Abstract
MicroRNAs (miRNAs) are a class of endogenous, small non-coding RNAs which play important roles in various biological and cellular processes, including chemoresistance. The expression level of miR-299-3p was dysregulated in doxorubicin-resistance lung cancer cell lines. However, the exact role of miR-299-3p in doxorubicin-resistance is still unknown. In the present study, miR-299-3p was down-expressed in doxorubicin-resistant or -sensitive lung cancer samples and it was identified to directly targeted adenosine triphosphate binding cassette E1 (ABCE1) 3’-untranslated region (UTR) in lung cancer H69 cells by luciferase assay. After transfection of miR-299-3p mimics or ABCE1-siRNA, MTT assay confirmed that the H69/ADR cell proliferation was inhibited, as well as the enhanced cell inhibitory rate in the presence of doxorubicin. H69/ADR cell apoptosis rate was promoted after miR-299-3p or ABCE1-siRNA transfection. The results indicated that miR-299-3p promotes the sensibility of lung cancer to doxorubicin through suppression of ABCE1, at least partly. Therefore, the disordered decreased of miR-299-3p and resulting ABCE1 up-expression may contribute to chemoresistance of lung cancer, and miR-299-3p-ABCE1 may represent a new potential therapeutic target for the treatment of chemoresistance of lung cancer.
Keywords: Lung cancer, miR-299-3p, chemoresistance, adenosine triphosphate binding cassette E1
Introduction
Lung cancer is a common malignant tumor and the leading causes of cancer death all over the world, and leading to more than 1 million deaths each year [1]. According to the clinicopathological characteristics, the major lung cancer divided into two histological subtypes: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). SCLC makes up approximately 15% of all lung cancers [2]. SCLC is high sensitively response to both radiotherapy and chemotherapy, however, drug resistance results in high relapse rates and poor prognosis. In general, the 2-year survival rate is less than 5% of lung cancer patients with SCLC after final diagnosis [3,4]. Doxorubicin, an anthracycline drug, is widely used in chemotherapy regimens to treat patients with SCLC. Although chemotherapy has more than 50% response rates, median survival of SCLC is only approximately 10-12 months or 18-24 months for patients with extensive disease or limited disease [5-7]. In despite of doxorubicin has an excellent role in anti-tumor activity, chemoresistance has become one of the major hurdles in the treatment of SCLC and is a very important issue for improving poor prognosis of SCLC in clinical.
MicroRNAs (miRNAs) are endogenous, non-coding small RNAs that play important roles in posttranscriptional regulation and are found in all eukaryotic cells [8]. In mammals, mature miRNAs approximately 22 nucleotides in length, usually imperfect binding to the 3’-untranslated regions (UTR) of target mRNAs, which resulting in suppressing translation or inducing degradation of the target mRNAs [9,10]. MiRNAs are frequently disordered expressed in different cancers and play important roles in various biological and pathological processes [11]. MiRNAs act as either tumor suppressors or oncogenes in lung cancer [12,13]. Some miRNAs are potential biomarkers for lung cancer in diagnosis, prognosis and treatment target [14,15]. Recently, some studies have reported that the expression of miRNAs was related to chemoresistant in different cancers [16,17]. miR-299-3p was significantly down-regulated in osteosarcoma tissues and it was demonstrated an association with chemotherapy [18]. A recent study reported that the expression level of miR-299-3p was dysregulated in doxorubicin-resistance lung cancer cell lines [19]. However, the specific function of miR-299-3p in doxorubicin resistance of lung cancer, especially its molecular mechanisms is remains unclear.
In this study, we test the hypothesis that miRNA-299-3p promotes the sensibility of lung cancer to doxorubicin through directly targeting adenosine triphosphate (ATP) binding cassette E1 (ABCE1). First, Quantitative real-time PCR was used to determine to expression level of miR-299-3p in doxorubicin-resistant or -sensitive lung cancer tissues, and found that it was expressed lower in doxorubicin-resistant samples than doxorubicin-sensitive tissues. Second, the expression levels of ABCE1 in lung cancer tissues were detected using qRT-PCR and Western blot, and it was expressed higher in doxorubicin-resistant samples than doxorubicin-sensitive tissues. Third, over-expression of miR-299-3p or tranfected with ABCE1-siRNA in doxorubicin-resistant human small lung cancer cell line H69/AR down-regulated the expression of ABCE1, inhibited the proliferation of H69/AR, and promoted the apoptosis rate of H69/AR to doxorubicin. Finally, dual-luciferase reporter assay results showed that ABCE1 is a direct target of miR-299-3p.
