Abstract
MicroRNAs (miRNAs) are known to function as negative gene regulators. Recently, miRNAs have been shown to regulate immunity processes; however, the mechanism is unclear. The role of microRNA-214 (miR-214) in dendritic cell (DC) maturation has not been investigated. We found that the miR-214 level was correlated with the maturation of DCs and inflammatory cytokine secretion, as depressed miR-214 levels induced DC tolerance. We also identified β-catenin as a target gene of miR-214 and demonstrated its association with Treg cell differentiation. MiR-214 regulates gene expression by binding to the 3’UTR of β-catenin. The results suggest that β-catenin is a critical regulator of tolerance in DCs via miR-214. The expression of miR-214 could be a potential therapeutic strategy in organ transplantation or autoimmunity patients.
Keywords: MicroRNA-214, dendritic cells, β-Catenin, Treg cells, transplantation tolerance
Introduction
MicroRNAs (miRNAs) are noncoding RNAs that are critical regulators and bind to the 3’ untranslated region (UTR) of mRNA, leading to mRNA translational repression and/or degradation in the cytoplasm [1,2]. MiRNAs play an essential role in several cell functions, including cell proliferation, differentiation, metabolism, apoptosis, and metastasis [3-5]. MiR-214 is a miRNA that is clustered with miR-199a and up-regulated by the transcriptional repressor Twist1 [6,7]. Epithelial ovarian cancer stem cells express high levels of β-catenin, which is one of the stem cell markers associated with low miR-214 levels [8,9].
Dendritic cells (DCs) are important for initiating robust immunity against pathogens by maintaining immunological tolerance. Antigen-presenting cells (APCs), such as DCs and macrophages, are specialized immune cells that play a vital role in stimulating immune responses [10-12]. Recent studies have shown that DC immune responses are suppressed by T regulatory cells (Treg cells) [13-15]. DCs express pathogen-recognition receptors (PRRs) [16-18] and initiate downstream signaling cascades that lead to adaptive immune responses [12,15,19]. PRRs can activate DCs and cytokines, which regulate the differentiation of naive CD4+ T cells into inflammatory T helper 1 (Th1), Th17 or Treg cells to help regulate immunity and tolerance.
A recent study found that Wnt-β-catenin signaling regulates the inflammatory responses of DCs via IL-10, TNF-β and Treg cells. It was also shown that the down-regulation of β-catenin levels in DCs enhances inflammatory responses [9]. In this study, we analyzed the roles of miR-214 in the regulation of DC differentiation and maturation as well as the roles of secreted cytokines in inducing T-cell proliferation in vitro. In addition, we analyzed the stimulation of regulatory T cells by DCs. Our results showed that miR-214 directly down-regulates the expression of β-catenin by targeting the 3’-UTR and further suppresses the generation of Treg cells.
Materials and methods
Animals
Female C57BL/6 mice (aged 6-8 weeks) were purchased from the Academy of Military Medical Science (Beijing, China). The experiments were performed in accordance with the guidelines for animal care and were approved by the Animal Ethics Committee of Tianjin Medical University (Tianjin, China).
miR-214 lentivirus-mediated overexpression and inhibition
The mouse miR-214 coding region and the downstream region of the cytomegalovirus promoter with a modified lentiviral vector were generated from pCDH-CMV-MCS-EF1-puro (CD510B-1; System Bioscience). The control or miR-214 inhibition encoding mutant was cloned into the PLKO.1 vector (addgene) according to the manufacturer’s instructions.
Cell culture and treatment
Primary DCs were cultured from C57BL/6 mouse bone marrow (BM) according to an established protocol with some modifications [20]. Briefly, BM was isolated from the hind legs bones, and the cells were resuspended after being passed through a 70 μm cell strainer. A mouse cell line (DC2.4) was purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). The cells were cultured to 1×106 cells/ml at 37°C with 5% CO2 in 6-well plates in RPMI 1640 medium (Gibco, Uxbridge, UK) containing 10% FBS (Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin, 10 ng/ml murine granulocyte-macrophage colony-stimulating factor (GM-CSF) and 2 ng/ml murine IL-4 (PeproTech Inc., Rocky Hill, NJ, USA). The cell medium was changed after 2 days.
