Abstract
To explore whether the over-expression of Sry-related HMG box (Sox9) in degenerative chondrocytes is able to improve cell regeneration and protects cells from inflammation induced apoptosis, we generated a Sox9 over-expressing vector delivery system in which the Sox9 gene was inserted into a lentiviral vector. After infecting mouse chondrocytes with the Sox9-encoding vector, we observed a high level of gene transduction efficiency and achieved a high level of Sox9 expression in the infected chondrocytes. To explore whether over-expression of Sox9 is able to induce cell regeneration and improve cell survival, we induced Sox9 over-expression by lentiviral vector infection 48 hours before IL-1β treatment. The cells were infected with the reporter gene GFP-encoded lentiviral vector as a negative control or left uninfected. 48-hours after IL-1β treatment, the chrondrocytes treated with IL-1β alone, underwent a degenerative process, with elevated expression of MMP-3, MMP-13, ADAMTS-5 and ALP, but the cell specific anabolic proteins collagen II and aggrecan were significantly suppressed. The cells infected with the GFP reporter vector had no increased regeneration after IL-1β treatment. The results indicated that Sox9 is an important chondrocyte transcription factor, promoting chondrocyte regeneration and cell survival, which were mediated through affecting multiple cell differentiation as well as anti-apoptotic signaling pathways.
Keywords: Osteoarthritis, Sox9, chondrocyte, cell degeneration, cell survival
Introduction
Osteoarthritis (OA) is a chronic degenerative disease in articular cartilage tissues, affects over 10% of adult populations nationwide and has become the fourth most common cause of hospitalization [1]. Chondrocytes are a predominant cell type in mature articular cartilage, responsible for type II collagen synthesis and extracellular matrix (ECM) turnover. In the early phase of OA, there is increased collagenase, causing type II collagen cleavage in focal cartilage and articular cartilage degeneration. Significantly increased inflammatory cytokines in the degenerated tissues are major players in the pathological processes acting through multiple signaling pathways including Wnt3A/beta-catenin and SIRT1/ERK signaling pathways [2-6]. Through activation of the downstream signaling pathways, many catabolic protein expressions such as matrix metalloproteinases (MMP-1 & MMP-3) are up-regulated in the degenerated cartilage tissues [6-8]. IL-1β is a predominant pro-inflammatory cytokine in the pathological process; studies in vitro revealed that IL-1β treatment significantly induced chondrocyte dedifferentiation and apoptosis, accompanied with increased NF-κB activation and other inflammatory cytokine expressions [9].
However, recent reports have indicated that the detrimental effects can be rescued by IGF-1 and some natural antioxidants through up-regulation of important chondrocyte specific transcription factors [9,10]. Some studies revealed that natural antioxidants curcumin and resveratrol increased chondrocyte survival and proliferation through suppression of NF-κB-regulated pro-inflammatory gene up-regulation. Cyclooxygenase-2, MMP-3, MMP-9, vascular endothelial growth factor was greatly suppressed by the antioxidants treatment in the IL-1β induced chondrocyte degeneration model [11].
Extensive studies in clinical patients with OA and mouse models also indicated that OA development is also associated with the suppressed expression of Sox9, a SRY-type high-mobility-group box transcription factor in degenerated chondrocytes; recovery of diseases are accompanied with up-regulated Sox9, a phenomenon which indicates the pivotal role of Sox9 in cell survival and regeneration [3,11]. The down-regulated Sox9 expression may be caused by increased methylation at the Sox9 promoter under the pro-inflammatory microenvironment, because 5-azacytidine (5-AzaC) can increase Sox9 expression in chondrocytes, leading to improved cell survival and regeneration [12]. Thus, up-regulation Sox9 possesses a potential therapeutic potential in the treatment of degenerative cartilage diseases. For example, TGF-β is able to improve chondrocyte survival and differentiation through up-regulation of Sox9, and subsequent up-regulation of type II collagen and aggrecan [13,14]. Some other studies in bone marrow and other tissue-derived stem cells also revealed the critical role of Sox9 in stem cell differentiation towards chondrocyte phenotype [15-17]. Thus Sox9 is a potential therapeutic target in gene therapy for chondrocyte degenerative diseases.
Due to the critical relationship between Sox9 and the pathogenesis of articular cartilage in OA patients [18], in the present study, we investigated the effects of over-expressing Sox9 on cell survival and regeneration of degenerated chondrocytes. Our results indicated that Sox9 over-expression significantly improved chondrocyte genesis and cell survival. The underlying molecular mechanisms were also analyzed and are discussed in this study.
Materials and methods
Culture of mouse chondrocytes and IL-1β treatment
Mouse articular cartilage chondrocytes were isolated from the femoral heads, femoral condyles, and tibial plateau of 5 to 6 day-old C57BL/B6 mice. Briefly, articular cartilage tissues were minced (< 1 mm3) and digested with 0.3% collagenase A overnight at 37°C. The cell suspension was filtered through a sterile 40 μM nylon mesh cell strainer, and the released cells were cultured in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere. Cell viability of isolated chondrocytes was determined by trypan blue exclusion assay. Primary cells were maintained as a monolayer culture throughout this study. The subconfluent monolayers of chondrocytes were infected with Sox9 expressing lentiviral vector (Lv-Sox9/GFP) or control lentiviral vector-expressing GFP (Lv-GFP) at the multiple of infection (MOI) of 5 for 48 hrs. The infected cells were again treated with 10 ng/ml IL-1β for 48 hrs for Western blotting and quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis.
Establishing an IL-1β-induced OA mouse model
The OA mouse model was established by an intra-articular cartilage bilateral injection of 1 µg recombinant IL-1β (BD Pagrmingen) on day 0, 3, 6 and 9 in 100 µl volumes under anesthesia (100 mg/kg ketamine and 20 mg/kg per mouse. 18 days after IL-1β, the articular cartilage tissues were collected for analysis of the tissue pathology with H&E and immune cell infiltration by immunohistochemistry. Some tissues were saved for RNA extraction and qRT-PCR.
