before adulthood, so that the tumour and possibly its metastases reach replicative senescence, with no further progression. The more slowly growing tumours will take longer to reach a detectable size than the more rapidly growing lesions. Small papillary carcinomas are extremely common; larger differentiated cancers and poorly differentiated and undifferentiated carcinomas are rare.
Table 1.
Proposed stages in the development of papillary thyroid carcinoma
| Stage, mutations and timing | Relevant factors | Comment |
|---|---|---|
| 1. Initial mutation; any age for RET or other rearrangement, predominantly infancy and childhood for BRAF or other point mutation | Spontaneous error rate together with exposure to radiation (rearrangement) or other carcinogen (point mutation) | Possible increased growth rate in resulting clone of cells |
| 2. Subsequent mutations in initiated cell clone giving rise to well-differentiated PTC but not complete escape from growth limitation; predominantly in infancy and childhood | Spontaneous error rate, contribution from both rearrangement breakpoints, possible additional growth of initiated cells, any additional carcinogen exposure | High levels of differentiation linked to low and limited net growth rate, very low risk of progression in adults; papillary micro-carcinoma |
| 3. Additional mutation(s) giving complete escape from growth limitation; almost all escape before adulthood; risk of further progression thereafter | Error rate related to tumour cell number and growth rate; any additional carcinogen exposure | Increased growth rate, variable loss of differentiation and growth control (complete loss in undifferentiated cancer); papillary macro-carcinoma |
The chance of progression will be influenced by various factors, including tumour cell number and growth rate. A solid tumour with a 2-cm diameter is predicted to have 64 times the number of cells compared to a tumour with a 0.5-cm diameter, as a larger tumour is likely to have a greater growth rate and its chance of acquiring an additional mutation and progressing to a clinically significant lesion would be even greater. It is suggested that the biological basis for the separation of so-called ‘micro-papillary carcinomas’ is that they lack the final mutation breaking the tight link between differentiation and growth limitation seen in the normal follicular cell in adults. The proposal that stage 2 and 3 cancers are not simply part of a continuous gradient of malignancy is supported by their differing risk of progression with increasing age, rising with macro-papillary carcinomas but diminishing with micro-carcinomas [39].
Clinical Relevance
This model provides the framework to explain some of the clinical observations on papillary carcinoma and provides support for proposals to reconsider standard treatment protocols. The proposal that nearly all papillary carcinomas start in childhood and those that have not acquired complete growth independence maintain their growth rate after adolescence implies that most micro-papillary cancers in older people must have a long latent period, a very low net growth rate, and an extremely low risk of progression to a more aggressive form. The huge increase in the incidence of papillary carcinoma in the USA over the last 3 decades has not been accompanied by any change in the death rate. The questionable benefits from lobectomy, thyroidectomy, or radioiodine therapy for small papillary carcinomas in adults have to be balanced against the known risk of complications.
Age as well as tumour size is an important variable when considering the risk to the patient from a well-differentiated papillary carcinoma. A papillary carcinoma of 1 cm in diameter in an adolescent will carry a very different risk for the patient than one of similar size in a patient of 70. The latent period is shorter, the growth rate is likely to be higher, and the chance of progression greater. After Chernobyl some of the primary papillary carcinomas in children associated with widespread metastases were less than 1 cm in diameter. In studies of micro-papillary carcinomas, the rate of increase in size and the frequency of metastasis is greater in younger than in older subjects [39]. This large study of papillary micro-carcinomas also suggested that older patients with micro-carcinoma may be candidates for observation only, and pointed out that if the tumour is later found to be expanding, treatment is still available. The patients in this study can be assumed to have the same frequency of central lymph node metastasis (up to 65%) as in studies where lymph node resection was carried out. In one such study, 551 patients had central lymph node resection for micro-papillary thyroid cancer and 13 suffered permanent hypocalcaemia or permanent vocal cord palsy [40]. Microscopic metastases do not necessarily indicate an aggressive tumour or justify aggressive therapy. Current guidelines should reconsider their advice on the treatment of thyroid carcinoma in view of the likely continuing increase in incidentally discovered tumours and the findings from Chernobyl and Fukushima. There is a strong case for a reduction in the use of surgery for the treatment of incidentally discovered small papillary carcinomas, taking tumour size and patients age into account. If the ever-smaller tumours being discovered are treated by surgery in older patients, the risks of morbidity are likely to outweigh the risks of morbidity from an unoperated tumour. All involved in treating thyroid cancer should be concerned that a tumour with the lowest risk of death of any major cancer is associated in the USA with a higher risk of bankruptcy than with major life-threatening cancers [41].