DC maturation
DCs were cultured with 100 ng/ml LPS (Sigma-Aldrich Co., St Louis, MO, USA) for 6 days, and cells were verified by flow cytometry via staining with CD80/83/86- and MHC II-specific antibodies.
DC and Treg response in mice
A single cell suspension was isolated from the spleens of lentivirus-treated or control mice by mashing and passing the tissue through a cell strainer. Splenocytes from the lentivirus-treated or control mice were subjected to surface staining. The expression of CD11c/80/83/86 and MHC-II was analyzed by staining cells (0.5-1×106) with FITC-conjugated specific mAbs or isotype controls (eBioscience, San Diego, CA) diluted in PBS containing 1% BSA. Treg cells were stained according to the manufacturer’s protocol (eBioscience, San Diego, CA).
Flow cytometry analysis
Cells were harvested on day 5 for the analysis of BM-derived DC differentiation or on day 7 for the analysis of DC maturation. DCs were stained for the surface markers CD80/83/86 and MHC-II using anti-mouse, fluorescence-conjugated antibodies and were stained simultaneously with PE-conjugated CD11c antibody (BD Biosciences, CA, USA). A fluorescence-activated cell sorter (FACS) was used for the analysis. The data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Reporter gene assay
HEK293T cells were maintained in DMEM and transfected with PEI (Polyplus). Wild-type or mutated 3’-UTR sequences of β-catenin were cloned into the pmirGLO vector (Promega). The dual-luciferase reporter gene construct (2 ng per well) and pCDH-miR-214 (100 ng per well) were cotransfected for 24 h, and luciferase activity was measured with the Dual-Luciferase Reporter Assay system (Promega).
Quantitative real-time PCR assay
RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. After RNA purification, the samples were treated with DNase to remove contaminating genomic DNA. Reverse transcription was performed with random hexamers and M-MLV reverse transcriptase (Promega, Madison, USA). All other reverse transcription reagents were supplied by Takara (Takara, Japan). The gene-specific primers were synthesized at BGI (Beijing, China). For relative quantitative real-time PCR, SYBR Green mix (Roche, USA) was used according to the manufacturer’s instructions. The reactions were performed in triplicate using an ABI PRISM 7500 Fast Real-Time PCR System (Applied Biosystems Inc., Foster City, California, USA), and the generated products were analyzed using ABI 7500 software (Version 2.0.6, Applied Biosystems Inc., Foster City, California, USA). The following primer pairs were used: Sense (5’-3’) Anti-sense (5’-3’), IL-1β, GCAACTGTTCCTGAACTCAACT, ATCTTTTGGGGTCCGTCAACT; IL-4, GGTCTCAACCCCCAGCTAGT, GCCGATGATCTCTCTCAAGTGAT; IL-6, TAGTCCTTCCTACCCCAATTTCC, TTGGTCCTTAGCCACTCCTTC; IL-10, GCTCTTACTGACTGGCATGAG, CGCAGCTCTAGGAGCATGTG; TNF-α, CCCTCACACTCAGATCATCTTCT, GCTACGACGTGGGCTACAG; GAPDH, AGGTCGGTGTGAACGGATTTG, TGTAGACCATGTAGTTGAGGTCA. Gene expression values were normalized based on GAPDH mRNA levels. Data are presented as the 2-ΔΔCt values and are representative of at least three independent experiments.
Western blot analysis
DC2.4 cells were lysed in a buffer containing 10 mM Tris-buffer (pH 7.6), 1% Triton X-100, 1% phosphatase inhibitor cocktail and 1 mM PMSF. The lysates were boiled in sodium dodecyl sulfate (SDS) sample buffer and subjected to SDS-PAGE. Rabbit monoclonal antibodies against β-actin and β-catenin were purchased from Abcam and were diluted 1:1000. Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Santa Cruz Biotechnology, CA, USA) was used as the secondary antibody. Immunoreactive bands were identified using the ECL Western Blotting Detection System (Millipore Corporation, Billerica, MA, USA).
Statistical analysis
One-way analysis of variance (ANOVA) was performed. P < 0.05 was considered statistically significant. In addition, the standard deviations from the mean, expressed as the SD values or as equivalent error bars, were calculated.
