Abstract
Apoptosis mediated by Fas/FasL has been implicated in pulmonary disorders. However, little is known about the relationship between Fas and FasL in the process of lung injury during malaria infection. Paraffin-embedded lung tissues from malaria patients were divided into two groups: those with pulmonary edema (PE) and those without pulmonary edema (non-PE). Normal lung tissues were used as the control group. Cellular expression of Fas, FasL, and the markers of apoptotic caspases, including cleaved caspase-3 and cleaved caspase-8 in the lung tissues were investigated by the immunohistochemistry (IHC) method. Semi-quantitative analysis of IHC staining revealed that cellular expression of Fas, FasL, cleaved caspase-8, and cleaved caspase-3 were significantly increased in the lungs of patients with PE compared with the lungs of patients with non-PE and control groups (all P < 0.05). In addition, significant positive correlations were obtained between Fas and apoptosis (rs = 0.937, P < 0.001) and FasL and apoptosis (rs = 0.808, P < 0.001). Significant positive correlations were found between Fas and FasL expression (rs = 0.827, P < 0.001) and between cleaved caspase-8 and cleaved caspase-3 expression (rs = 0.823, P < 0.001), which suggests that Fas-dependent initiator and effector caspases, including cleaved caspase-8 and caspase-3, are necessary for inducing apoptosis in the lungs of patients with severe P. falciparum malaria. The Fas/FasL system and downstream activation of caspases are important mediators of apoptosis and may be involved in the pathogenesis of pulmonary edema in severe P. falciparum malaria patients. The proper regulation of the Fas/FasL pathway can be a potential treatment for pulmonary complications in falciparum malaria patients.
Keywords: Malaria, Fas, FasL, apoptosis, pulmonary edema
Introduction
Approximately 25% of adult and 40% of child patients with severe Plasmodium falciparum (P. falciparum) malaria develop respiratory complications [1]. Pulmonary edema is one of the major causes of pulmonary manifestation of malaria and is usually associated with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), which occurs in approximately 20% of severe P. falciparum malaria patients [2]. It has been proposed that increased alveolar permeability resulting in intravascular fluid loss into the lungs is the key pathophysiological mechanism [1,3]. Evidence of the sequestration of parasitized red blood cells (PRBCs) in the pulmonary capillaries and recruitment of the host inflammation response have been reported as playing major roles in the pathogenesis of pulmonary manifestation during malaria infection [4]. However, the pathogenetic mechanisms underlying lung injury in malaria are poorly understood. Fas (CD95)/Fas ligand (FasL/CD95L) system-mediated apoptosis has been implicated in pulmonary disorders [5]. Fas activation also leads to a form of lung injury characterized by increased alveolar permeability [6].
The Fas/FasL system plays an important role in the regulation of apoptosis in various cell types [7]. Fas is a 45-kD type I membrane receptor that is a member of the tumor necrosis factor family of surface receptors [8,9]. This protein is expressed on many cell types of the lung, including inflammatory cells, alveolar macrophages, and alveolar epithelial cells [5,10-12]. FasL is a 37-kD type II membrane glycoprotein that belongs to a member of the tumor necrosis factor family of cytokines [13]. FasL can be found in the soluble form in circulation or the membrane-bound form in some cells such as neutrophil and activated T cells [14,15]. Membrane-bound FasL is converted to a soluble form by a matrix metalloproteinase-like enzyme [15]. Both forms of FasL have been reported to induce apoptosis when binding with Fas receptors on the cell surface [9,16]. Previous studies have demonstrated that the Fas/FasL system acts as a pro-apoptotic system, which has been implicated in the development of ALI and ARDS. The level of soluble Fas was increased in bronchoalveolar lavage (BAL) fluid [17,18] and pulmonary edema fluid [5] of ARDS patients and has the ability to induce apoptosis of distal lung epithelium [18] and alveolar epithelium [6]. Several reports have revealed that Fas and FasL are expressed on the alveolar and inflammatory cells in the lung tissues of mice [10] and humans [5]. Detection of cleaved caspase-3 has been demonstrated in lung epithelium of children with ARDS [19]. According to previous studies, these findings suggested that Fas/FasL system-mediated apoptosis and downstream activation of apoptosis caspases may contribute to the pathogenesis of ALI.
In this study, since Fas and FasL have not been previously studied in lungs of severe falciparum malaria patients, cellular expression of the Fas/FasL system and the markers of apoptotic caspases were investigated by IHC staining. Results of semi-quantitative analysis of cellular expression of each apoptotic marker (Fas, FasL, cleaved caspase-8, and cleaved caspase-3) in the lungs of severe falciparum malaria patients with pulmonary edema (PE) were compared to results of non-pulmonary edema (non-PE) and the control group. In addition, the correlation between each apoptotic markers and clinical data and severity of lung injury were analyzed.
Materials and methods
Lung tissue specimens
The formalin fixed, paraffin-embedded lung tissues from autopsy of 37 malaria patients were obtained from the Department of Tropical Pathology, Faculty of Tropical Medicine, Mahidol University, Thailand. On the basis of histopathological findings obtained from the autopsy records, lung tissues from malaria patients with P. falciparum infection were classified into two groups: those who presented with pulmonary edema (PE) (n = 18 cases) and those who presented without pulmonary edema (non-PE) (n = 19 cases). Normal lung tissues (n = 10 cases) from patients who died accidentally with no pathological changes to the lungs were used for a control group. Clinical data for each malaria patient were collected from medical records. Demographic data, including age, gender, parasite density, days of hospitalization, hemoglobin, hematocrit, and white blood cell (WBC) count, are shown in Table 1. The use of leftover lung specimens and the study protocol were reviewed and approved by the Ethics Committee of Faculty of Tropical Medicine, Mahidol University (MUTM 2013-015-01) and the Ethics Committee on Human Rights Related to Research Involving Human Subjects, Walailak University, Thailand (061/2012).