There is another reason for resection of small papillary carcinomas; once a patient has been told that their thyroid nodule was a cancer, they may remain fearful until it is removed and may not fully accept reassurances that they need not be concerned. The reason for the patient's concern is the association of the word cancer with prolonged suffering and death. If an invasive and potentially metastatic tumour kills 1 in 2 patients, there is no doubt that it should be called a cancer – both for theoretical reasons and for communication with the patient. Suppose the tumour causes a death rate in untreated patients of 1 in 100, 1 in 1,000, or 1 in 10,000 – is the term ‘cancer’ still clinically appropriate whatever the risk? One way of reducing the concern that follows from using the word ‘cancer’ in a clinical setting for a tumour that is technically malignant but clinically benign is to accept the patient's usage, restrict the use of the term ‘cancer’ to lesions with a significant chance of causing suffering and death, and change the name used for small papillary carcinomas with a prognosis indistinguishable from normal life expectancy. We suggested in 2003 that with certain exceptions the term ‘papillary micro-tumour’ should be used for lesions in adults currently referred to as micro-papillary carcinomas [42]. Other names have been suggested. There is a precedent; in 1907 Oberndorfer [43] described a small primary tumour of the intestines with a distinctive histology. It was invasive but in contrast to other invasive intestinal tumours was generally associated with a relatively good prognosis. Because of this contrast he called the tumour a ‘carcinoid’ rather than a ‘cancer’. An imaginative use of nomenclature is a possible way of dealing with the problem posed by the inappropriate use of the word ‘carcinoma’; the term ‘papillary micro-tumour’ has proven acceptable in practice [44]. Other names are possible, but wide consultation, including with patient groups, and international agreement is needed.
Conclusions
This review and analysis of the relationship between thyroid growth and thyroid cancer is based on multiple strands of evidence. The main conclusions are (1) that thyroid tumours originate not in stem cells but in the differentiated follicular cell; (2) that the initial mutation leading to thyroid carcinogenesis in the great majority of cases occurs in infancy and childhood; (3) that the chance of the remaining mutations occurring is related to thyroid growth, so the earlier the first mutation occurs the greater the likelihood of malignancy; (4) that if a tumour has acquired the mutations for malignancy but not complete loss of the tight linkage between differentiation and growth seen in the normal follicular cell by adult life, tumour growth rate will remain low and the chance of progression will be very small; (5) that treatment protocols for the management of papillary micro-carcinomas need to be reviewed, and (6) that micro-papillary carcinomas should be given a name better reflecting their indolent behaviour.
This analysis suggests that there is a biological distinction between so-called micro- and macro-papillary carcinomas; a separation based purely on size is not tenable. The choice of size as a criterion reflects the divergence in later life of those tumours with and those without escape from growth limitation. It is not applicable to small cancers in children, and its importance in young adults is not clear. This is relevant to the treatment of screen-detected micro-carcinomas in young people, as after Fukushima. It has been suggested that resection is overtreatment since these are micro-carcinomas [15], but the evidence based largely on studies in older patients should not be applied to adolescents and very young adults. Follow-up studies of the cohort in Fukushima should provide very valuable evidence on the incidence of very small lesions, and the effect of resection of early micro-lesions on the later frequency of larger tumours. Studies to provide direct evidence for consistent differences between stage 2 and stage 3 cancers could assist diagnosis and management and elucidate the mechanisms involved. These should include whole genome sequence and expression studies of large and small cancers in older patients, quantitative analysis of the proportion of cells in cycle, and the rate of cell loss. It will be important to determine whether morphological or molecular features that are linked to a more aggressive behaviour in large tumours do or do not carry the same significance in small tumours in older patients. The high proportion of BRAF V600E mutations in micro-papillary thyroid cancers suggests that driver mutations alone are not a reliable guide to clinical behaviour [45].
The aim of treatment is always to ensure that the benefit is greater than the chance of detriment. For most cancers this normally involves attempting to remove or kill every cancer cell. This does not necessarily apply to a stage 2 papillary cancer in older patients where cancer cells are so few and so slowly growing that treatment may be detrimental. Ideally treatment decisions should be based on clinical trials, but these are difficult in such a slowly growing tumour and with the changing pattern of disease due to the increasing use of imaging techniques. The treatment protocols for small papillary carcinomas and their nomenclature need to be reconsidered in the light of the new evidence available, the continuing discovery of smaller lesions, and the model of thyroid carcinogenesis proposed.