Results
The successful overexpression and inhibition of miR-214 in DCs in vitro and in vivo
Lentiviral vectors were constructed via in vitro and in vivo miR-214 manipulation. Pre-miR-214 (LV-214), a mutant miR-214 control (LV-ctrl) and its specific inhibitor (LV-anti-214) were designed, and the overexpression or inhibition of miR-214 in DCs were confirmed (Figure 1A). Recombinant lentivirus was injected into mice daily for 7 days, and the efficacy of lentivirus was measured by quantitative PCR analysis. Infection with LV-214 led to three-fold greater expression of mature miR-214 in spleen and 1.9-fold greater expression in peripheral lymph nodes (Figure 1B).
Figure 1.
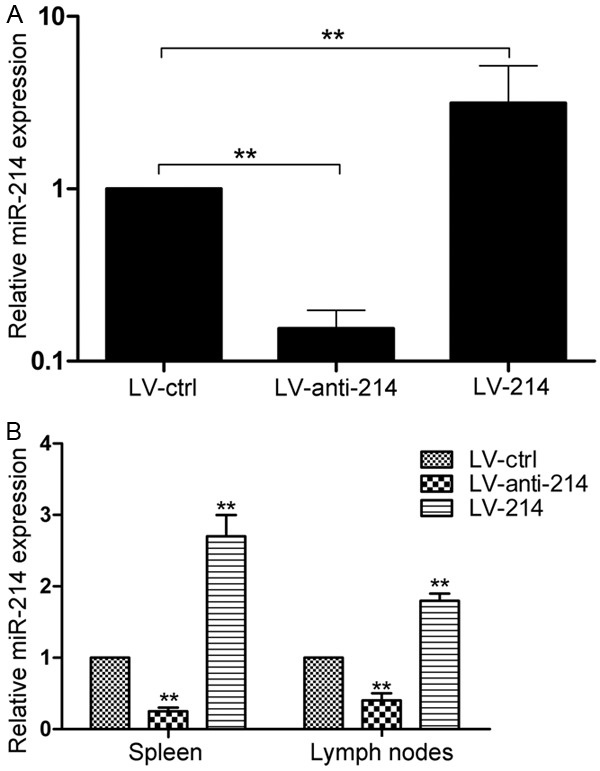
The mRNA expression levels of miR-214 in DC2.4 (A) and in splenocytes and lymph nodes cells (B) were determined by quantitative PCR after transfection with three lentiviral vectors. All data were representative of those obtained in three independent experiments and expressed as mean ± SEM. **P < 0.01 vs. LV-ctrl.
Effect of miR-214 on the maturation of mouse BM-derived DCs
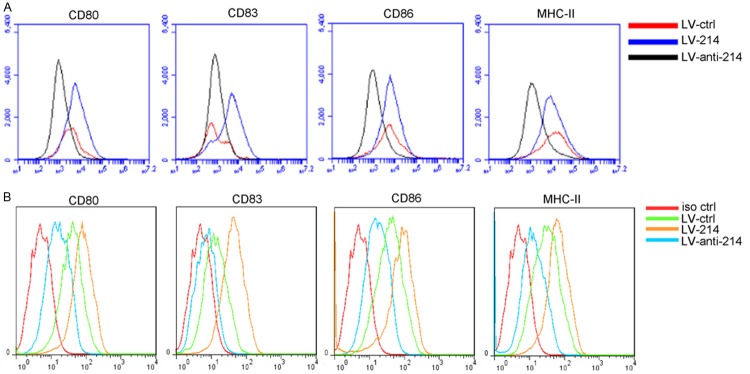
The flushed BM cells were incubated at 37°C with 5% CO2 in the presence of GM-CSF and IL-4 for 4 days. The various types of recombinant lentivirus were added into culture medium with 8 μg/ml polybrene. The maturation of DCs was induced by culturing for an additional 24 h with LPS at a final concentration of 100 ng/ml on the sixth day of DC culture. DC maturation status was verified by flow cytometry after staining with FITC-CD80/83/86 and MHC-II specific Abs. We found that elevated miR-214 promoted iDC maturation, whereas depressed miR-214 inhibited iDC maturation (Figure 2A). The results obtained using DC2.4 was similar, thus validating the effects of miR-214 on DC maturation results (Figure 2B).
Figure 2.
The expression levels of CD80, CD83, CD86 and MHC-ll in mouse BM-derived DCs (A) and DC2.4 (B) were examined by flow cytometry after transfection with three lentiviral vectors.