Generation of lentiviral vector encoding Sox9 and viral infection
Lentiviral vector encoding Sox9 was generated by co-transfection of a subconfluent monolayer of 293 packaging cells maintained in a 150 mm dish with three helper plasmids (14.8 µg pMDLg/pRRE, 6.9 µg pRSV.REV and 9.6 µg pMD2.G) and 22.5 µg bicistronic plasmid vector pLv-Sox9/GFP or one reporter vector pLv-GFP. Three days after co-transfection, the cell supernatants containing the released lentiviral vectors were collected and concentrated by ultracentrifugation at a speed of 25000 rpm (70,000 g) twice. The concentrated lentiviral vectors were re-suspended in 300 µl PBS and stored at -80°C in aliquots until used.
Immunohistochemical staining of Sox9, PI3KCA in cartilage tissues
Sox9 and PI3KCA expression in human cartilage tissues were detected by incubation with primary antibodies. Briefly stated, tissue sections were fixed with 3.7% formaldehyde and non-specific bindings were blocked by incubation with 10% goat serum and followed by primary antibodies anti-Sox9 and anti-PI3KCA (dilution 1:500, BD Transduction Laboratories) for two-hours. After washing with phosphate-buffered saline (PBS), the indicated HRP-conjugated second antibodies (Jackson Immuno, Inc) were added for 1 hour. After washing with PBS three times, the stained sections were developed by adding DAB (Vector laboratories) and mounted under a mounting medium. The stained section was visualized under light-contrast microscope. For fluorescence visualization, the indicated FITC-conjugated second antibody was incubated with sections for 1 hr after primary antibody incubation. The stained sections were visualized under a fluorescence microscope.
Quantitative real-time RT-PCR (qRT-PCR) analysis for Sox9 and PI3KCA mRNA in cartilage tissue
RNA was extracted from samples by using Trizol reagent (Invitrogen, Grand Island, NY) and cDNA was synthesized by using cDNA synthesis kits (Invitrogen, Grand Island, NY). The primer sequences used to analyze expression of murine and human Sox9 and other gene transcripts are shown in Table 1. SYBR green PCR master mix (Applied Biosystems, Warrington, UK) were used for real-time PCR reaction following the reaction condition: 94°C 5 min, 50 cycles (94°C for 40 sec; 57°C for 30 sec, 72°C for 30 sec). Data was analyzed by using 7500 systems DS software (Applied Biosystems, Warrington, UK) and normalized by an internal control GAPDH and presented as ΔΔCt. Measurements were repeated at least three times and one instance of the data is shown.
Table 1.
Primer sequenses
| Sense | Antisense | |
|---|---|---|
| Sox9 | 5’-TCAGCCACCCAGCAAATCCT-3’ | 5’-GTCATCTGGATCTCAGCACG-3’ |
| MMP-3 | 5’-ACATGGAGACTTTGTCCCTTTTG-3’ | 5’-TTGGCTGAGTGGTAGAGTCCC-3’ |
| MMP-13 | 5’-CTTCTTCTTGTTGAGCTGGACTC-3’ | 5’-CTGTGGAGGTCACTGTAGACT-3’ |
| ADAMTS-5 | 5’-GGAGCGAGGCCATTTACAAC-3’ | 5’-CGTAGACAAGGTAGCCCACTTT-3’ |
| Collagen II | 5’-GGGAATGTCCTCTGCGATGAC-3’ | 5’-GAAGGGGATCTCGGGGTTG-3’ |
| Aggrecan | 5’-CCTGCTACTTCATCGACCCC-3’ | 5’-AGATGCTGTTGACTCGAACCT-3’ |
| GAPDH | 5’-AGGTCGGTGTGAACGGATTTG-3’ | 5’-TGTAGACCATGTAGTTGAGGTCA-3’ |
| Sox9 | 5’-AGCGAACGCACATCAAGAC-3’ | 5’-CTGTAGGCGATCTGTTGGGG-3’ |
| PI3KCA | 5’-CCACGACCATCATCAGGTGAA-3’ | 5’-CCTCACGGAGGCATTCTAAAGT-3’ |
| GAPDH | 5’-TGTTCCTACCCCCAATGTGT-3’ | 5’-TGTGAGGGAGATGCTCAGTG-3’ |
Western blotting for Sox9, PI3KCA, PI3KCB, p-AKT, AKT, Bcl-2 and Bax
Cellular protein was extracted from tissues or cultured cells by using RIPA lysis buffer (Promega) and the protein amount was measured by BCA assay (Pierce). A western blot analysis was performed using primary antibodies against Sox9, PI3KCA, PI3KCB, p-AKT, AKT, Bcl-2 and Bax respectively (Cell Signaling Technology, Beverly, MA). Antibodies against GAPDH and β-Actin (Sigma) were used as a loading control. Band intensity was quantitatively analyzed by using Image J software, and the data is presented as a mean ratio of densimetric intensity of target protein to internal control GAPDH for each sample.
Flow cytometry analysis for cell apoptosis
Chondrocytes were plated on 35-mm dishes at 4 × 105 cells/plate and treated with indicated reagents for 48 h. Cells were stained with FITC conjugated Annexin-V according to the instructions provided by the kit (BD Pharmingen) and then incubated with propidium iodide (2.5 mg/mL) for 5 min at room temperature. After washing with PBS twice stained samples were analyzed with a FACScan flow cytometer (Becton Dickson, Franklin Lakes, NJ). Data was presented as a percentage of positive stained cells.
Statistical analysis
All data are expressed as means ± standard deviations. Statistical analysis was conducted using a one-way analysis of variance. P value < 0.05 was considered significant difference.
Results
Sox9 expression was reduced in OA patients
To determine if Sox9 expression is reduced in articular cartilage of OA patients, we analyzed Sox9 expression in articular cartilage tissue from OA patients, and compared the expression with those of normal human controls. The Sox9 protein was positively detected in the normal tissues, but not in the sections incubated with the second antibody alone. Compared to the normal controls, The Sox9 expression was largely suppressed in the tissues of OA patients (Figure 1A). The results were further confirmed by Western blotting and qRT-PCR at protein and mRNA levels (Figure 1B, 1C). There were at least 2-fold decreases in OA patients as compared to normal controls at mRNA level (P < 0.05). Thus, Sox9 was significantly suppressed in OA patients; Sox9 expression was negatively associated with degeneration of articular cartilage.