Disclosure Statement
The author declares no relevant financial interests.
References
- 1.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 2.Zhu C, Zheng T, Kilfoy BA, et al. A birth cohort analysis of the incidence of papillary thyroid carcinoma in the United States, 1973-2004. Thyroid. 2009;19:1061–1066. doi: 10.1089/thy.2008.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid: a ‘normal' finding in Finland. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Beerenwinkel N, Antal T, Dingli D, et al. Genetic progression and the waiting time to cancer. PLoS Comput Biol. 2007;3:e225. doi: 10.1371/journal.pcbi.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21. doi: 10.1038/359021a0. [DOI] [PubMed] [Google Scholar]
- 6.Baverstock K, Egloff B, Pinchera A, Ruchti C, Williams D. Thyroid cancer after Chernobyl. Nature. 1992;359:21–22. doi: 10.1038/359021b0. [DOI] [PubMed] [Google Scholar]
- 7.Williams ED. Effects on the thyroid in populations exposed to radiation as a result of the Chernobyl accident. In: Williams ED, Tronko ND, editors. One Decade after Chernobyl: Proceedings of an International Conference. Vienna: IAEA; 1996. [Google Scholar]
- 8.Williams D. Cancer after nuclear fallout: lessons from the Chernobyl accident. Nat Rev Cancer. 2002;2:543–549. doi: 10.1038/nrc845. [DOI] [PubMed] [Google Scholar]
- 9.Holm LE, et al. Cancer risk after iodine 131 therapy for hyperthyroidism. J Nat Cancer Inst. 1991;83:1072–1077. doi: 10.1093/jnci/83.15.1072. [DOI] [PubMed] [Google Scholar]
- 10.Ron E, Lubin JH, Shore RE, et al. Thyroid carcinoma after exposure to external radiation. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 11.Furukawa K, Preston D, Funamoto S, et al. Long-term trend of thyroid cancer risk among Japanese atomic bomb survivors: 60 years after exposure. Int J Cancer. 2013;132:1222–1226. doi: 10.1002/ijc.27749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita S, Suzuki S. Risk of thyroid cancer after the Fukushima nuclear power plant accident. Respir Investig. 2013;51:128–133. doi: 10.1016/j.resinv.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima Medical University http://www.fmu.ac.jp/radiationhealth/results/20141225_Thyroid_Ultrasound_Examination.html (accessed April 15, 2015).
- 14.Center for Cancer Control and Information Services , National Cancer Center, Japan. http://ganjoho.jp/pro/statistics/en/table_download.html (accessed April 15, 2015).
- 15.Shibuya K, Gilmour S, Oshima A. Time to reconsider thyroid cancer screening in Japan. Lancet. 2014;383:1883–1884. doi: 10.1016/S0140-6736(14)60909-0. [DOI] [PubMed] [Google Scholar]
- 16.UNSCEAR 2013 Report to the General Assembly. Volume 1, Scientific Annex A. New York, United Nations, 2014.