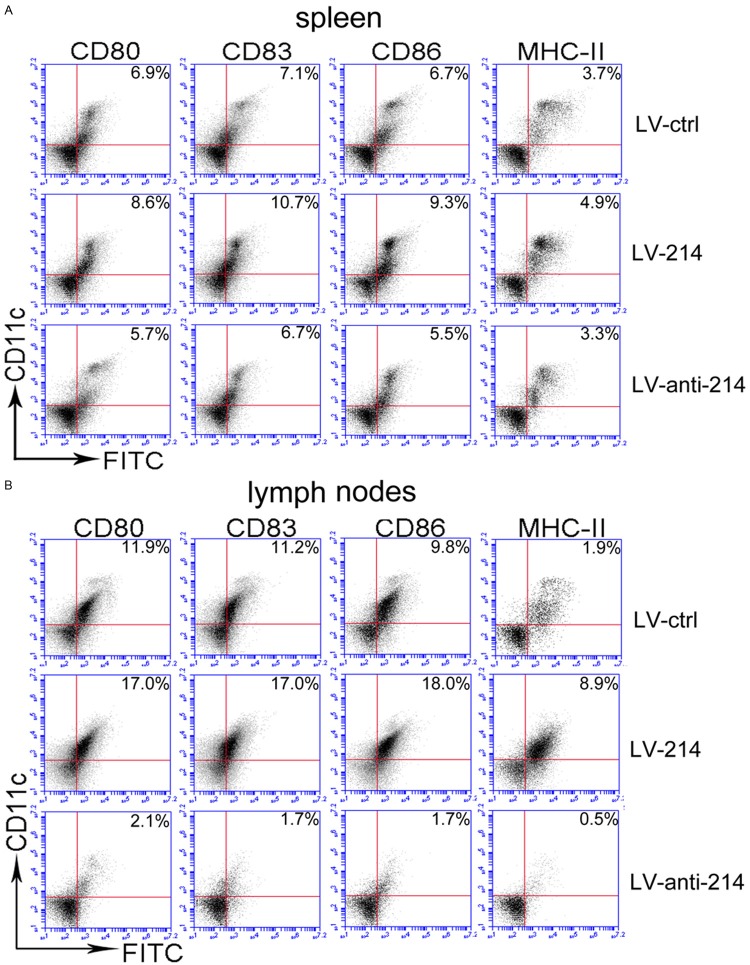
Effect of miR-214 on the function of DC in vivo
We subsequently determined whether the difference was similar in the three groups of lentivirus-infected mice with ova antigen sensitization. Splenocytes and lymph node cells were obtained from lentivirus-infected mice and were analyzed ex vivo for the expression of CD80/83/86 and MHC-II (Figure 3A, 3B). The result showed that the surface expression of CD80/83/86 and MHC-II was reduced in CD11c+ cell populations from the LV-anti-214-treated mice compared with the LV-ctrl-treated mice. The expression levels of CD80/83/86 and MHC-II were increased in CD11c+ cell populations from the LV-214-treated mice compared with the LV-ctrl-treated mice.
Figure 3.
The expression levels of CD80, CD83, CD86 and MHC-ll in CD11c+ cells derived from mouse spleen (A) and lymph nodes (B) of mice treated with three lentiviral vectors were examined by flow cytometry.
Effect of miR-214 on DC-secreted cytokines
DC-derived cytokines are required for the adaptive immune response. Therefore, we investigated the potential effects of miR-214 on the regulation of the expression of T helper cell-polarizing cytokines. The iDCs were treated with three types of lentivirus and stimulated with LPS for 24 h, and the steady-state levels of cytokine mRNAs were analyzed using quantitative PCR (Figure 4). The results showed that anti-miR-214 substantially inhibited the production of the inflammatory cytokines TNF-α, IL-1β and IL-6 and induced the production of the anti-inflammatory cytokines IL-4 and IL-10. These data suggest that anti-miR-214 suppresses the DC-mediated polarization of Th1 and Th17 cells and might be useful for the treatment of autoimmune inflammatory diseases that are mediated by Th1 and Th17 cells.
Figure 4.