Table 1.
Clinical characteristic of 37 malaria patients with non-PE and PE
| Clinical characteristics | non-PE (n = 19) | PE (n = 18) |
|---|---|---|
| Sex (male: female) | 13:6 | 11:7 |
| Age (years) | 22.58 ± 3.52 | 28.55 ± 2.88 |
| Parasitemia (/µl) | 218,675.72 ± 91,531.65 | 464,300.84 ± 83,675.36* |
| Days of hospitalization (hr) | 47.05 ± 9.18 | 71.72 ± 9.95 |
| Hemoglobin (g/dL) | 10.21 ± 0.75 | 8.84 ± 0.69 |
| Hematocrit (%) | 31.89 ± 2.39 | 27.0 ± 2.07 |
| White blood cell (WBC) (/µl) | 16,627.47 ± 2,168.83 | 14,816.11 ± 1,926.16 |
Data are expressed as mean ± SEM.
Significant difference of P < 0.05, compared to non-PE group.
Histopathological examinations
A histopathological scoring method was used to quantify changes in lung tissue stained with hematoxylin and eosin (H&E) under a light microscope at high magnification (400 ×) by two observers who were blinded to the clinical data. The degree of microscopic injury was scored based on the following variables: PRBC sequestration in the septal capillaries, septal hemorrhage, alveolar edema, alveolar hemorrhage, leukocyte infiltration, alveolar macrophage, and septal thickening. The severity of injury was graded for each variable: no injury = 0; injury to 25% of the high power field = 1; injury to 50% of the high power field = 2; injury to 75% of the high power field = 3; and diffuse injury to > 75% of the high power field = 4 [20]. Subsequently, a lung injury score ranging from 0 to 28 points was calculated by adding the sum of each variable to determine the overall histopathological changes in lung tissue from malaria patients with P. falciparum. A score of 0 meant the absence of histopathological changes while a score of 28 signified the most severe histopathological changes. In addition, 10 images of H&E lung slides for each case were used to measure the thickness of alveolar septa using the computer software program “Image Tool” (version 3.0, UTHSCSA). Average septal width in each group was calculated and presented as mean ± standard error of the mean (SEM).
Immunohistochemical staining for apoptotic markers
Lung tissues were sectioned at 4-μm thickness, deparaffinized in xylene, and rehydrated through graded concentrations of alcohol. Subsequently, antigen unmasking was performed in a citrate-based solution, pH 6.0 (Vector Laboratories, CA, USA) using a microwave method. The endogenous peroxidase was inactivated with 3% hydrogen peroxide in distilled water for 30 min at room temperature (RT). After washing, the nonspecific binding site was blocked with normal goat serum for 30 min at RT. Lung sections were incubated with primary antibody: rabbit anti-human Fas (1:200 optimal dilution; Santa Cruz Biotechnology Inc., CA, USA) or rabbit anti-human FasL (1:200 optimal dilution; Santa Cruz Biotechnology Inc., CA, USA) or rabbit anti-human cleaved caspase-8 (1:200 optimal dilution; Cell Signaling Technology, MA, USA) or rabbit anti-human cleaved caspase-3 (1:200 optimal dilution; Cell Signaling Technology, MA, USA) overnight at 4°C. The following day, the lung sections were washed three times with phosphate-buffered saline (PBS) and incubated with secondary antibody for 30 min at RT and reacted with avidin-biotin complex (ABC) conjugated with horseradish peroxidase (HRP) (Vecterstain ABC Kit; Vector Laboratories, CA, USA) according to the manufacturer’s instructions. After washing with PBS, the peroxidase reaction was developed by the Impact DAB Kit (Vector Laboratories, CA, USA) as chromogen for 3 mins. Finally, lung sections were counterstained with Mayer’s hematoxylin (Merck, Darmstadt, Germany) then dehydrated and mounted with a coverslip. Negative controls were processed with the omission of the primary antibody and were also stained in each run.
Semi-quantitative analysis of immunohistochemical staining for apoptotic markers
Immunostained sections of lung tissue were examined under a light microscope. All evaluation was performed by two independent observers (CP and PV) who were unaware of the patients’ clinical outcomes. To determine apoptosis in the lung tissues, each immunostained section was randomly counted in 10 microscopic fields at high magnification (400 ×) for immunopositive cells stained for the target marker. The expressions of Fas, FasL, cleaved caspase-8, and cleaved caspase-3 were separately examined in alveolar epithelium, alveolar macrophages, and leukocytes in lung tissues based on morphological identification. Consequently, the percentage of positive stained cells for each protein marker was calculated by dividing the number of positive cells by the total cell count and multiplying this number by 100. Finally, the percentage of the total positive cells for each protein marker was calculated by adding the sum of the percentage of positive stained cells for each protein. The average percentage of total positive cells for each protein markers was then calculated. The results were presented as mean ± SEM.
Statistical analysis
Results were presented as mean ± SEM. Statistical analysis was performed using SPSS version 17.0 software (SPSS, IL, USA). The normality of distribution was tested with Kolmogorov-Smirnov test. Difference between groups was analyzed by the Mann-Whitney U test. Spearman’s rank correlation coefficient was computed to estimate the direction and strength of correlation between the expression of all apoptotic markers and histopathological changes and clinical data. Statistical significance was defined as P value of ≤ 0.05.