Figure 1.
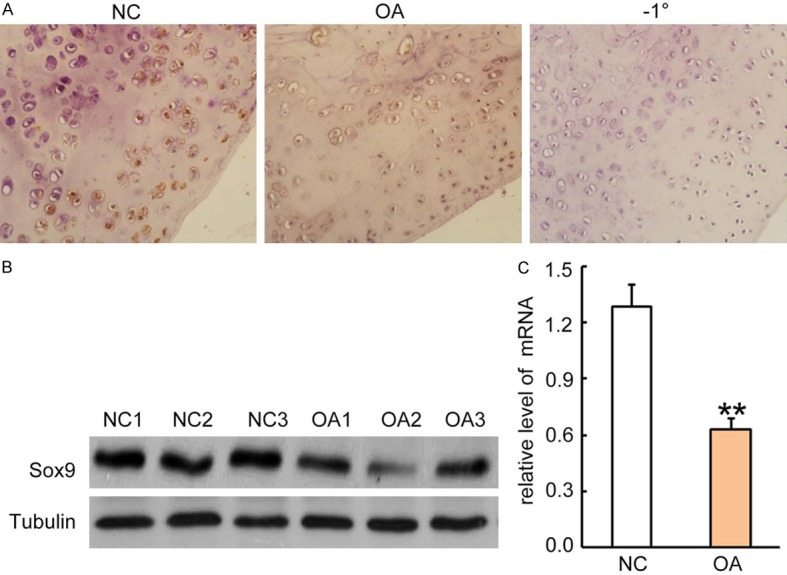
Sox9 expression was reduced in OA patients. A. Immunohistochemical staining for Sox9 expression in articular cartilage tissues from patients with OA and normal controls. Rabbit anti-human Sox-9 antibody (1:500 dilution) was incubated with sections for 2 hrs and followed by incubation with HRP-conjugated second antibody for 1 hour. The Sox9 positive staining was developed by the addition of DAB substrate. Sections from normal controls were incubated with second antibody only as a negative control (right panel). One representative photograph of two groups is shown. B. Western blotting analysis for Sox9 expression in cell lysates from articular cartilage tissues. Each lane represents a sample from individual human subjects. After stripping, the GAPDH antibody was incubated for sample loading controls. C. Sox9 mRNA levels in articular cartilage tissues were quantitatively analyzed by qRT-PCR. Data was normalized by internal control GAPDH and presented as mean DDCt relative to GAPDH ± standard error, n = 3. *P < 0.05, **P < 0.01.
Lentiviral vectors effectively infected mouse chondrocytes and expressed Sox9
Chondrocytes are major sources of Sox9 in articular cartilage tissues. We hypothesize that over-expression of Sox9 in degenerated chondrocytes would reverse the pathological process and promote degenerated tissue repair. We explored the lentiviral vector gene delivery system. The generated lentiviral vectors have a titer reaching 3 × 1010 pfu/ml after purification and ultracentrifuge concentration. To determine the transduction efficiency of the generated lentiviral vectors, we infected mouse chondrocytes isolated from normal articulate tissues with Lv-Sox9/GFP or a control vector Lv-GFP at a multiple of infection (MOI) of 5 for 48 hrs. Transduction efficiency reached up to 90% with both vectors, indicating a high transduction efficiency of our produced lentiviral vectors (Figure 2). Thus, lentiviral vector is an ideal gene delivery vehicle for chondrocytes and very useful for the delivery of other genes into chondrocytes.
Figure 2.
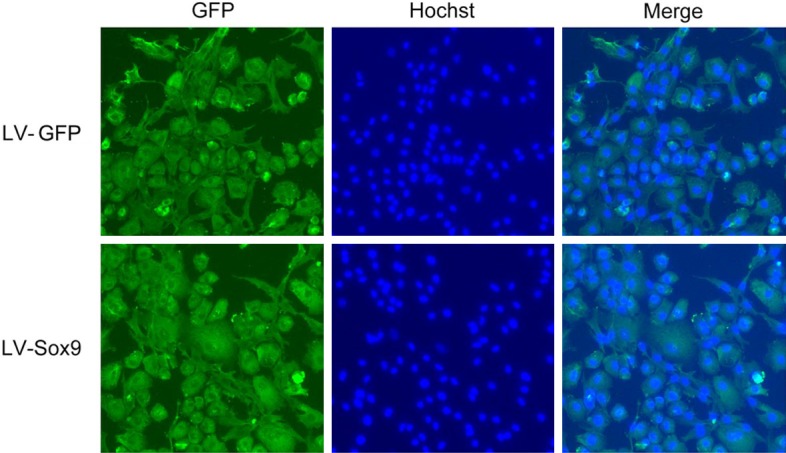
Lentiviral vectors effectively infect mouse articular cartilage chondrocytes in vitro. Mouse chondrocytes were isolated from articular cartilage tissues of normal mice and infected with Sox9-encoding lentiviral vector (Lv-Sox9/GFP) or control lentiviral vector-encoding GFP (Lv-GFP) at a multiple of infection (MOI) of 5 for 48 hrs. GFP expressions in the infected cells were visualized under fluorescence microscope (magnification 20 ×). Nuclei were stained with Hochst for identification of the cells. One representative photograph of two treatments is shown.