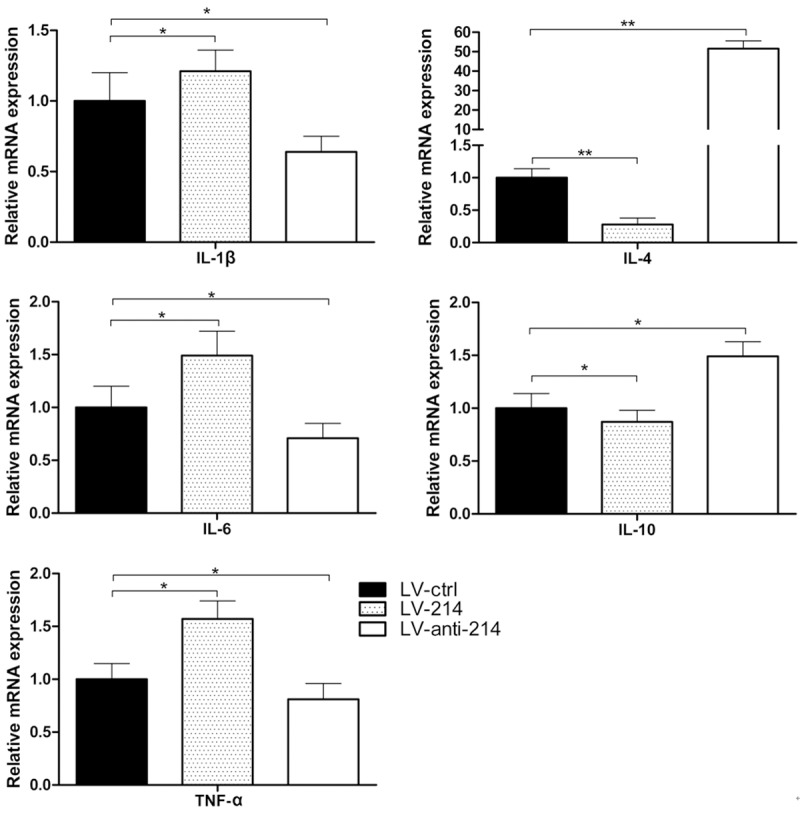
The mRNA expression levels of IL-1β, IL-4, IL-6, IL-10 and TNF-α in mouse BM-derived DCs were determined by quantitative PCR after transfection with three lentiviral vectors. All data were representative of those obtained in three independent experiments and expressed as mean ± SEM. *P < 0.05, **P < 0.01 vs. LV-ctrl.
Expression of β-catenin in the overexpression and inhibition of miR-214 in DCs
The potential miR-214 targets were predicted using the program TargetScan28. It has been reported that negative regulators of DC differentiation (Science, 2010), such as β-catenin, have putative miR-214-binding elements in the 3’-untranslated regions (UTRs). In our study, we found that miR-214 inhibited the luciferase activity of a reporter containing the wild-type β-catenin 3’-UTR but not that of a mutated 3’-UTR (Figure 5A), indicating that miR-214 targets β-catenin. Consistent with this finding, miR-214 overexpression suppressed the expression of β-catenin, whereas miR-214 inhibition promoted the expression of β-catenin (Figure 5B). Moreover, there was a similar correlation between miR-214 and β-catenin protein levels (Figure 5C).
Figure 5.
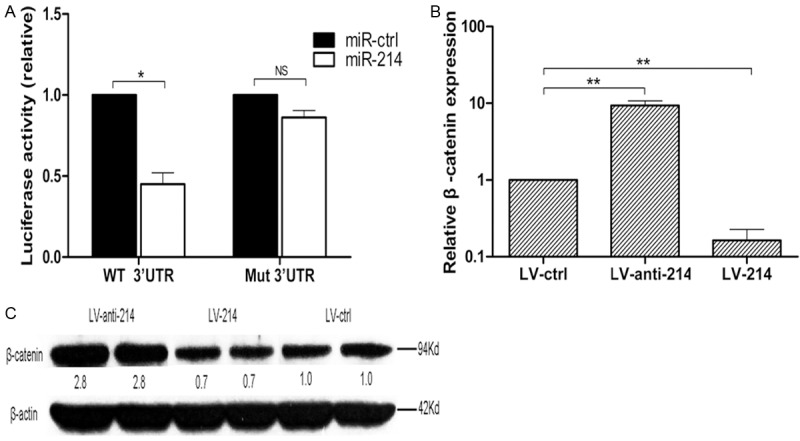
(A) Luciferase activity of reporter carrying the mutated (Mut) or wild-type (WT) β-catenin 3’ UTR cotransfected into HEK293T cells with wildtype miR-214. The mRNA (B) and protein (C) expression levels of β-catenin in DC2.4 were determined by quantitative PCR and Western blot analysis after transfection with three lentiviral vectors. All data were representative of those obtained in three independent experiments and expressed as mean ± SEM. *P < 0.05 vs. miR-ctrl, **P < 0.01 vs. LV-ctrl, NS indicated P >0 .05 vs. miR-ctrl.