Results
Patient characteristics
Thirty-seven severe P. falciparum malaria patients with non-PE and PE were included in this study. Demographic and clinical characteristics of all malaria patients are summarized in Table 1. There was a significant difference in parasite density between malaria patients with PE and non-PE (P < 0.05). There was no significant difference in age, days of hospitalization, hemoglobin, hematocrit, and WBC count between malaria patients with PE and non-PE (all P > 0.05).
Histopathological features in the lung tissues of severe falciparum malaria patients
The lung histopathology of severe falciparum malaria patients with PE revealed alveolar edema fluid, alveolar and septal hemorrhages, activation of alveolar macrophages laden with hemosiderin pigments, and PRBC sequestration in the septal capillary (Figure 1B and 1C). A large number of alveolar macrophages and mixed inflammatory infiltration was present in the alveolar space (Figure 1B). The alveoli were filled with PRBCs, red blood cells (RBCs), and pigment-laden macrophages (Figure 1B). In addition, alveolar hemorrhage was always seen in the lung tissues of patients with severe falciparum malaria (Figure 1D). Semi-quantitative analysis of histopathological changes in the lung tissues of severe falciparum malaria patients with non-PE and PE are shown in Table 2. The lung injury score was significantly higher for the PE group (13.72 ± 0.45) compared to the non-PE group (6.42 ± 0.41) (P < 0.001). In addition, quantitative analysis for septa thickness demonstrated greater septal width for the PE group (20.9 ± 0.36 μm) than the non-PE (14.43 ± 0.34 μm) and control groups (9.3 ± 0.09 μm) (all P < 0.001).
Figure 1.
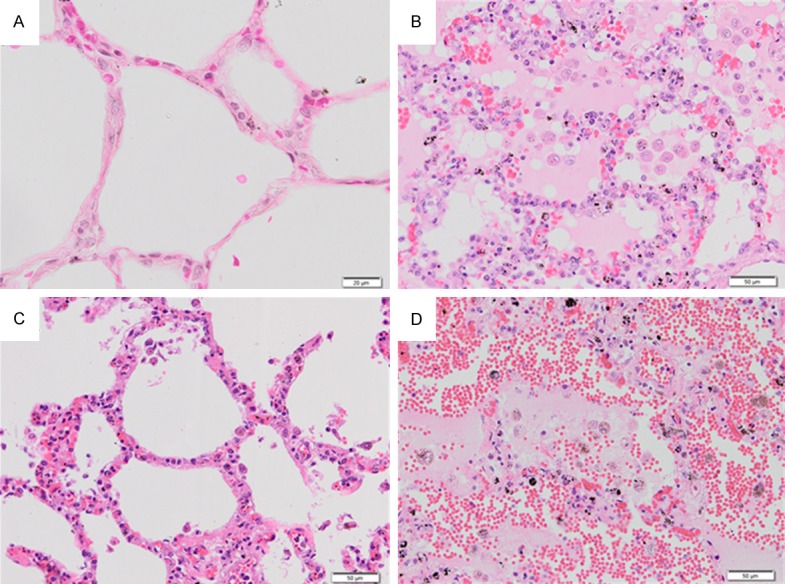
Histopathological changes in H&E-stained lung tissues of severe falciparum malaria patients. Normal lung tissue (A). The lung tissue of severe falciparum malaria patient showing edema fluid in the alveoli (B). The alveoli are filled with PRBCs, RBCs, and pigment-laden macrophages. PRBCs sequester in the septal capillaries and small blood vessels in the lung of severe falciparum malaria (C). Alveolar hemorrhage was always seen in the lung tissues of severe falciparum malaria patients (D). All images are 200 × magnification. Bar = 50 μm.
Table 2.
Semi-quantitative analysis of histopathological changes in the lung tissues of severe falciparum malaria patients with non-PE and PE
| Histopathological changes | Grading score (mean ± SEM) | P value | |
|---|---|---|---|
|
| |||
| non-PE (n = 19) | PE (n = 18) | ||
| PRBC sequestration | 0.00 ± 0.00 | 0.39 ± 0.24 | 0.067 |
| Alveolar hemorrhage | 0.32 ± 0.17 | 1.56 ± 0.29 | 0.001* |
| Septal hemorrhage | 2.21 ± 0.20 | 3.00 ± 0.28 | 0.014* |
| Aveolar edema | 0.00 ± 0.00 | 2.72 ± 0.24 | 0.001* |
| Alveolar macrophage | 1.95 ± 0.12 | 3.28 ± 0.16 | 0.001* |
| Leukocyte infiltration | 1.89 ± 0.11 | 2.61 ± 0.16 | 0.001* |
| Septal thickening | 0.05 ± 0.05 | 0.17 ± 0.12 | 0.500 |
| Lung injury score | 6.42 ± 0.41 | 13.72 ± 0.45 | 0.001* |
Significant difference of P < 0.05, compared to non-PE group.
Immunohistochemical staining for Fas and FasL
Figure 2 represents the pattern of immunoperoxidase staining for Fas or FasL in the lung tissues of patients with severe falciparum malaria with PE, non-PE and the control groups. Immunoreactivity for Fas and FasL was detected in alveolar cells located in the alveolar walls, alveolar macrophages distributed in the alveolar spaces, and leukocytes inside the septal capillary in the lung tissues of patients with PE (Figure 2E and 2F). Immunoreactivity for Fas and FasL was also found in the alveolar cells, alveolar macrophage, and leukocytes in the lung tissues of patients with non-PE (Figure 2C and 2D), but a low number of positive cells was found compared with the lung tissues of patients with PE. In contrast, immunoreactivity for Fas and FasL was infrequently demonstrated in the lung tissues of the control group (Figure 2A and 2B) when compared with the lung tissues of the non-PE and PE groups.