Over-expression of Sox9 reversed protein expression affected by IL-1β treatment in chondrocytes
IL-1β is a key pro-inflammatory cytokine in the pathogenesis of OA. To establish an OA in vitro mouse model, we treated mouse chondrocytes with 10 ng/mL IL-1β for 48 hrs. As a result, we observed great increases of some catabolic protein expressions, such as MMP-3, MMP-13, ADAMTS-5 (a disintegrin and metalloproteinase with thrombospondin motifs) and ALP (alkaline phosphatase); whereas the anabolic protein such as collagen II and aggrecan were greatly suppressed in the treated cells, demonstrating the degenerative effects of IL-1β on treated chondrocytes (Figure 3A). However, the over-expression of Sox9 was able to reverse IL-1β induced up-regulation of MMP-3, MMP-13, ADAMTS-5 and ALP. In addition, Sox9 also reversed IL-1β induced down-regulation of type II collagen and aggrecan expressions. The beneficial effects were not observed in the control vector Lv-GFP infected cells (Figure 3A). The results were further confirmed by qRT-PCR at mRNA level, showing at least a 2-fold increase in collagen II and aggrecan mRNA and at least a 3-fold decrease in MMP-3, MMP-13 and ADAMTS-5 mRNA (Figure 3B) (P < 0.05). Therefore, the over-expression of Sox9 by lenetiviral vector gene transfer increased anabolic metabolism, but suppressed catabolic metabolisms of chondrocytes, beneficial to degenerated cell repair.
Figure 3.
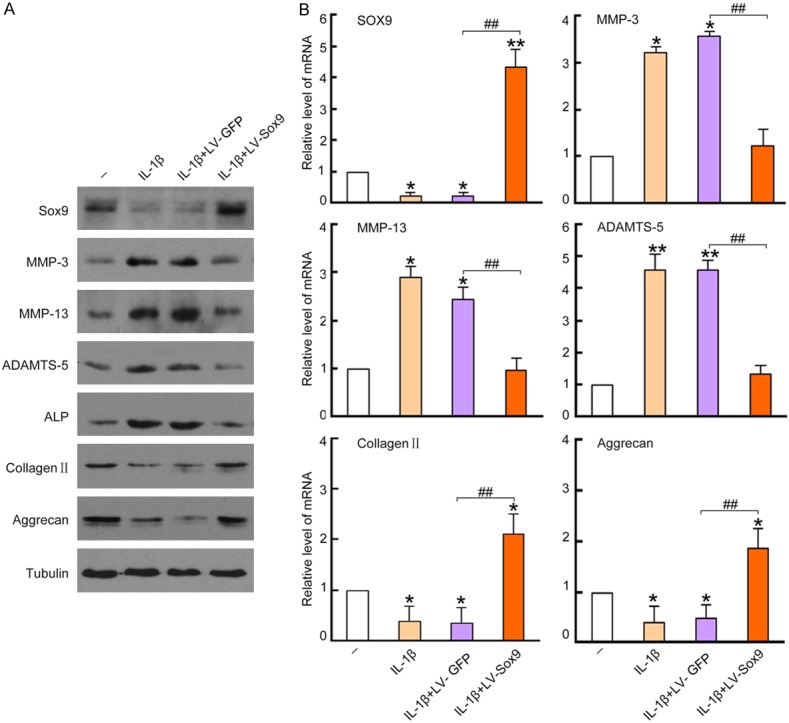
Over-expression of Sox9 in chondrocytes reversed IL-1β induced protein expression. Cultured chondrocytes were untreated as negative controls, or pre-infected with Lv-GFP or Lv-Sox9/GFP at MOI = 5 for 48 hrs and followed by a treatment of 10 ng/mL IL-1β for 48 hrs. The cells treated with 10 ng/mL IL-1β only were used as a control. 20 μg cell lysates were loaded into 10% SDS/PAGE gel for Western blotting analysis. A. Membranes were incubated with antibodies against Sox9, MMP-3, MMP-13, ADAMTS-5, ALP, collagen II and aggrecan, blots were developed by ECL for film exposure. Antibodies against Tubulin were used for detection of internal control proteins. One representative Western blot of three independent experiments is shown. B. mRNA level of Sox9, MMP-3, MMP-13, ADAMTS-5, collagen II and aggrecan in the treated chondrocytes were quantitatively analyzed by qRT-PCR. Data was normalized by internal control GAPDH and presented as mean DDCt relative to GAPDH ± standard error, n = 3. *P < 0.05, **P < 0.01 compared to untreated negative control. #P < 0.05, ##P < 0.01 compared to Lv-GFP infected cell controls.
Over-expression of Sox9 in chondrocytes suppressed IL-1β induced chondrocyte apoptosis
To investigate whether over-expression of Sox9 has benefits on chondrocyte survival, we performed Annexin V and propidium iodide (PI) staining to determine cell apoptosis. As a result, IL-1β treatment greatly increased Annexin V+PI- apoptotic cell population from untreated 4.85% to IL-1β treated 59.8%; whereas the cells with over-expression of Sox9 have 29% Annexin V+PI- population. In contrast, there were no marked changes of Annexin V+PI- cell population in control vector infected cells (Figure 4A). The results were also quantitatively analyzed for apoptotic cell population in the treated cells, showing at least 50% reduction of apoptotic cells in the Sox9-overexpressed cells as compared to the negative controls (P < 0.01) (Figure 4B). The results confirmed the beneficial role of Sox9 over-expression in chondrocyte survival in a degenerative environment, in consistent with favorable increased type II collagen and aggrecan expressions.
Figure 4.