Effect of miR-214 on Treg cell differentiation in vivo
To directly demonstrate that β-catenin is a functional target of miR-214 in immunity, we isolated splenocytes and lymph nodes cell from lentivirus-infected mice with ova antigen sensitization and analyzed the Treg cells. As shown in Figure 6A and 6B, miR-214 overexpression promoted Treg differentiation, whereas miR-214 inhibition suppressed Treg differentiation. This result is consistent with the DC numbers, which indicated that miR-214 might infect DCs to suppress immune responses through the generation of Treg cells.
Figure 6.
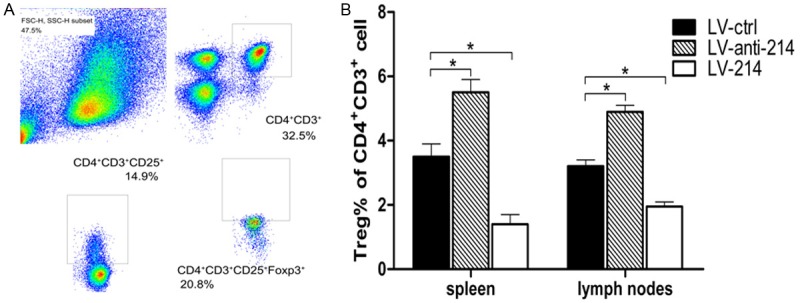
Effects of miR-214 on Treg cell differentiation in vivo. The abundance of Treg cells as a percentage of CD3+CD4+ cells was detected by flow cytometry. CD3+CD4+ cells were obtained from spleen and lymph nodes of mice treated with one of three lentiviral vectors. Data are presented as a representative plot (A) and summary graph (B). All data were representative of those obtained in three independent experiments and expressed as mean ± SEM. *P < 0.05 vs. LV-ctrl.
Discussion
MiRNAs are vital for controlling immune system processes, including cell differentiation and homeostasis, cytokine responses, interactions with pathogens and tolerance induction [21]. Defects in miRNAs have been associated with oncogenesis and other diseases [22-24]. In this study, our data suggest that miR-214 is a DC-associated miRNA that functions as a regulator of β-catenin signaling. First, we observed that miR-214 expression was correlated with β-catenin levels. We also demonstrated that miR-214 overexpression suppresses the expression of β-catenin and that miR-214 inhibition enhances the expression of β-catenin. Second, we demonstrated that miR-214 expression affects DC functions, and miR-214 influences iDC maturation and cytokine secretion in response to LPS stimulation. Third, we showed that miR-214 might regulate Treg cells via DCs. MiR-214 influenced Treg cell differentiation in vivo, which might be mediated by DCs. The novel finding of this study is that miR-214 regulates functional β-catenin to influence DCs and thus balance immunity and tolerance. Consistent with previous studies [9,25-27], β-catenin signaling was found to program DCs into a tolerogenic state and limit the inflammatory response.