Materials and methods
Human lung cancer tissues
Lung cancer tissues and compared adjacent normal tissues were obtained from patients surgically treated, and the study was approved by the Research Ethics Boards at the Department of Respiratory, Nanyang City Center Hospital. All the patients had an accurate histological diagnosis according to the clinicopathological criteria of the International Union for Cancer Control (UICC). All patients underwent conventional chemotherapy with 4-6 postoperative cycles of doxorubicin after optimal cytoreductive surgery. They were followed up for a minimum of 1 year after the completion of chemotherapy. The patients were assigned to either the doxorubicin-resistant group (n=20) or the doxorubicin-sensitive group (n=20) in accordance with the 2013 guidelines of the National Comprehensive Cancer Network (NCCN). Tumors and pericarcinous tissues were classified as doxorubicin-resistant lung cancer tissue (ARLC), doxorubicin-resistant pericarcinous tissue (RRPT), doxorubicin-sensitive lung cancer tissue (ASLC) and doxorubicin-sensitive pericarcinous tissue (ASPT). The median age of the patients was 48.3 years (range, 35-73 years). Samples were collected from primary lesions during surgery and stored in a liquid nitrogen tank until they were used for mRNA or protein isolation. All patients provided consent for the use of their specimens in research, and this use was approved by the institute research ethics committee of the Nanyang City Center Hospital.
Cell lines and culture conditions
Human small lung cancer cell line NCI-H69 was purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The H69 cells were maintained according to the vendor’s instructions. In brief, H69 cells were cultured in RPMI-1640 Medium (ATCC, Manassas, VA, USA) with 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2. The H69 cells were selected for resistance to doxorubicin (trade name, Adriamycin) (Sigma-Aldrich: Doxorubicin hydrochloride, D1515) as previously described. In brief, selection began at a drug dose (dose 1) that was 1000-fold less than the concentration at which 50% of parental H69 cells are killed (the IC50). The dose was then increased 1.5- or 3-fold until the maximally tolerated dose was achieved. Table 1 depicts the doxorubicin concentrations (doses) to which the cells were exposed. The resistance index of H69/ADR lung cancer cells was 14.77.
Table 1.
Concentrations of doxorubicin used at each selection dose
| Dose | Doxorubicin |
|---|---|
| IC50 | 0.94 μM |
| Dose 1 | 0.94 nM |
| Dose 2 | 2.82 nM |
| Dose 3 | 8.46 nM |
| Dose 4 | 25.38 nM |
| Dose 5 | 76.14 nM |
| Dose 6 | 228.42 nM |
| Dose 7 | 685.26 nM |
| Dose 8 | 2.06 μM |
| Dose 9 | 6.17 μM |
| Dose 10 | 9.25 μM |
| Dose 11* | 13.88 μM |
H69 lung cancer cells were exposed to progressively higher concentrations (doses) of doxorubicin drugs, beginning at a dose equivalent to 1/1000th of the concentration required to kill or inhibit the growth of 50% of wild-type H69 cells (the IC50). The symbol
denotes the highest concentration of drug at which the cells could survive (the maximally tolerated dose).
Plasmid construction
The 3’-untranslated region (3’-UTR) of ABCE1 containing putative binding site (ABCE1-3’-UTR-WT) or a mutant (ABCE1-3’-UTR-MUT), was cloned into the psi-CHECK2 vectors (Promega, USA). All plasmids were confirmed by DNA sequencing.
Cell transfection
The miR-299-3p mimics and scrambled sequence (NC) were purchased from a commercial manufacturer (GenePharm, China). For knockdown of ABCE1, the siRNA was obtained from Santa Cruz Biotech (USA). H69 cells were seeded in 24-well plates (1×105 per well) for 24 h, then cells were transfected with miRNA mimics, NC or siRNA (100 nM) using Lipofectamine 2000 (Invitrogen, CA, USA) in serum-free medium in accordance with the manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen Inc., USA). The concentration was measured by Nanodrop 2000 (Thermo Scientific, USA) at 260 nm. M-MLV Reverse Transcriptase (Promega, USA) was used for reverse transcription reaction according to the manufacturer’s instructions. PCR analysis was performed on Applied Biosystems 7500 Sequence Detection system (ABI, USA) using SYBR green real-time Master Mix (TOYOBO, Japan). The stem-loop primers used for the PCR amplification were synthesized by RiboBio (China). U6 and GAPDH were used to normalize the expression of miR-299-3p and ABCE1, separately. All the primers used were presented in Table 2.
Table 2.
Primer sequences used for miRNA and mRNA expression analysis
| Name | Primer sequence (5’-3’) |
|---|---|
| miR-299-3p-RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTTGGCGAA |
| U6-RT | CGCTTCACGAATTTGCGTGTCAT |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
| miR-299-3p-F | ACACTCCAGCTGGGTATGTGGGATGGTAAAC |
| Universal-R | GTGCAGGGTCCGAGGT |
| ABCE1-F | CCAGGTGAAGTTTTGGGATTAG |
| ABCE1-R | AGGTTTGATGATGGCTTTTAGG |
| GAPDH-F | ACACCCACTCCTCCACCTTT |
| GAPDH-R | TTACTCCTTGGAGGCCATGT |
F forward primer, R reverse primer, RT reverse transcription primer.