- 17.Baverstock K. 2013 UNSCEAR report on Fukushima: a critical appraisal. Kagaku. 2014;84:1–8. [Google Scholar]
- 18.Cardis E, Kesminiene A, Ivanov V, et al. Risk of thyroid carcinoma after exposure to 131I in childhood. J Nat Cancer Inst. 2005;97:1–9. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 19.Jacob P, Kaiser JC, Ulanovsky A. Ultrasonography survey and thyroid cancer in the Fukushima Prefecture. Radiat Environ Biophys. 2014;53:391–401. doi: 10.1007/s00411-013-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bondeson L, Ljungberg O. Occult papillary thyroid carcinoma in the young and the aged. Cancer. 1984;53:1790–1792. doi: 10.1002/1097-0142(19840415)53:8<1790::aid-cncr2820530831>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Franssila KO, Harach HR. Occult thyroid carcinoma in children and young adults. A systematic autopsy study in Finland. Cancer. 1986;58:715–719. doi: 10.1002/1097-0142(19860801)58:3<715::aid-cncr2820580319>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabes HM, Demidchik EP, Siderov JD, et al. Pattern of radiation induced RET and NTRK1 rearrangements in 191 post-Chernobyl papillary thyroid carcinomas. Clin Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 25.Hamatani K, Eguchi H, Ito R, et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008;68:7176–7182. doi: 10.1158/0008-5472.CAN-08-0293. [DOI] [PubMed] [Google Scholar]
- 26.Ciampi R, Knauf JA, Kerler R, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2003;115:94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jhiang SM, Jho JY, Furminger TL, et al. Thyroid carcinomas in RET/PTC transgenic mice. Recent Results Canc Res. 1998;154:265–270. doi: 10.1007/978-3-642-46870-4_17. [DOI] [PubMed] [Google Scholar]
- 28.Williams D. Radiation carcinogenesis: lessons from Chernobyl. Oncogene. 2009;27:S9–S18. doi: 10.1038/onc.2009.349. [DOI] [PubMed] [Google Scholar]
- 29.Onken MD, Winkler AE, Kanchi KL, et al. A surprising cross species conservation in the genomic landscape of mouse and human oral cancer identifies a transcriptional signature predicting metastatic disease. Clin Cancer Res. 2014;20:2873–2884. doi: 10.1158/1078-0432.CCR-14-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabbarah O, Nogueira C, Feng B, et al. Integrative genome comparison of primary and metastatic melanoma. PLoS One. 2010;5:e10770. doi: 10.1371/journal.pone.0010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright NA. Stem cell identification – in vivo lineage analysis versus in vitro isolation and clonal expansion. J Pathol. 2012;227:255–266. doi: 10.1002/path.4018. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Mitsutake N, Saenko V, et al. Dedifferentiation of human primary thyrocytes into multilineage progenitor cells without gene introduction. PLoS One. 2011;6:e19354. doi: 10.1371/journal.pone.0019354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas GA, Williams D, Williams ED. The clonal origin of thyroid nodules and adenomas. Am J Pathol. 1989;134:141–147. [PMC free article] [PubMed] [Google Scholar]
- 34.Saad AG, Kumar S, Ron E, et al. Proliferative activity of human thyroid cells in various age groups, and its correlation with the risk of thyroid cancer after radiation exposure. J Clin Endocrinol Metab. 2006;91:2672–2677. doi: 10.1210/jc.2006-0417. [DOI] [PubMed] [Google Scholar]
- 35.Williams ED. Biological mechanisms underlying radiation induction of thyroid carcinoma. In: Thomas G, Karaoglou A, Williams ED, editors. Radiation and Thyroid Cancer. Singapore: World Scientific; 1999. [Google Scholar]
- 36.Gandhi M, Evdokimova V, Nikiforov YE. Mechanisms of chromosome rearrangements in solid tumors: the model of papillary thyroid carcinoma. Mol Cell Endocr. 2010;321:36–43. doi: 10.1016/j.mce.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wynford-Thomas D, Stringer BJ, Williams ED. Dissociation of growth and function in the rat thyroid. Acta Endocrinol. 1982;101:562–569. doi: 10.1530/acta.0.1010210. [DOI] [PubMed] [Google Scholar]
- 38.Nichols CW, Lindsay S, Sheline GE, Chaikoff IL. Induction of neoplasms in the thyroid gland by irradiation of a single lobe. Arch Path. 1965;80:177–191. [PubMed] [Google Scholar]
- 39.Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27–34. doi: 10.1089/thy.2013.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.So YK, Son YI, Hong SD. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery. 2010;148:526–531. doi: 10.1016/j.surg.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosai J, LiVolsi VA, Sobrinho-Simoes M, Williams ED. Renaming papillary carcinoma of the thyroid gland: the Porto proposal. Int J Surg Pathol. 2003;11:249–251. doi: 10.1177/106689690301100401. [DOI] [PubMed] [Google Scholar]
- 43.Oberndorfer S. Karzinoide Tumoren des Dünndarms. Frankf Z Pathol. 1907;1:426–432. [Google Scholar]
- 44.Asioli S, Odasso C, Macri L, Palestini N, Bussolati G. Merits of the PmiT (Papillary Microtumor) terminology in the defibinition of a subset of incidental papillary microcarcinomas of the thyroid. Int J Surg Path. 2009;17:378–383. doi: 10.1177/1066896908321181. [DOI] [PubMed] [Google Scholar]
- 45.Virk RK, Van Dyke AL, Finkelstein A, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype-phenotype correlation. Modern Pathol. 2013;26:62–70. doi: 10.1038/modpathol.2012.152. [DOI] [PubMed] [Google Scholar]