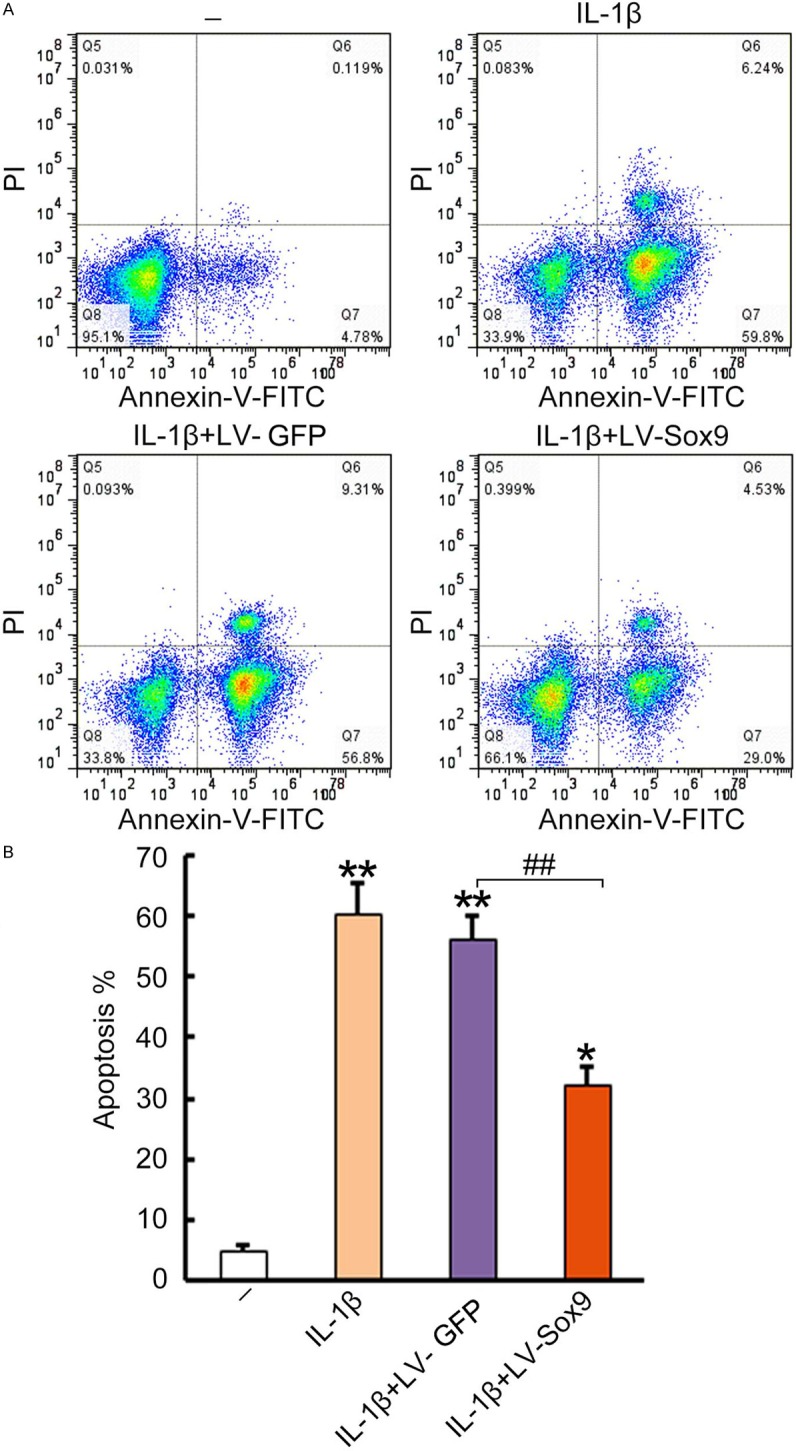
Over-expression of Sox9 in chondrocytes suppressed IL-1β induced chondrocyte apoptosis. Cultured chondrocytes were untreated as negative control, or pre-infected with Lv-GFP or Lv-Sox9/GFP at MOI = 5 for 48 hrs and followed by treatment of 10 ng/mL IL-1β for 48 hrs. The cells treated with 10 ng/mL IL-1β were only used as a control. A. The treated cells were stained with FITC conjugated Annexin-V and then incubated with propidium iodide (2.5 mg/mL) for 5 minutes at room temperature. After washing with PBS twice, the stained samples were analyzed with a FACScan flow cytometer. Data was presented as a percentage of positive stained cells for each group in a pseudo dot plot. One representative dot plot data of 3 independent experiments are shown. B. Statistical analysis of apoptotic cells in the treated chondrocytes. Data was presented as a mean percentage of Annexin V+PI- cells ± standard error, n = 3. *P < 0.05, **P < 0.01, compared to untreated negative control. #P < 0.05, ##P < 0.01 compared to Lv-GFP infected cell control.
Over-expression of Sox9 in chondrocytes suppressed cell apoptotic signaling pathways
To investigate whether the role of Sox9 over-expression was beneficial we used apoptotic signaling pathways, we analyzed PI3KCA, PI3KCB, p-AKT, AKT, Bcl-2 and Bax protein concentrations and activation by Western blotting analysis. Our results revealed that IL-1β treatment greatly suppressed concentrations of survival proteins, such as PI3KCA, p-AKT and Bcl-2, but have no effects on total AKT or PI3KCB. In addition, the pro-apoptotic Bax protein was greatly up-regulated after IL-1β treatment (Figure 5A). However, the increased apoptotic pathways were effectively reversed by over-expression of Sox9, as evidenced by at least 4-times the normal amount of PI3KCA, p-AKT and Bcl-2 protein. In contrast, the pro-apoptotic Bax protein was a least 4-fold attenuated with Lv-Sox9/GFP treatment, as compared to the cells treated with negative the control vector (P < 0.01) (Figure 5B). The results further confirmed the protective role of Sox9 over-expression in chondrocyte survival that was mediated by suppressed intrinsic apoptotic pathways.
Figure 5.