Figure 2.
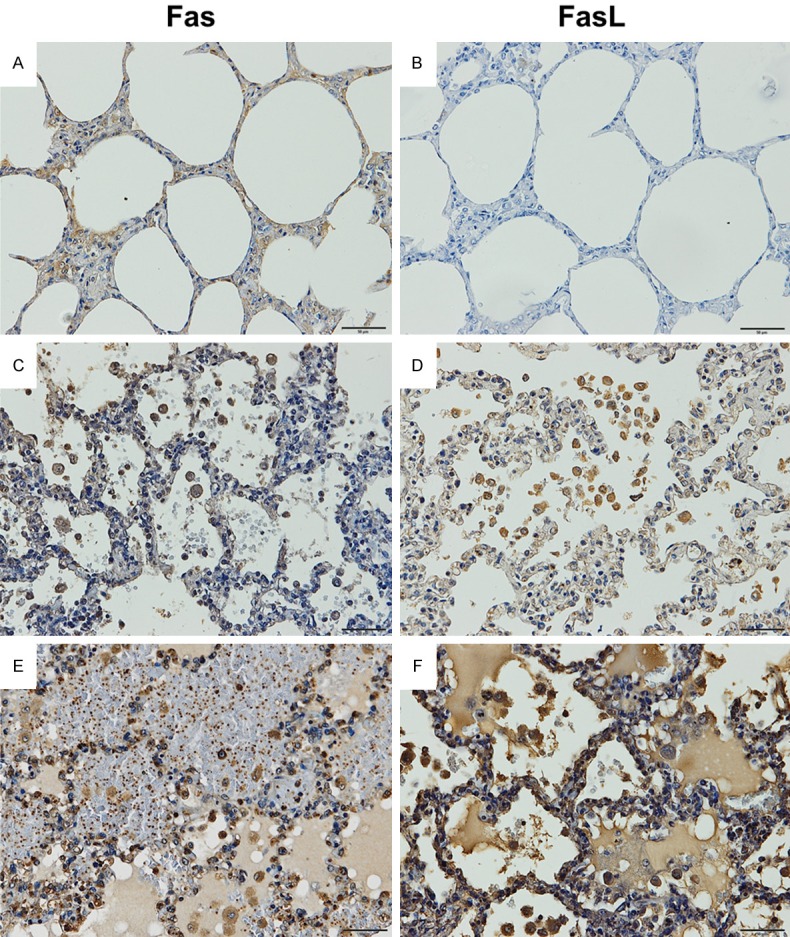
Representative results of immunoperoxidase staining for Fas and FasL in lung tissue of severe falciparum malaria patients. Normal lung tissue (A and B). The lung tissue of a severe falciparum malaria patient with non-PE (C and D). The lung tissue of a severe falciparum malaria patient with PE (E and F). All images are 200 × magnification. Bar = 50 μm.
For semi-quantitative analysis, the percentage of positive stained cells for Fas was significantly increased in the lung tissues of the PE group (61.53 ± 1.15%) compared to the tissues of the non-PE group (12.69 ± 0.88%) and the control group (6.00 ± 1.94%) (P < 0.05). The percentage of positive stained cells for FasL was significantly increased in the lung tissues of the PE group (59.81 ± 1.82%) compared to the non-PE (20.22 ± 1.37%) and control groups (3.50 ± 1.07%) (P < 0.05). The mean percentages of Fas and FasL immunostaining in alveolar cells, alveolar macrophages, and leukocytes are shown in Figure 3. The mean number of Fas- and FasL-immunopositive cells was significantly up-regulated in alveolar cells, alveolar macrophages, and leukocytes in the lung tissues of the PE group compared with the non-PE and control groups (all P < 0.05).
Figure 3.
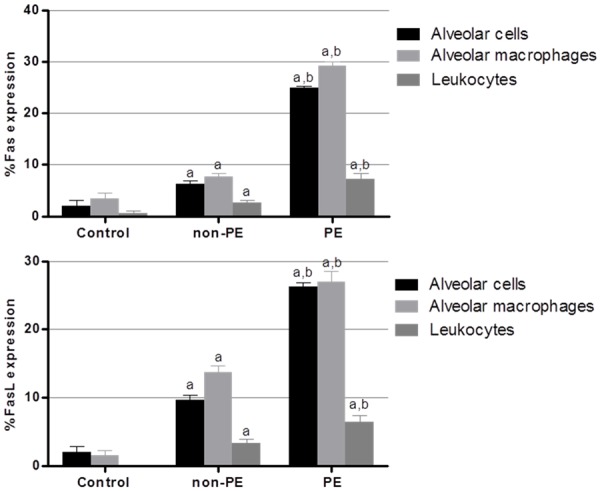
Semi-quantitative analysis for expression of Fas and FasL in alveolar cells, alveolar macrophage, and leukocytes in the lung of PE, non-PE, and control groups. aSignificance of P < 0.001 compared with the control group (Mann-Whitney U test). bSignificance of P < 0.001 compared with non-PE group (Mann-Whitney U test). Data are presented as mean ± SEM.