Immature DCs, as well as cytokine (TGF-β) and/or drug-treated ‘tolerogenic’ DCs, can induce T cell tolerance. ‘Tolerogenic’ DCs produce less pro-inflammatory cytokines in favor of cytokines such as TGF-β and IL-10 [28,29]. Overexpression of miR-30b in DCs leads to increased IL-10 and NO production, whereas inhibition reduces IL-10 and NO production. In addition, miR-125a and miR-99a expression has been shown to increase in ‘tolerogenic’ DCs [30]. MiR-23b has also been associated with a ‘tolerogenic’ DC phenotype. Expression of miR-23b in mouse BM-DCs and human moDCs results in DCs with a reduced IL-12 but increased IL-10 production capacity as well as reduced MHC-II, CD80 and CD86 expression. Increased expression of Foxp3 was observed when CD4+ T cells were co-cultured in the presence of miR-23b-expressing DCs [31]. Stumpfova et al. [32] showed that 27 miRNAs, including miR-17, miR-133b and miR-203, were specifically increased in ‘tolerogenic’ DCs compared with mature DCs. They also found four miRNAs that were down-regulated in ‘tolerogenic’ DCs, miR-99b, miR-135a, miR-147 and miR-214 [32]. A low level of the miRNA let-7 in human moDCs has been shown to favor the expansion of Treg cells following interaction with these DCs, again demonstrating a link between miRNAs and tolerance induction [33,34]. In this study, we first found that miR-214 can regulate ‘tolerogenic’ DCs to favor the Treg cell, similar to the action of miR-let-7. The overexpression of miR-214 promoted Treg differentiation, whereas the inhibition of miR-214 suppressed Treg differentiation.
An increasing body of literature now highlights the importance of specific miRNAs in DC development as well as their maturation process, antigen presentation capacity and cytokine release. Given the unique role of DCs within the immune system as a link between the adaptive and the innate immune response, understanding how specific miRNAs affect DCs function is important for understanding disease. In this study, we provide the first identification of the unique role of miR-214 in DC maturation, leading to altered cytokine profiles of DCs and activation of Treg cells, which may be of clinical relevance and warrants further investigation in defined disease states, such as autoimmunity and organ transplantation rejection.
Conclusion
To our knowledge, this is the first study describing miR-214 association with tolerogenic DCs through β-catenin signaling. We provide further evidence that miRNAs are linked with the ‘tolerogenic’ DC phenotype. We consider it important to share these findings and encourage further and larger studies.
Acknowledgements
This work was financially supported by grants from the National High Technology Research and Development Program 863 (No. 2012AA021003). We thank Song Juan, Jing Yaqing, and Zhao Yuxia (College of Basic medicine, Tianjin Medical University) for their excellent technical assistance and helpful discussions.
Disclosure of conflict of interest
None.
References
- 1.Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153:516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Xie M, Li M, Vilborg A, Lee N, Shu MD, Yartseva V, Šestan N, Steitz JA. Mammalian 5’-capped microRNA precursors that generate a single microRNA. Cell. 2013;155:1568–1580. doi: 10.1016/j.cell.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun K, Lai EC. Adult-specific functions of animal microRNAs. Nat Rev Genet. 2013;14:535–548. doi: 10.1038/nrg3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Zuo X, Yang B, Li Z, Xue Y, Zhou Y, Huang J, Zhao X, Zhou J, Yan Y, Zhang H, Guo P, Sun H, Guo L, Zhang Y, Fu XD. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell. 2014;158:607–619. doi: 10.1016/j.cell.2014.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T, Parenti AR, Daidone MG, Bicciato S, Piccolo S. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141:1195–1207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe T, Sato T, Amano T, Kawamura Y, Kawamura N, Kawaguchi H, Yamashita N, Kurihara H, Nakaoka T. Dnm3os, a non-coding RNA, is required for normal growth and skeletal development in mice. Dev Dyn. 2008;237:3738–3748. doi: 10.1002/dvdy.21787. [DOI] [PubMed] [Google Scholar]