Protein extraction and Western blot
Total protein was extracted using protein extraction kit (Beyotime, China) and protein concentrations were determined using BCA Protein Assay kit (Pierce, USA). Proteins were separated by 12% SDS-PAGE gel, transferred to polyvinylidene difluoride membranes (PVDF) (Millipore, USA). Membranes were blotted with 10% non-fat milk overnight at 4°C, incubated with primary polyclonal antibodies (ABCE1, 1:3000, Abcam; GAPDH, 1:3000, Abcam) at 37°C for 2 h, and incubated with secondary antibody (horseradish peroxidase conjugated IgG) for 1 h at room temperature. Finally, they were detected using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, USA).
Luciferase reporter assay
1×105 per well of H69 cells were cultured in 24-well plates for 24 h. ABCE1-WT or -MUT psi-CHECK2 vectors were co-transfected with miR-299-3p mimics or negative control into H69 cells using Lipofectamine 2000 reagent, as described previously. Dual Luciferase Reporter Assay System (Promega, USA) was used to measure luciferase activities according to manufacturer’s instructions. Firefly luciferase activity was normalized to renilla luciferase activity.
Cell proliferation assay
After transfection with miR-299-3p mimics and ABCE1-siRNA, H69/ADR was treated with 6.89 μmol/L of doxorubicin for 0, 24, 48, 72, 96 hours. At different times, cells were harvested and assessed using CCK-8 kit (Sigma, USA). Cell viability was determined by measuring the optical absorbance of cells at 450 nm wavelength. The 50% inhibitory concentrations (IC50) and the inhibitory rate of the cells treated in different conditions were also determined using the CCK-8 kit.
Cell apoptosis assay
Annexin V-FITC/PI apoptosis detection kit (BestBio, China) was used to detect cell apoptosis by flow cytometry in accordance with the manufacturer’s instruction. H69/ADR cells tranfected with miR-299-3p and ABCE1-siRNA in presence of 6.89 μmol/L of doxorubicin for 48 hours. Cells were harvested and stained using the Annexin V-FITC/PI apoptosis detection kit. Then the cells were analyzed using Becton-Dickinson flow cytometer.
Statistical analysis
All the data were presents as Mean ± SD. Statistical significance between different groups was determined using one-way analysis of variance (ANOVA) or an unpaired Student’s t-test using SPSS 20.0 (SPSS Inc., USA). All experiments were repeated at least three times. P<0.05 was considered statistically significant.
Results
Expression level of miR-299-3p in lung cancer tissues
To explore the expression of miR-299-3p in lung cancer samples, qRT-PCR was used to detect the expression level of miR-299-3p in doxorubicin-resistant and -sensitive lung cancer tissue (Figure 1). The results showed that miR-299-3p was significantly down-regulated in lung cancer tissues compared with matched normal tissues. Moreover, the expression level of miR-299-3p in doxorubicin-resistant lung cancer was decreased more than that in doxorubicin-sensitive lung cancer.
Figure 1.
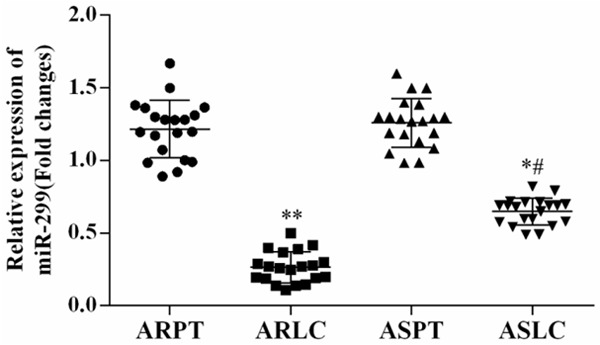
Expression level of miR-299-3p in lung cancer tissues. The expression level of miR-299-3p in lung cancer tissues was determined using qRT-PCR. The results showed that the expression of miR-299-3p was decreased in both doxorubicin-resistant and -sensitive lung cancer samples (n=20). Strikingly, miR-299-3p was down-regulated more in doxorubicin-resistant samples than in doxorubicin-sensitive tissues, *P<0.05 ARLC vs. ARPT or ASLC vs. ASPT; #P<0.05 ASLC vs. ARLC. ARLC: doxorubicin-resistant lung cancer; ARPT: doxorubicin-resistant pericarcinous tissue; ASLC: doxorubicin-sensitive lung cancer; ASPT: doxorubicin-sensitive pericarcinous tissue.
Expression level of ABCE1 in lung cancer samples
The expression level of ABCE1 in doxorubicin-resistant and -sensitive lung cancer tissue was determined using qRT-PCR and Western blot (Figure 2). Both mRNA and protein level, ABCE1 was significantly up-regulated in lung cancer tissues compared with matched normal tissues. Strikingly, the expression level of ABCE1 in doxorubicin-resistant lung cancer was increased more than that in doxorubicin-sensitive lung cancer.
Figure 2.