Immunohistochemistry staining for cleaved caspase-8 and cleaved caspase-3
To determine the downstream activation of apoptosis caspases, cleaved caspase-8 and cleaved caspase-3 were investigated in this study. Figure 4 demonstrates the pattern of immunoperoxidase staining for cleaved caspase-8 and cleaved caspase-3 in the lung tissues of the PE, non-PE, and control groups. The results of immunoperoxidase staining for cleaved caspase-8 and cleaved caspase-3 were associated with the results of immunoperoxidase staining for Fas and FasL. Immunoreactivity for cleaved caspase-8 and cleaved caspase-3 was detected in the alveolar cells that line the alveolar walls, distributed alveolar macrophages, and leukocytes in the septal capillary in the lung tissues of the PE group (Figure 4E and 4F). In addition, a low number of positive cells for cleaved caspase-8 and cleaved caspase-3 were also found in the alveolar cells, alveolar macrophage, and leukocytes in the lung tissues of the non-PE group (Figure 4C and 4D) when compared with the lung tissues of the PE group. In the control lung tissues, immunoreactivity for Fas and FasL was infrequently demonstrated in the alveolar cells, alveolar macrophage, and leukocytes compared with the lung tissues of the PE and non-PE groups (Figure 4A and 4B).
Figure 4.
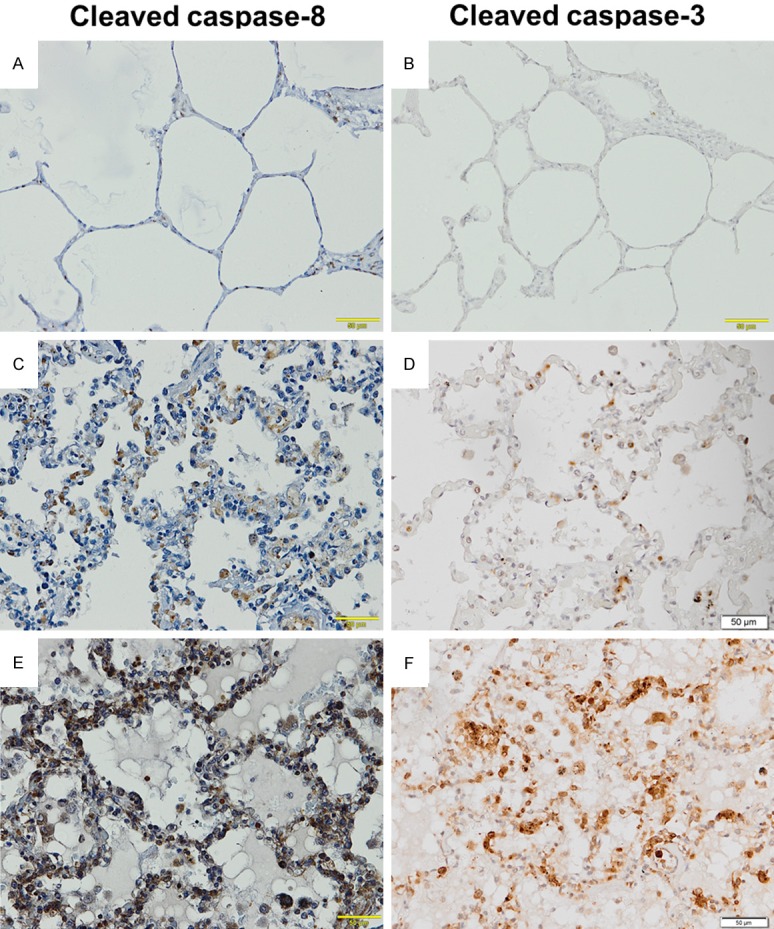
Representative results of immunoperoxidase staining for Fas and FasL in the lung tissues. Normal lung tissue (A and B). The lung tissue of a severe malaria patient with non-PE (C and D). The lung tissue of a severe malaria patient with PE (E and F). All images are 200 × magnification. Bar = 50 μm.
For semi-quantitative analysis, the percentage of positive stained cells for cleaved caspase-8 was significantly increased in the lung tissues of the PE group (77.58 ± 2.56%) compared to those of the non-PE (21.45 ± 1.18%) and control groups (6.00 ± 1.94%) (P < 0.05). The percentage of positive stained cells for cleaved caspase-3 was significantly increased in the lung tissues of the PE group (77.95 ± 2.24%) compared to those of the non-PE (22.26 ± 1.53%) and control groups (3.50 ± 1.07%) (P < 0.05). The mean percentages of cleaved caspase-8 and cleaved caspase-3 immunostaining in alveolar cells, alveolar macrophages, and leukocytes are shown in Figure 5. The mean number of positive cells for cleaved caspase-8 and cleaved caspase-3 was significantly up-regulated in alveolar cells, alveolar macrophages, and leukocytes in the lung tissues of the PE group compared with non-PE and control groups (all P < 0.05).
Figure 5.
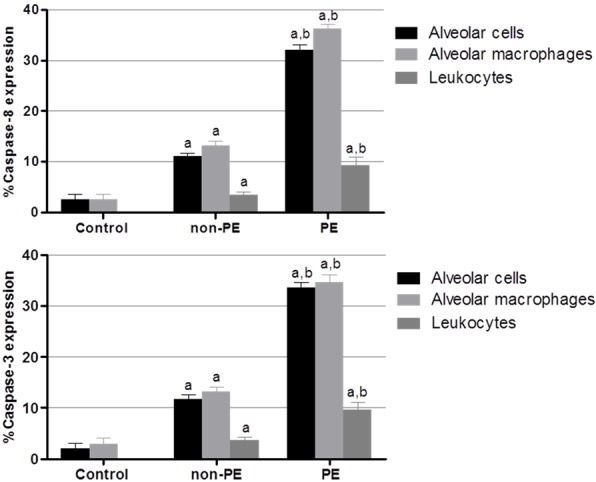
Semi-quantitative analysis of expression of cleaved caspase-8 and cleaved caspase-3 in alveolar cells, alveolar macrophage, and leukocytes in the PE, non-PE, and control groups. aSignificance of P < 0.001 compared with the control group (Mann-Whitney U test). bSignificance of P < 0.001 compared with non-PE group (Mann-Whitney U test). Data are presented as mean ± SEM.