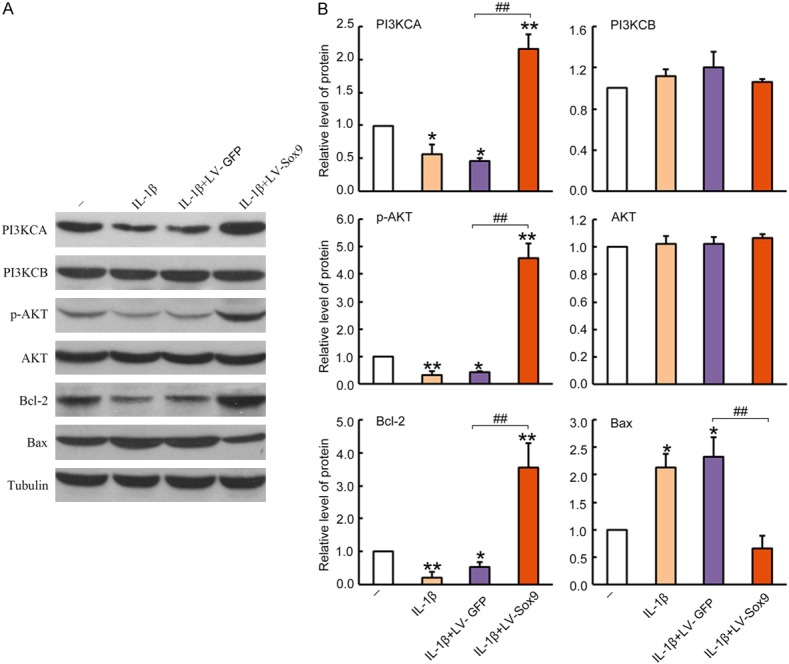
Over-expression of Sox9 in chondrocytes suppressed apoptosis pathways. The cultured chondrocytes were untreated as a naïve control, or pre-infected with Lv-GFP or Lv-Sox9/GFP at MOI = 5 for 48 hrs and followed by a treatment of 10 ng/mL IL-1β for 48 hrs. The cells treated with 10 ng/mL IL-1β were used only as a control. 20 μg cell lysates were loaded into 10% SDS/PAGE gel for Western blotting analysis. A. Blots were incubated with antibodies against PI3KCA, PI3KCB, p-AKT, AKT, Bcl-2, Bax. Antibodies against Tubulin were used for the detection of internal controls. One representative Western blot of three independent experiments is shown. B. Quantitative analysis for densimetric intensity of the positive bands by Image J. Data are presented as a mean ratio of target protein intensity to internal controls tubulin ± standard error, n = 3. *P < 0.05, **P < 0.01 compared to untreated negative control. #P < 0.05, ##P < 0.01 compared to Lv-GFP infected cell control.
Sox9 was positively correlated to PI3KCA in articular cartilage tissues
Our results in vitro mouse degenerated chondrocyte model displayed a significant role of Sox9 over-expression in PI3KCA up-regulation, a key intracellular protein involved in cell survival and anti-apoptotic process. To further define the correlation in human articular cartilage tissues, we analyzed the Sox9 and PI3KCA expressions in articular cartilage tissues from patients with OA and normal tissues as a control. The results indicated that there were moderately decreases in PI3KCA in the articular cartilage tissues of OA patients as compared to normal controls as detected by immunohistochemical staining (Figure 6A). The attenuated PI3KCA expression in OA patients was further confirmed by Western blotting and qRT-PCR analysis, showing at least a 2-fold reduction in PI3KCA expression at the protein and mRNA levels (P < 0.05) (Figure 6B). Thus Sox9 and PI3KCA were positively related and show significant correlation with an R square of 0.54 (P < 0.001) (Figure 6C).
Figure 6.
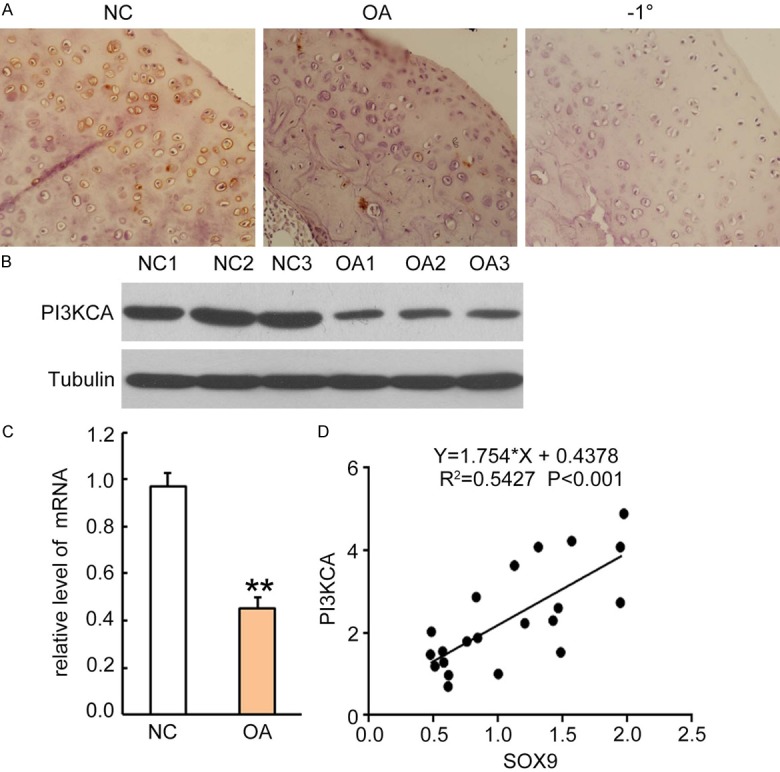
Sox9 expression level was positively correlated to PI3KCA in articular cartilage tissues from patients with OA. A. Rabbit anti-human PI3KCA antibody (1:300 dilution) was incubated on sections for 2 hours and followed by incubation with HRP-conjugated second antibody for 1 hour. The PI3KCA positive staining was developed by addition of DAB substrate. Sections from normal controls were incubated with second antibody only as negative control (right panel). One representative photograph of each group sample is shown. B. Western blotting analysis for PI3KCA expression in cell lysates from articular cartilage tissues of normal subjects and patients with OA. Each lane represents sample from an individual human subject. After stripping, internal control antibodies against tubulin were incubated for detection of internal control proteins. C. PI3KCA mRNA was quantitatively analyzed by qRT-PCR. Data was normalized by internal control GAPDH and presented as mean DDCt relative to GAPDH ± standard error, n = 3. *P < 0.05, **P < 0.01. D. Correlations between Sox9 and PI3KCA was plotted as a scatter plot, and statistical significances were analyzed by the R-squared test, *P < 0.05 is considered as significant regression.
Discussion
OA is a detrimental degenerative disease in articular cartilage. Many factors contribute to the initiation and progression of OA, including genetic component, environmental influence and aging [19]. Pro-inflammatory cytokines such as IL-6, IL-8, IL-1β and TNF-α are key mediators in the pathological process [7,20]. These cytokines are significantly elevated in the inflamed tissues, contributing to the chondrocyte degeneration and tissue damage. A series of activated downstream transcription factors modulate both cellular catabolic and anabolic metabolisms. Activated c-Jun/AP-1 and Wnt5/β-catenin signaling are responsible for the up-regulation of MMPs and increased ECM turnover [4,5]. In contrast, Sox9, a pivotal intracellular transcription factor, responsible for anabolic metabolism and chondrocyte differentiation is significantly suppressed in the oxidative stress microenvironment. This causes increased degeneration and apoptosis of affected chondrocytes [12,21].