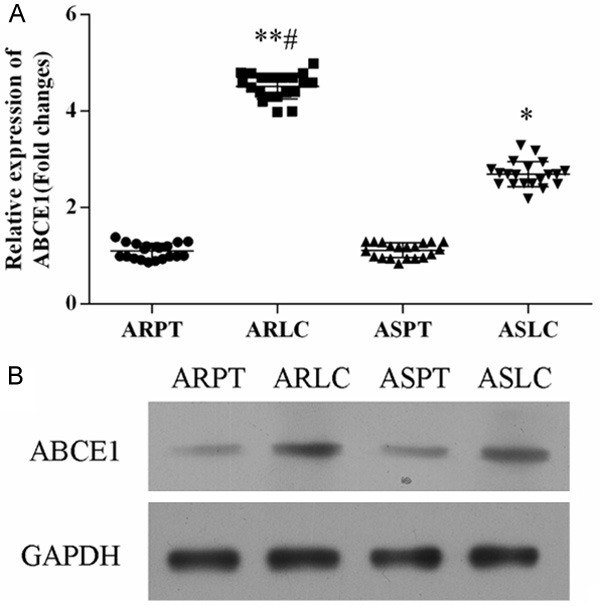
Expression level of ABCE1 in lung cancer samples. A. qRT-PCR revealed that the mRNA expression of ABCE1 was up-regulated more in doxorubicin-resistant samples than in doxorubicin-sensitive tissues, *P<0.05 ARLC vs. ARPT or ASLC vs. ASPT; #P<0.05 ASLC vs. ARLC. B. Western blot analysis the protein expression level of ABCE1 in lung cancer tissues.
Establishment of doxorubicin-resistant lung cancer cell line
To evaluate the role of miR-299-3p on doxorubicin-resistant, the doxorubicin-resistant lung cancer cell line H69/ADR was established. The expression level of miR-299-3p was significantly decreased in H69/ADR cell line compared with H69 cells (Figure 3A), and ABCE1 was significantly up-regulated in H69/ADR cells (Figure 3B).
Figure 3.
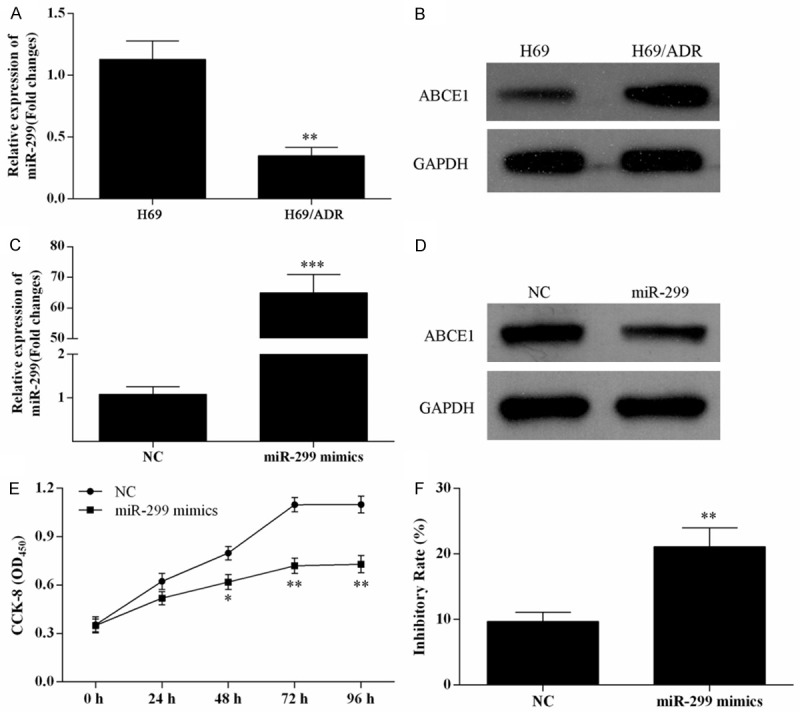
Over expression of miR-299-3p suppressed H69/ADR cell proliferation and promoted cell inhibitory rate. The expression level of miR-299-3p in H69 and H69/ADR cells was analysis using qRT-PCR (A), **P<0.01. Western blot was used to detect the expression of ABCE1 in H69 and H69/ADR cells (B). qRT-PCR and Western blot were used to analysis the expression of miR-299-3p (C) and ABCE1 (D) in H69 and H69/ADR cells, separately. (E) MTT assay was used to detect cell viability of H69/ADR cells transfected with miR-299-3p mimics. (F) The inhibitory rate of H69/ADR cells transfected with miR-299-3p was enhanced in the presence of 6.89 μmol/L of doxorubicin for 48 h, **P<0.01.
Over-expression of miR-299-3p inhibits cell proliferation and promotes cell inhibitory rate
After treated with various concentrations of doxorubicin, the IC50 of doxorubicin for H69 cells, H69/ADR cells and H69/ADR cells transfected with miR-299-3p mimics were 0.94, 13.88 and 6.89 μmol/L, respectively. Thus, the 6.89 μmol/L of doxorubicin was used to administrate the cells to detect its effect on cell proliferation and inhibitory rate of H69/ADR cells using a MTT assay.