- 7.Lee YB, Bantounas I, Lee DY, Phylactou L, Caldwell MA, Uney JB. Twist-1 regulates the miR-199a/214 cluster during development. Nucleic Acids Res. 2009;37:123–128. doi: 10.1093/nar/gkn920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, Glackin C, Rutherford T, Mor G. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010;29:3545–3553. doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckwalter MR, Albert ML. Orchestration of the immune response by dendritic cells. Curr Biol. 2009;19:R355–R361. doi: 10.1016/j.cub.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Laffont S, Powrie F. Immunology: dendritic-cell genealogy. Nature. 2009;462:732–733. doi: 10.1038/462732a. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Sun B, Wang D, Ji Y, Kong Q, Wang G, Wang J, Zhao W, Jin L, Li H. Murine bone marrow mesenchymal stem cells cause mature dendritic cells to promote T-cell tolerance. Scand J Immunol. 2008;68:607–615. doi: 10.1111/j.1365-3083.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 13.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Pandey G, Cohain A, Miller J, Merad M. Decoding dendritic cell function through module and network analysis. J Immunol Methods. 2013;387:71–80. doi: 10.1016/j.jim.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Svajger U, Rozman P. Tolerogenic dendritic cells: molecular and cellular mechanisms in transplantation. J Leukoc Biol. 2014;95:53–69. doi: 10.1189/jlb.0613336. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Shaw MH, Kim YG, Nuñez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manicassamy S, Pulendran B. Modulation of adaptive immunity with toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CZ, Schaffert S, Fragoso R, Loh C. Regulation of immune responses and tolerance: the microRNA perspective. Immunol Rev. 2013;253:112–128. doi: 10.1111/imr.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro F, Lieberman J. Small RNAs guide hematopoietic cell differentiation and function. J Immunol. 2010;184:5939–5947. doi: 10.4049/jimmunol.0902567. [DOI] [PubMed] [Google Scholar]
- 23.Slezak-Prochazka I, Durmus S, Kroesen BJ, van den Berg A. MicroRNAs, macrocontrol: regulation of miRNA processing. RNA. 2010;16:1087–1095. doi: 10.1261/rna.1804410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha TY. The role of MicroRNAs in regulatory T Cells and in the immune response. Immune Netw. 2011;11:11–41. doi: 10.4110/in.2011.11.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu C, Jiang A. Generation of tolerogenic dendritic cells via the E-cadherin/beta-catenin-signaling pathway. Immunol Res. 2010;46:72–78. doi: 10.1007/s12026-009-8126-5. [DOI] [PubMed] [Google Scholar]
- 26.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 27.Xue Z, Ge Z, Zhang K, Sun R, Yang J, Han R, Peng M, Li Y, Li W, Zhang D, Hao J, Da Y, Yao Z, Zhang R. Embelin suppresses dendritic cell functions and limits autoimmune encephalomyelitis through the TGF-β/β-catenin and STAT3 signaling pathways. Mol Neurobiol. 2014;49:1087–1101. doi: 10.1007/s12035-013-8583-7. [DOI] [PubMed] [Google Scholar]
- 28.Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol. 2014;5:7–25. doi: 10.3389/fimmu.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fändrich F. Cell therapy approaches aiming at minimization of immunosuppression in solid organ transplantation. Curr Opin Organ Transplant. 2010;15:703–708. doi: 10.1097/MOT.0b013e328340669a. [DOI] [PubMed] [Google Scholar]
- 30.Su X, Qian C, Zhang Q, Hou J, Gu Y, Han Y, Chen Y, Jiang M, Cao X. miRNomes of haematopoietic stem cells and dendritic cells identify miR-30b as a regulator of Notch1. Nat Commun. 2013;4:2903–2914. doi: 10.1038/ncomms3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng J, Jiang HY, Li J, Tang HC, Zhang XM, Wang XR, Du JT, Li HB, Xu G. MicroRNA-23b promotes tolerogenic properties of dendritic cells in vitro through inhibiting Notch1/NF-κB signalling pathways. Allergy. 2012;67:362–370. doi: 10.1111/j.1398-9995.2011.02776.x. [DOI] [PubMed] [Google Scholar]
- 32.Stumpfova Z, Hezova R, Meli AC, Slaby O, Michalek J. MicroRNA profiling of activated and tolerogenic human dendritic cells. Mediators Inflamm. 2014;2014:259689–259698. doi: 10.1155/2014/259689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Liu F, Jia H, Zhang Q, Yin L, Liu W, Li H, Yu B, Wu J. Inhibition of microRNA let-7i depresses maturation and functional state of dendritic cells in response to lipopolysaccharide stimulation via targeting suppressor of cytokine signaling 1. J Immunol. 2011;187:1674–1683. doi: 10.4049/jimmunol.1001937. [DOI] [PubMed] [Google Scholar]
- 34.Ge Z, Da Y, Xue Z, Zhang K, Zhuang H, Peng M, Li Y, Li W, Simard A, Hao J, Yao Z, Zhang R. Vorinostat, a histone deacetylase inhibitor, suppresses dendritic cell function and ameliorates experimental autoimmune encephalomyelitis. Exp Neurol. 2013;241:56–66. doi: 10.1016/j.expneurol.2012.12.006. [DOI] [PubMed] [Google Scholar]