Due to the positive role of Sox9 in collagen II and aggrecan synthesis, [22] attenuated Sox9 levels are negatively related to disease severity in patients with OA or in vivo mouse models [23]. Our results also revealed that Sox9 was also significantly decreased in the degenerated cartilage tissues from OA patients at mRNA and protein levels, as compared to the normal controls. Thus over-expression of Sox9 may be beneficial to tissue repair. Previous reports have showed that increasing Sox9 expression in bone marrow derived stem cells and adipocytes promoted cells differentiation towards chondrocyte-like cell phenotype in vitro [15,17]. The results provided an important approach in stem cell-based therapeutics. However, the authors think that over-expressing Sox9 in stem cells is limited by stem cell availability and potential risk of graft rejection. Therefore, the direct modulation of Sox9 expression in chondrocytes may be considered as an alternative approach in the gene therapy for OA. To determine whether over-expressing Sox9 can improve survival and differentiation of degenerated chondrocytes, generated lentiviral vectors encoding the Sox9 gene under the control of a CMV promoter. After the infection of the primary murine chondrocytes, we achieved over a 95% virus transduction efficiency. The results indicated that lentiviral vector was an ideal gene delivery vector candidate for chondrocytes.
MMPs and ADAMTS contribute to aggrecan degradation in articular cartilage through proteolysis [24]. In this study, we observed increased expression of MMP-3, MMP-13, ADAMTS-5 and ALP in the IL-1β treated cells, accompanied with suppressed type II collagen, aggrecan. Furthermore, we observed that over-expressing Sox9 in chondrocytes by lentiviral vector-mediated gene transfer can successfully reverse the detrimental effects of IL-1β on chondrocytes, as demonstrated by elevated levels of type II collagen, aggrecan, but suppressed expressions of MMP-3, MMP-13, ADAMTS-5, as compared to negative control vector treated cells. Other intracellular factors such as Smad3/4, Sp1/Sp3 and p300 are thought to be critically involved in the beneficial effects of Sox9 by forming an immune complex and increasing bindings to the promoter region of type II collagen and other related anabolic genes [21,25,26]. Therefore, Sox9 over-expression is presented as a useful therapeutic strategy for the regeneration of degenerated chondrocytes.
In addition, our study also revealed that Sox9 over-expression significantly increased cell survival and prevented IL-1β induced cell apoptosis. The beneficial effects were correlated to the increased expression of survival genes, such as PI3KCA, p-AKT and Bcl-2, in the Sox9 over-expressed cells. In contrast, pro-apoptotic gene protein Bax was significantly suppressed by Sox9 over-expression. The results further confirmed that Sox9 has a significant role in protecting chondrocyte from IL-1β induced apoptosis. According to previous reports, the increased survival maybe mediated by the increased PI3K/Akt signaling, and subsequent up-regulation of anti-apoptotic protein Bcl-2 expression, as well as subsequent suppression of pro-apoptotic Bax translocation from cytoplasm to mitochondria [27,28]. In addition, we also speculate that Bcl-2-associated athanogene-1 (BAG-1) maybe also involved in the protective role of Sox9 in IL-1β induced apoptosis, because BAG-1 was greatly up-regulated in Sox9 over-expressed adipose tissue derived cells, in combination with increased chondrocyte differentiation and cartilage mineralization [29].
Taken together, Sox9 over-expression in the degenerated chondrocytes greatly improved cell survival through suppressing apoptotic signaling pathways. The beneficial effects were associated with up-regulated type II collagen and aggrecan in the IL-1β induced degenerated chondrocytes, but suppressed inflammatory and pro-apoptotic molecules. Therefore, over-expression of Sox9 by lentiviral vector mediated gene transfer is presented as a potential therapeutic approach in the gene therapy for patients with OA.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81272040; 30600632), the Natural Science Foundation of Guangdong Province, P. R. China (No. S2013010016385), and the Science and Technology Projects of Guangdong Province, P. R. China (No. 2012B031800451).
Disclosure of conflict of interest
None.
References
- 1.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(Suppl 1):S13–S19. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 2.Zanotti S, Canalis E. Interleukin 6 mediates selected effects of Notch in chondrocytes. Osteoarthritis Cartilage. 2013;21:1766–1773. doi: 10.1016/j.joca.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong EH, Song JY, Lee SJ, Park IC, Um HD, Park JK, Lee KH, Nam SY, Hwang SG. Low-dose gamma-radiation inhibits IL-1beta-induced dedifferentiation and inflammation of articular chondrocytes via blockage of catenin signaling. IUBMB Life. 2014;66:128–137. doi: 10.1002/iub.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong EH, Yun HS, Kim J, Um HD, Lee KH, Kang CM, Lee SJ, Chun JS, Hwang SG. Nicotinamide phosphoribosyltransferase is essential for interleukin-1beta-mediated dedifferentiation of articular chondrocytes via SIRT1 and extracellular signal-regulated kinase (ERK) complex signaling. J Biol Chem. 2011;286:28619–28631. doi: 10.1074/jbc.M111.219832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang SG, Yu SS, Lee SW, Chun JS. Wnt-3a regulates chondrocyte differentiation via c-Jun/AP-1 pathway. Febs Lett. 2005;579:4837–4842. doi: 10.1016/j.febslet.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 6.Thomas RS, Clarke AR, Duance VC, Blain EJ. Effects of Wnt3A and mechanical load on cartilage chondrocyte homeostasis. Arthritis Res Ther. 2011;13:R203. doi: 10.1186/ar3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen S, Guo J, Luo Y, Zhang W, Cui Y, Wang Q, Zhang Z, Wang T. Functional proteomics revealed IL-1beta amplifies TNF downstream protein signals in human synoviocytes in a TNF-independent manner. Biochem Biophys Res Commun. 2014;450:538–544. doi: 10.1016/j.bbrc.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol. 2005;32:876–886. [PubMed] [Google Scholar]