After tranfected with miR-299-3p mimics, miR-299-3p was over-pressed in H69/ADR cells (Figure 3C). The expression level of ABCE1 was decreased in H69/ADR cells by transfection with miR-299-3p mimics (Figure 3D). MTT assay was used to detect the cell proliferation of H69/ADR cells after miR-299-3p mimics transfection (Figure 3E). After transfection, the cell proliferation was significantly inhibited after 48 h. In addition, the inhibitory rate of H69/ADR cells tranfected with miR-299-3p mimics was significantly enhanced in 6.89 μmol/L of doxorubicin for 48 h (Figure 3F).
Over-expression of miR-299-3p promotes cell apoptosis
The apoptosis of H69/ADR cells tranfected with miR-299-3p mimics in 6.89 μmol/L of doxorubicin for 48 h was detected using Annexin V-FITC/PI flow cytometry (Figure 4A), and proportion of apoptosis cells was measured (Figure 4B). It was observed that over-expression of miR-299-3p promoted the H69/ADR cell apoptosis rate.
Figure 4.
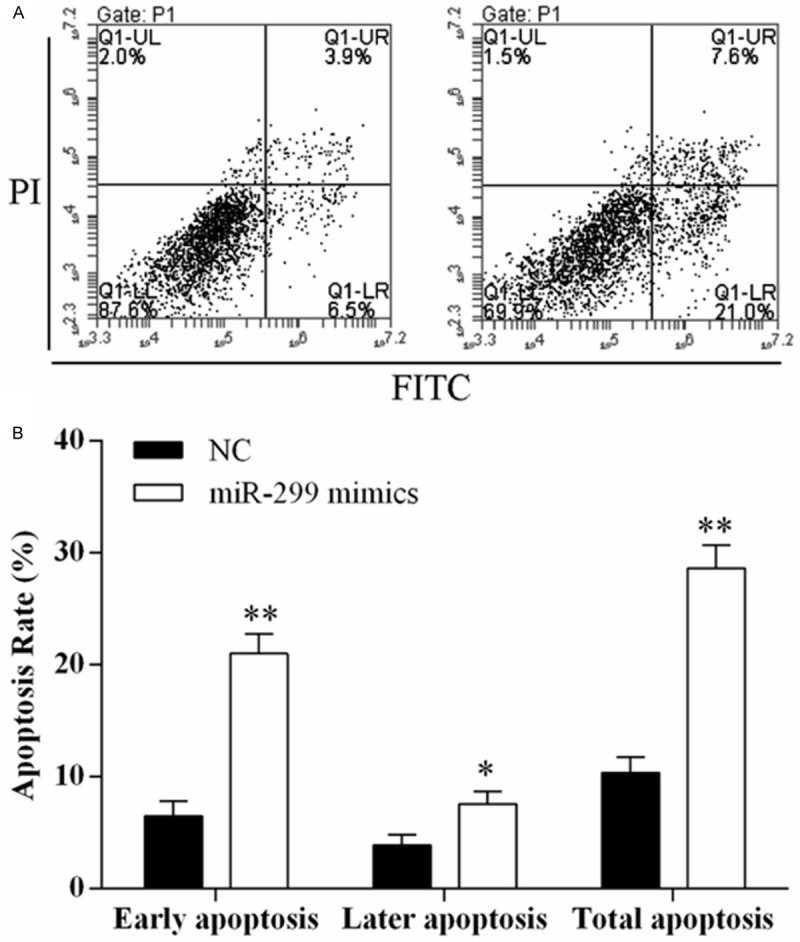
Over expression of miR-299-3p promoted H69/ADR cell apoptosis. Cell death is monitored by Annexin V-FITC/PI staining and flow cytometry. Quadrant statistics: early apoptosis cells in lower right (LR), late apoptosis cells in upper right (UR), viable cells in lower left (LL), and in necrosis cells in upper left (UL). A. Flow cytometry analysis of H69/ADR cells transfected with NC or miR-299-3p mimics. B. The percentage of early apoptosis cells, late apoptosis cells, and total apoptosis cells. Data are representative of three experiments, and are expressed as mean ± SD.
ABCE1 is a target gene of miR-299-3p
To verify whether or not ABCE1 is a target gene of miR-299-3p, the dual-luciferase reporter vectors were constructed containing wild type or mutant type seed sequences in the 3’-UTR of ABCE1 (Figure 5A). The activity of luciferase reporter gene was significantly reduced in H69 cells after co-transfected of ABCE1-3’UTR-WT with miR-299-3p mimics (Figure 5B). The results indicated that ABCE1 is a target gene of miR-299-3p.
Figure 5.