Correlation between the expression of apoptosis markers and lung injury and clinical data
In the lung tissues of the PE group (n = 18), the percentage of positive stained cells for Fas correlated with the percentage of positive stained cells for cleaved caspase-3 (Spearman’s rank correlation, rs = 0.937; P < 0.001) (Figure 6A). A positive correlation was ob-tained between the percentage of positive stained cells for FasL and the percentage of positive stained cells for cleaved caspase-3 (Spearman’s rank correlation, rs = 0.808; P < 0.001) (Figure 6B). Significant positive correlation was found between the percentage of positive stained cells for Fas and the percentage of positive stained cells for FasL (Spearman’s rank correlation, rs = 0.827; P < 0.001) (Figure 6C) and between the percentage of positive stained cells for cleaved caspase-8 and the percentage of positive stained cells for cleaved caspase-3 (Spearman’s rank correlation, rs = 0.823; P < 0.001) (Figure 6D). However, no association was established between apoptotic markers and lung injury and the following clinical parameters: age, gender, parasite density, days of hospitalization, hemoglobin, hematocrit, and WBC count.
Figure 6.
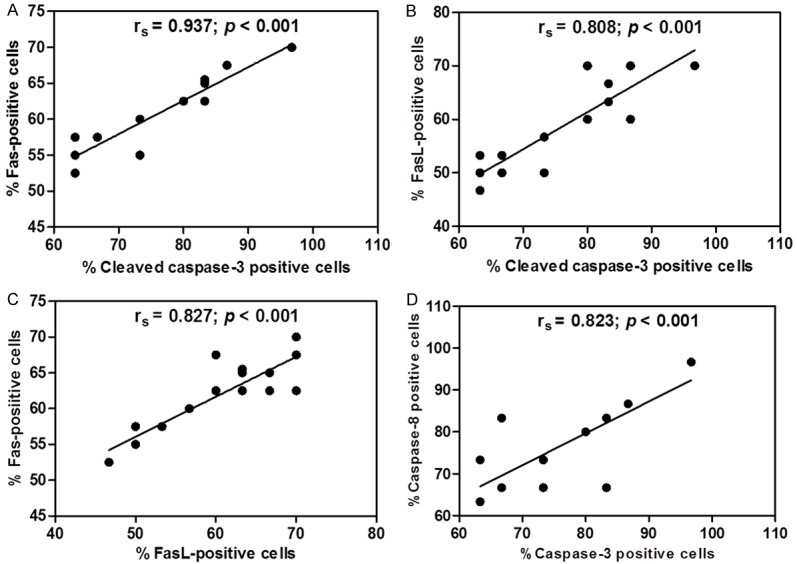
The graphs illustrate positive correlations in the lung tissues of the PE group (n = 18) using Spearman’s rank correlation test. Positive correlations between the percentage of positive stained cells for Fas and the percentage of positive stained cells for cleaved caspase-3 (A), the percentage of positive stained cells for FasL and the percentage of positive stained cells for cleaved caspase-3 (B), the percentage of positive stained cells for FasL and the percentage of positive stained cells for FasL (C), and the percentage of positive stained cells for cleaved caspase-8 and the percentage of positive stained cells for cleaved caspase-3 (D).
Discussion
Histopathological changes revealed that the sequestration of PRBCs in pulmonary capillaries, a large number of pigment-laden macrophages in the alveolar space, alveolar, pulmonary edema, and leukocyte infiltration were found in the lung tissue of patients with severe falciparum malaria. Our findings agree with previous reports in post-mortem studies on human patients who died due to severe P. falciparum malaria that demonstrated sequestration of PRBCs, heavy edematous, and hemorrhage and leukocyte infiltration [21]. It has been reported that both PRBC sequestration, which is likely associated with CD36-mediated sequestration [22], and inflammation mediated by hemozoin [23] are pathological features observed in the lungs of severe falciparum malaria patients. A recent report that studied the ultrastructure of the lungs of mice that died due to malaria-associated ALI/ARDS demonstrated the presence of infected red blood cell/endothelium contact, swollen endothelium with distended cytoplasmic extensions, thickening of the endothelial basement membrane, thickening of septa with congested capillaries and leukocytes, and alveolar spaces containing blood cells, edema, and cell debris [24]. The alveolar congestion and pulmonary edema in the lungs of severe falciparum malaria patients may be due to the breakdown of barriers and damage of alveolar epithelium. In addition, capillary congestion was one of the most prominent observations by light microscopy. It is likely that this congestion results in inefficient blood flow and blood gas exchange in the lungs [24]. In addition, the septal width of the lungs of the PE group was thicker (20.9 ± 0.36 μm) than that of the non-PE (14.43 ± 0.34 μm) and control groups (9.3 ± 0.09 μm). Septal thickening in the lungs of patients with PE may be the result of congested capillaries or leucocyte infiltration, which contributes to the dilatation of alveolar septum.