- 9.Shakibaei M, Allaway D, Nebrich S, Mobasheri A. Botanical Extracts from Rosehip (Rosa canina), Willow Bark (Salix alba), and Nettle Leaf (Urtica dioica) Suppress IL-1beta-Induced NF-kappaB Activation in Canine Articular Chondrocytes. Evid Based Complement Alternat Med. 2012;2012:509383. doi: 10.1155/2012/509383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolettas E, Muir HI, Barrett JC, Hardingham TE. Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of Sox-9 transcription factor. Rheumatology (Oxford) 2001;40:1146–1156. doi: 10.1093/rheumatology/40.10.1146. [DOI] [PubMed] [Google Scholar]
- 11.Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11:R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KI, Park YS, Im GI. Changes in the epigenetic status of the SOX-9 promoter in human osteoarthritic cartilage. J Bone Miner Res. 2013;28:1050–1060. doi: 10.1002/jbmr.1843. [DOI] [PubMed] [Google Scholar]
- 13.Roman-Blas JA, Stokes DG, Jimenez SA. Modulation of TGF-beta signaling by proinflammatory cytokines in articular chondrocytes. Osteoarthritis Cartilage. 2007;15:1367–1377. doi: 10.1016/j.joca.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Wang Q, Wang JF. Transforming growth factor-beta (TGF-beta) induces the expression of chondrogenesis-related genes through TGF-beta receptor II (TGFRII)-AKT-mTOR signaling in primary cultured mouse precartilaginous stem cells. Biochem Biophys Res Commun. 2014;450:646–651. doi: 10.1016/j.bbrc.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Peran M, Ruiz S, Kwiatkowski W, Marchal JA, Yang SL, Aranega A, Choe S, Izpisua BJ. Activin/BMP2 chimeric ligands direct adipose-derived stem cells to chondrogenic differentiation. Stem Cell Res. 2013;10:464–476. doi: 10.1016/j.scr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Cha BH, Kim JH, Kang SW, Do HJ, Jang JW, Choi YR, Park H, Kim BS, Lee SH. Cartilage tissue formation from dedifferentiated chondrocytes by codelivery of BMP-2 and SOX-9 genes encoding bicistronic vector. Cell Transplant. 2013;22:1519–1528. doi: 10.3727/096368912X647261. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Im GI. Electroporation-mediated transfer of SOX trio genes (SOX-5, SOX-6, and SOX-9) to enhance the chondrogenesis of mesenchymal stem cells. Stem Cells Dev. 2011;20:2103–2114. doi: 10.1089/scd.2010.0516. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Im GI. SOX trio decrease in the articular cartilage with the advancement of osteoarthritis. Connect Tissue Res. 2011;52:496–502. doi: 10.3109/03008207.2011.585409. [DOI] [PubMed] [Google Scholar]
- 19.Brandi ML, Gennari L, Cerinic MM, Becherini L, Falchetti A, Masi L, Gennari C, Reginster JY. Genetic markers of osteoarticular disorders: facts and hopes. Arthritis Res. 2001;3:270–280. doi: 10.1186/ar316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbruggen G. Chondroprotective drugs in degenerative joint diseases. Rheumatology (Oxford) 2006;45:129–138. doi: 10.1093/rheumatology/kei171. [DOI] [PubMed] [Google Scholar]
- 21.Furumatsu T, Asahara H. Histone acetylation influences the activity of Sox9-related transcriptional complex. Acta Med Okayama. 2010;64:351–357. doi: 10.18926/AMO/41320. [DOI] [PubMed] [Google Scholar]
- 22.Furumatsu T, Matsumoto-Ogawa E, Tanaka T, Lu Z, Ozaki T. ROCK inhibition enhances aggrecan deposition and suppresses matrix metalloproteinase-3 production in human articular chondrocytes. Connect Tissue Res. 2014;55:89–95. doi: 10.3109/03008207.2013.852544. [DOI] [PubMed] [Google Scholar]
- 23.Elkasrawy M, Fulzele S, Bowser M, Wenger K, Hamrick M. Myostatin (GDF-8) inhibits chondrogenesis and chondrocyte proliferation in vitro by suppressing Sox-9 expression. Growth Factors. 2011;29:253–262. doi: 10.3109/08977194.2011.599324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durigova M, Nagase H, Mort JS, Roughley PJ. MMPs are less efficient than ADAMTS5 in cleaving aggrecan core protein. Matrix Biol. 2011;30:145–153. doi: 10.1016/j.matbio.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaur T, Rich L, Lengner CJ, Hussain S, Trevant B, Ayers D, Stein JL, Bodine PV, Komm BS, Stein GS, Lian JB. Secreted frizzled related protein 1 regulates Wnt signaling for BMP2 induced chondrocyte differentiation. J Cell Physiol. 2006;208:87–96. doi: 10.1002/jcp.20637. [DOI] [PubMed] [Google Scholar]
- 26.Maneix L, Servent A, Poree B, Ollitrault D, Branly T, Bigot N, Boujrad N, Flouriot G, Demoor M, Boumediene K, Moslemi S, Galera P. Up-regulation of type II collagen gene by 17beta-estradiol in articular chondrocytes involves Sp1/3, Sox-9, and estrogen receptor alpha. J Mol Med (Berl) 2014 doi: 10.1007/s00109-014-1195-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Guo WP, Fu XG, Jiang SM, Wu JZ. Neuregulin-1 regulates the expression of Akt, Bcl-2, and Bad signaling after focal cerebral ischemia in rats. Biochem Cell Biol. 2010;88:649–654. doi: 10.1139/O09-189. [DOI] [PubMed] [Google Scholar]
- 28.Tsuruta F, Masuyama N, Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002;277:14040–14047. doi: 10.1074/jbc.M108975200. [DOI] [PubMed] [Google Scholar]
- 29.Tare RS, Townsend PA, Packham GK, Inglis S, Oreffo RO. Bcl-2-associated athanogene-1 (BAG-1): a transcriptional regulator mediating chondrocyte survival and differentiation during endochondral ossification. Bone. 2008;42:113–128. doi: 10.1016/j.bone.2007.08.032. [DOI] [PubMed] [Google Scholar]