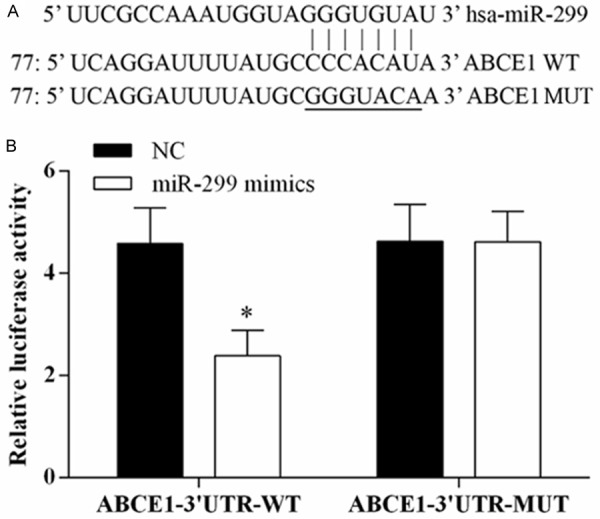
ABCE1 as direct target of miR-299-3p. Luciferase reporter assay was used to determine whether ABCE1 is a target gene of miR-299-3p. A. miR-299-3p and predicted duplex formation between wild-type ABCE1 3’-UTR or mutant ABCE1 3’-UTR. B. Luciferase activity with co-transfection of wild-type ABCE1 3’-UTR (WT) or mutant (MUT), and miR-299-3p or negative control mimics in H69 cells, *P<0.05.
Knockdown of ABCE1 inhibits cell proliferation, promotes cell inhibitory rate and cell apoptosis
The roles of ABCE1 in cell proliferation and cell apoptosis were performed by ABCE1 knockdown. The expression level of ABCE1 was knocked down by ABCE1-siRNA (Figure 6A, 6B). MTT assay showed that the cell proliferation was inhibited (Figure 6C) and cell inhibitory rate was promoted (Figure 6D) after H69/ADR cells tranfected with ABCE1-siRNA. Flow cytometry showed knockdown of ABCE1 promoted the apoptosis rate of H69/ADR cells (Figure 6E, 6F).
Figure 6.
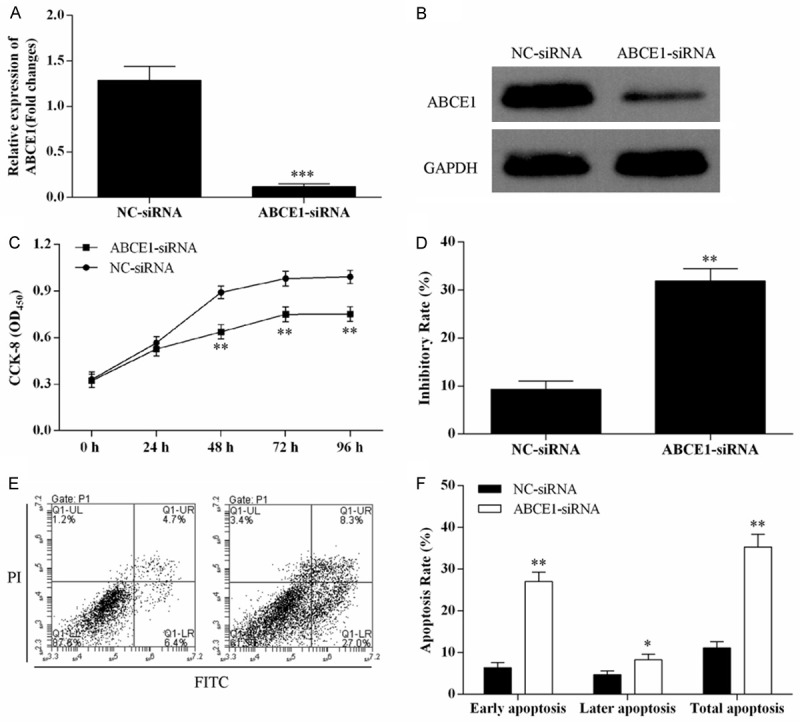
Down-regulation of ABCE1 inhibited H69/ADR cell proliferation, increased cell inhibitory rate, and promoted H69/ADR cell apoptosis. After tranfected with ABCE1-siRNA, the mRNA and protein expression of ABCE1 in H69/ADR cells was detected using qRT-PCR (A) and Western blot (B). Knocked down the expression of ABCE1 suppressed H69/ADR cell proliferation (C), and increased cell inhibitory rate (D). H69/ADR cell apoptosis rate was promoted after tranfected with ABCE1-siRNA (E, F).
Discussion
MicroRNAs (miRNAs) are a class of endogenous, small non-coding RNAs that target the 3’-untranslated regions (3’UTR) of mRNAs to negatively regulate gene expression, including mRNA degradation and translation inhibition [8]. More and more studies showed that miRNAs are involved in various cellular processes and play important role on occurrence and development of human cancers [20,21], also impact on cancer chemoresistance [22].