Our study using IHC staining demonstrates that immunoreactivity for both Fas and FasL was significantly increased in alveolar cells, alveolar macrophages, and leukocytes in the lung tissue of severe falciparum patients with PE compared with the lung tissue of severe falciparum patients with non-PE and control groups. The Fas and FasL system has also been implicated in pulmonary disorders. Our finding showing the increased expression of Fas expressed in alveolar cells, alveolar macrophages, and leukocytes is consistent with several previous reports [3,8-10]. Expression of Fas mRNA in isolated primary murine alveolar type II cells and induction of apoptosis in mouse alveolar type II cells by activated Fas has also been demonstrated [25]. FasL exists as a soluble or membrane-bound form in some cells. Apoptosis is induced when membrane-bound or soluble FasL binds to Fas-bearing cells [15]. Previous studies using IHC staining have demonstrated that FasL is expressed on infiltrating mononuclear cells, alveolar epithelium, and columnar and basal cells of the airway [5,10,11,26]. Our study found that the Fas and FasL are frequently seen in the alveolar macrophages inside the alveoli of the lungs of patients with PE, suggesting that alveolar macrophages may be a possible source of Fas, which is bound to the Fas receptor on alveolar epithelium. Previous studies have demonstrated that the soluble form of FasL is present in the BAL fluid from patients with ARDS, idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis, and bronchiolitis obliterans organizing pneumonia [5,11,18,27]. In addition, circulating soluble FasL has also been detected in the serum of patients with ARDS, IPF, pulmonary fibrosis associated with collagen vascular disease, and pneumococcal pneumonia [11,28-30] and also in the pulmonary edema fluid of patients with ARDS [5]. However, it has been reported that a high concentration of soluble FasL is required for inducing apoptosis of distal lung epithelial cells in vitro [18]. Therefore, our study suggests that FasL on the cell surface migrates into the lungs and that the soluble form of FasL may induce apoptosis of lung epithelium, resulting in permeability changes. However, the expression of both Fas and FasL in the lungs of malaria patients with PE has not been studied previously. For further work, the levels of soluble of Fas and FasL in BAL and pulmonary fluids should be investigated and in vitro study of the effect of these proteins on apoptosis of alveolar cells in the lungs of patients with severe P. falciparum malaria should be undertaken. In addition, there was a significant positive correlation between the percentage of positive stained cells for Fas and the percentage of positive stained cells for FasL (Spearman’s rank correlation, rs = 0.827; P < 0.001). This finding supports the relationship between the Fas/FasL system for inducing apoptosis in the lungs. Activation of the Fas/FasL system could be one mechanism of disruption of the alveolar epithelial-capillary barrier by inducing apoptosis or dysfunction of alveolar epithelial cells [15]. It has been suggested that Fas-mediated apoptosis of the alveolar epithelium could be an initial event in the development of some form of lung injury [31].
To determine Fas/FasL system-mediated apoptosis in lung tissue, the expression of apoptosis caspases, including cleaved caspase-8 and cleaved caspase-3 was investigated. This study demonstrates that expression of cleaved caspase-8 and cleaved caspase-3 in the lung tissues of severe falciparum malaria patients with PE was significantly increased in alveolar cells, alveolar macrophages, and leukocytes compared with the lung tissues of severe falciparum patients with non-PE and the control groups. FasL binding to its Fas receptor triggers a cascade of well-characterized intracellular signaling events that ends in cell death by apoptosis [7,32]. Evidence for increased marker of cleaved caspase-3 has been reported in the lung tissues of patients with ARDS [5,15,19]. Trimerization of the Fas receptor by Fas ligand results in activation of caspase-8 and subsequently stimulates the activation of downstream caspases, including caspase-3, leading to cell death [32]. It is possible that the increased expression of cleaved caspase-8 and caspase-3 in the lung tissue of severe falciparum malaria patients may be modulated by the Fas and FasL pathway. In addition, a positive correlation was found between expression of Fas and FasL with cleaved caspase-3 and between expression of cleaved caspase-8 and caspase-3 in the lung tissue of patients with PE. Therefore, the activated caspase-3 as a downstream target of active caspase-8 during induction of apoptosis can be linked to the elevated expression of Fas, FasL, and cleaved caspase-8 in the lung tissue of severe falciparum malaria patients with PE. Our current data suggest that Fas-dependent initiator and effector caspases, including cleaved caspase-8 and caspase-3, are necessary for inducing apoptosis in the lung tissue of severe falciparum malaria patients with PE.
In conclusions, this study demonstrates that increased expression of Fas and FasL is associated with the activation of downstream caspases, including caspase-8 and caspase-3, for induction of apoptosis in the lungs of severe falciparum malaria patients with PE. Fas and FasL pathway-mediated apoptosis may be involved in the pathogenesis of pulmonary edema in patients with severe falciparum malaria. The proper regulation of Fas and FasL signaling may be important for potential treatment of pulmonary complications in malaria patients.
Acknowledgements
The study was supported by the Institute of Research and Development, Walailak University, Nakhon Si Thammarat, Thailand (Grant No. WU56307).
Disclosure of conflict of interest
None.