Although miR-299-3p was identified for a long time, its biological function is still known little. miR-299-3p was found significantly down-expressed in myeloid leukemia [23]. Previous study showed that miRNA-299-3p was down-regulated in osteosarcoma tissues and it was demonstrated an association with chemotherapy [18]. A recent study reported that dysregulation of miR-299-3p was involved in doxorubicin resistance in lung cancer [19]. However, the exact role of miR-299-3p in doxorubicin resistance of lung cancer is poorly understood. In the current study, the expression level of miR-299-3p in doxorubicin-resistant and -sensitive lung cancer tissues was changed, respectively compared with adjacent normal samples. The results showed that the expression of miR-299-3p in doxorubicin-resistant lung cancer samples was decreased more than that in doxorubicin-sensitive lung cancer tissues. In order to functional studies, we established the doxorubicin-resistant H69/ADR lung cancer cell line. miR-299-3p was significantly decreased in H69/ADR cells. The results of MTT assay and flow cytometry assay illustrated that over-expression of miR-299-3p was significantly inhibited the cell proliferation, promoted cell inhibitory rate, and increased cell apoptosis in H69/ADR lung cancer cells. All the results suggesting that miR-299-3p plays a critical role in cell proliferation and cell apoptosis of lung cancer, and indicating a greater suppression of miR-299-3p involved in doxorubicin-resistant lung cancers.
Subsequently, we demonstrated ABCE1 as direct target of miR-299-3p in human lung cancer. The results showed that ABCE1 was significantly up-regulated in human lung cancer tissues in both mRNA level and protein level, especially higher expressed in doxorubicin-resistant samples compared with doxorubicin-sensitive tissues. MTT assay and flow cytometry assay presented significant differences via RNAi approach of ABCE1. Knockdown of ABCE1 decreased the H69/ADR cell proliferation, and enhanced the H69/ADR cell inhibitory rate, and promoted the cell apoptosis, and that miR-299-3p dramatically decreased the expression of ABCE1 in protein level by bounding the complementary sites of its 3’-UTR. The ABCE1 gene is a member of the ATP-binding cassette (ABC) family and encodes a protein which was originally identified based on its capacity to inhibit ribonucleic L (RNase L), an interferon-induced nuclease in mammalian cells [24,25]. ABCE1 plays important roles in cell apoptosis and cell proliferation and is a candidate tumor suppressor protein. ABCE1 has previously been reported to be involved in the development of breast cancer [26], human esophageal carcinoma [27], human oral cancer [28], and lung cancer [29]. But, this is the first time to investigate the role of ABCE1 in chemoresistance of lung cancer. Although a more detailed mechanism must be discovered to explain the phenomena that the reduction of ABCE1 expression impaired cell proliferation, led to increased cell apoptosis, and promoted cell sensitive to doxorubicin, these observations provide the evidence that miR-570 exerts its function in cell motility and cell apoptosis via regulating the protein expression level of ABCE1.
In conclusion, we demonstrated that miR-299-3p in doxorubicin-sensitive lung cancer was decreased less than that in doxorubicin-resistant lung cancer samples, which directly regulated the expression of ABCE1. Over-expression of miR-299-3p was significantly inhibited the cell proliferation and increased cell apoptosis in H69/ADR lung cancer cells, and also promoted cell inhibitory rate. Overall, we concluded that over-expression of miR-299-3p promotes the sensibility of lung cancer to doxorubicin. Our investigation provides novel insight into the biological functions mediated by miR-299-3p, and may lead to new therapeutic approaches for doxorubicin-resistant lung cancer.
Acknowledgements
This work was partly supported by Nanyang City Center Hospital and we thank Guangzhou Vipotion Biotechnology Co., Ltd. for the assistance in establishing doxorubicin-resistant H69 cell line. All the authors did the same contribution to this work.
Disclosure of conflict of interest
None.
References
- 1.Marshall AL, Christiani DC. Genetic susceptibility to lung cancer-light at the end of the tunnel? Carcinogenesis. 2013;34:487–502. doi: 10.1093/carcin/bgt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQM, Downey RJ, Gandhi L, Ganti AKP, Govindan R, Grecula JC, Hayman J, Heist RS, Horn L, Jahan T, Koczywas M, Loo BW, Merritt RE, Moran CA, Niell HB, O’Malley J, Patel JD, Ready N, Rudin CM, Williams CC, Gregory K, Hughes M. Small Cell Lung Cancer: Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2013;11:78–98. doi: 10.6004/jnccn.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandler AB. Chemotherapy for small cell lung cancer. Seminars in Oncology. 2003;30:9–25. doi: 10.1053/sonc.2003.50012. [DOI] [PubMed] [Google Scholar]
- 5.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 6.Schmittel A. Second-line therapy for small-cell lung cancer. Expert Rev Anticancer Ther. 2011;11:631–637. doi: 10.1586/era.11.7. [DOI] [PubMed] [Google Scholar]
- 7.Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. 2007;12:1096–1104. doi: 10.1634/theoncologist.12-9-1096. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 10.Lai EC. Micro RNAs are complementary to 3’UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 11.Croce CM. 37 Causes and Consequences of microRNA Dysregulation in Cancer. European Journal of Cancer. 2012;48(Suppl 5):S8–S9. doi: 10.1097/PPO.0b013e318250c001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan B, Manley J, Lee J, Singh SR. The emerging roles of microRNAs in cancer metabolism. Cancer Lett. 2015;356:301–308. doi: 10.1016/j.canlet.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Gailhouste L, Ochiya T. Cancer-related microRNAs and their role as tumor suppressors and oncogenes in hepatocellular carcinoma. Histol Histopathol. 2013;28:437–451. doi: 10.14670/HH-28.437. [DOI] [PubMed] [Google Scholar]
- 14.Qi J, Mu D. MicroRNAs and lung cancers: from pathogenesis to clinical implications. Front Med. 2012;6:134–155. doi: 10.1007/s11684-012-0188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guz M, Rivero-Müller A, Okoń E, Stenzel-Bembenek A, Polberg K, Słomka M, Stepulak A. MicroRNAs-Role in Lung Cancer. Dis Markers. 2014;2014:218169. doi: 10.1155/2014/218169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka S, Campbell NR, An F, Kuo SC, Potter JJ, Mezey E, Maitra A, Selaru FM. Coordinated effects of microRNA-494 induce G(2)/M arrest in human cholangiocarcinoma. Cell Cycle. 2012;11:2729–2738. doi: 10.4161/cc.21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y, Tobin LA, Camps J, Wangsa D, Yang J, Rao M, Witasp E, Awad KS, Yoo N, Ried T, Kwong KF. MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in cancer cells. Oncogene. 2013;32:2442–2451. doi: 10.1038/onc.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nugent M. MicroRNA function and dysregulation in bone tumors: the evidence to date. Cancer Manag Res. 2014;6:15–25. doi: 10.2147/CMAR.S53928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo L, Liu Y, Bai Y, Sun Y, Xiao F, Guo Y. Gene expression profiling of drug-resistant small cell lung cancer cells by combining microRNA and cDNA expression analysis. Eur J Cancer. 2010;46:1692–1702. doi: 10.1016/j.ejca.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 20.Nagadia R, Pandit P, Coman WB, Cooper-White J, Punyadeera C. miRNAs in head and neck cancer revisited. Cell Oncol (Dordr) 2013;36:1–7. doi: 10.1007/s13402-012-0122-4. [DOI] [PubMed] [Google Scholar]
- 21.Wang YW, Shi DB, Chen X, Gao C, Gao P. Clinicopathological significance of microRNA-214 in gastric cancer and its effect on cell biological behaviour. PLoS One. 2014;9:e91307. doi: 10.1371/journal.pone.0091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garofalo M, Croce CM. MicroRNAs as therapeutic targets in chemoresistance. Drug Resist Updat. 2013;16:47–59. doi: 10.1016/j.drup.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garzon R, Volinia S, Liu CG, Fernandez-Cymering C, Palumbo T, Pichiorri F, Fabbri M, Coombes K, Alder H, Nakamura T, Flomenberg N, Marcucci G, Calin GA, Kornblau SM, Kantarjian H, Bloomfield CD, Andreeff M, Croce CM. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood. 2008;111:3183–3189. doi: 10.1182/blood-2007-07-098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barthelme D, Scheele U, Dinkelaker S, Janoschka A, Macmillan F, Albers SV, Driessen AJ, Stagni MS, Bill E, Meyer-Klaucke W, Schunemann V, Tampe R. Structural organization of essential iron-sulfur clusters in the evolutionarily highly conserved ATP-binding cassette protein ABCE1. J Biol Chem. 2007;282:14598–14607. doi: 10.1074/jbc.M700825200. [DOI] [PubMed] [Google Scholar]
- 25.Shichijo S, Ishihara Y, Azuma K, Komatsu N, Higashimoto N, Ito M, Nakamura T, Ueno T, Harada M, Itoh K. ABCE1, a member of ATP-binding cassette transporter gene, encodes peptides capable of inducing HLA-A2-restricted and tumor-reactive cytotoxic T lymphocytes in colon cancer patients. Oncol Rep. 2005;13:907–913. [PubMed] [Google Scholar]
- 26.Huang BO, Zhou H, Lang X, Liu Z. siRNA-induced ABCE1 silencing inhibits proliferation and invasion of breast cancer cells. Mol Med Rep. 2014;10:1685–1690. doi: 10.3892/mmr.2014.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang B, Gong X, Zhou H, Xiong F, Wang S. Depleting ABCE1 expression induces apoptosis and inhibits the ability of proliferation and migration of human esophageal carcinoma cells. Int J Clin Exp Pathol. 2014;7:584–592. [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Zhang M, Liu DX. Knock-down of ABCE1 gene induces G1/S arrest in human oral cancer cells. Int J Clin Exp Pathol. 2014;7:5495–5504. [PMC free article] [PubMed] [Google Scholar]
- 29.Huang B, Gao Y, Tian D, Zheng M. A small interfering ABCE1-targeting RNA inhibits the proliferation and invasiveness of small cell lung cancer. Int J Mol Med. 2010;25:687–693. [PubMed] [Google Scholar]