References
- 1.Taylor WR, Hanson J, Turner GD, White NJ, Dondorp AM. Respiratory manifestations of malaria. Chest. 2012;142:492–505. doi: 10.1378/chest.11-2655. [DOI] [PubMed] [Google Scholar]
- 2.Aursudkij B, Wilairatana P, Vannaphan S, Walsh DS, Gordeux VR, Looareesuwan S. Pulmonary edema in cerebral malaria patients in Thailand. Southeast Asian J Trop Med Public Health. 1998;29:541–545. [PubMed] [Google Scholar]
- 3.Taylor WR, Canon V, White NJ. Pulmonary manifestations of malaria: recognition and management. Treat Respir Med. 2006;5:419–428. doi: 10.2165/00151829-200605060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Van den Steen PE, Deroost K, Deckers J, Van Herck E, Struyf S, Opdenakker G. Pathogenesis of malaria-associated acute respiratory distress syndrome. Trends Parasitol. 2013;29:346–358. doi: 10.1016/j.pt.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matute-Bello G, Lee JS, Liles WC, Frevert CW, Mongovin S, Wong V, Ballman K, Sutlief S, Martin TR. Fas-mediated acute lung injury requires fas expression on nonmyeloid cells of the lung. J Immunol. 2005;175:4069–4075. doi: 10.4049/jimmunol.175.6.4069. [DOI] [PubMed] [Google Scholar]
- 7.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 8.Itoh N, Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 9.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, Nakajima H. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med. 2001;163:762–769. doi: 10.1164/ajrccm.163.3.2003065. [DOI] [PubMed] [Google Scholar]
- 11.Kuwano K, Hagimoto N, Kawasaki M, Nakamura N, Shirakawa K, Maeyama T, Hara N. Expression of FasL and Fas protein and their soluble form in patients with hypersensitivity pneumonitis. Int Arch Allergy Immunol. 2000;122:209–215. doi: 10.1159/000024399. [DOI] [PubMed] [Google Scholar]
- 12.Matute-Bello G, Frevert CW, Liles WC, Nakamura M, Ruzinski JT, Ballman K, Wong VA, Vathanaprida C, Martin TR. Fas/Fas ligand system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria. Infect Immun. 2001;69:5768–5776. doi: 10.1128/IAI.69.9.5768-5776.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 14.Serrao KL, Fortenberry JD, Owens ML, Harris FL, Brown LA. Neutrophils induce apoptosis of lung epithelial cells via release of soluble Fas ligand. Am J Physiol Lung Cell Mol Physiol. 2001;280:L298–305. doi: 10.1152/ajplung.2001.280.2.L298. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto S, Kobayashi A, Kooguchi K, Kitamura Y, Onodera H, Nakajima H. Upregulation of two death pathways of perforin/ granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:237–243. doi: 10.1164/ajrccm.161.1.9810007. [DOI] [PubMed] [Google Scholar]
- 18.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 19.Bem RA, van der Loos CM, van Woensel JB, Bos AP. Cleaved caspase-3 in lung epithelium of children who died with acute respiratory distress syndrome. Pediatr Crit Care Med. 2010;11:556–560. doi: 10.1097/PCC.0b013e3181d5063c. [DOI] [PubMed] [Google Scholar]
- 20.Su X, Bai C, Hong Q, Zhu D, He L, Wu J, Ding F, Fang X, Matthay MA. Effect of continuous hemofiltration on hemodynamics, lung inflammation and pulmonary edema in a canine model of acute lung injury. Intensive Care Med. 2003;29:2034–2042. doi: 10.1007/s00134-003-2017-3. [DOI] [PubMed] [Google Scholar]
- 21.Milner D Jr, Factor R, Whitten R, Carr RA, Kamiza S, Pinkus G, Molyneux M, Taylor T. Pulmonary pathology in pediatric cerebral malaria. Hum Pathol. 2013;44:2719–2726. doi: 10.1016/j.humpath.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovegrove FE, Gharib SA, Pena-Castillo L, Patel SN, Ruzinski JT, Hughes TR, Liles WC, Kain KC. Parasite burden and CD36-mediated sequestration are determinants of acute lung injury in an experimental malaria model. PLoS Pathog. 2008;4:e1000068. doi: 10.1371/journal.ppat.1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deroost K, Tyberghein A, Lays N, Noppen S, Schwarzer E, Vanstreels E, Komuta M, Prato M, Lin JW, Pamplona A, Janse CJ, Arese P, Roskams T, Daelemans D, Opdenakker G, Van den Steen PE. Hemozoin induces lung inflammation and correlates with malaria-associated acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2013;48:589–600. doi: 10.1165/rcmb.2012-0450OC. [DOI] [PubMed] [Google Scholar]
- 24.Aitken EH, Negri EM, Barboza R, Lima MR, Alvarez JM, Marinho CR, Caldini EG, Epiphanio S. Ultrastructure of the lung in a murine model of malaria-associated acute lung injury/acute respiratory distress syndrome. Malar J. 2014;13:230. doi: 10.1186/1475-2875-13-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fine A, Anderson NL, Rothstein TL, Williams MC, Gochuico BR. Fas expression in pulmonary alveolar type II cells. Am J Physiol. 1997;273:L64–71. doi: 10.1152/ajplung.1997.273.1.L64. [DOI] [PubMed] [Google Scholar]
- 26.Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, White SR. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol. 1998;19:537–542. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]
- 27.Kuwano K, Kawasaki M, Maeyama T, Hagimoto N, Nakamura N, Shirakawa K, Hara N. Soluble form of fas and fas ligand in BAL fluid from patients with pulmonary fibrosis and bronchiolitis obliterans organizing pneumonia. Chest. 2000;118:451–458. doi: 10.1378/chest.118.2.451. [DOI] [PubMed] [Google Scholar]
- 28.Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 29.Kemp K, Bruunsgaard H, Skinhoj P, Klarlund Pedersen B. Pneumococcal infections in humans are associated with increased apoptosis and trafficking of type 1 cytokine-producing T cells. Infect Immun. 2002;70:5019–5025. doi: 10.1128/IAI.70.9.5019-5025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuwano K, Maeyama T, Inoshima I, Ninomiya K, Hagimoto N, Yoshimi M, Fujita M, Nakamura N, Shirakawa K, Hara N. Increased circulating levels of soluble Fas ligand are correlated with disease activity in patients with fibrosing lung diseases. Respirology. 2002;7:15–21. doi: 10.1046/j.1440-1843.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- 31.Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol. 2001;158:153–161. doi: 10.1016/S0002-9440(10)63953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waring P, Mullbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77:312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]


