Abstract
Peripheral neuropathies are a major cause of morbidity in patients with diabetes mellitus. Up to now, drugs for improving the impaired nerve functions has been lacking for diabetic neuropathy. The antioxidant and neuroprotective effects of luteolin make it an attractive candidate for diabetic neuropathy. The present study was designed to investigate the putative beneficial effect of luteolin on diabetic neuropathy. Diabetic rats were intraperitoneally treated with daily luteolin (50 mg/kg, 100 mg/kg and 200 mg/kg) or vehicle for 3 weeks from the 28th day after streptozotocin injection. Behavioral, electrophysiological and biochemical studies were performed to evaluate the effect of luteolin on the impaired nerve functions in diabetic neuropathy. It was found that luteolin dose dependently alleviated abnormal sensation, improved nerve conduction velocities and nerve blood flow in diabetic rats. Biochanical analysis showed that luteolin significantly lowered the reactive oxygen species production and malondialdehyde level, as well as increased antioxidants activities in a dose dependent manner. In addition, luteolin significantly up-regulated the protein levels of nuclear factor-E2-related factor-2 (Nrf2) and heme oxygenase-1 (HO-1) in diabetic nerves. Taken together, luteolin is capable of improving diabetes-induced deficit in motor and sensory functions, which could be attributable, at least in part, to its Nrf2-dependent antioxidant capacity. The findings in the present study highlight the therapeutic value of luteolin for diabetic neuropathy.
Keywords: Luteolin, diabetic neuropathy, antioxidant, Nrf2, neuroprotection
Introduction
Diabetes mellitus is an increasingly important health problem globally, which affected 366 million in 2011 and is expected to rise to 552 million by 2030 [1]. Peripheral neuropathies are a major cause of morbidity in patients with diabetes mellitus, affecting as many as 50% of patients with diabetes [2]. Peripheral neuropathy can cause acute and chronic neuropathic pain, hyperalgesia and impaired nerve conduction velocities, which can cause significant morbidity of these patients and affect their daily activities [3]. Although analgesics have been used for diabetic neuropathy, they are only partially effective in pain relief, and have little effect on motor nerve conduction velocities [4]. Therefore, it is imperative to develop effective drug to simultaneously improve the impaired sensory and motor function in diabetic mellitus.
Oxidative damage is the most important pathway for various pathogenetic mechanisms of neuronal injury in diabetic neuropathy [5,6]. Therefore, antioxidants are surfacing out to be one of the successful strategies for treatment of diabetic neuropathy [7]. However, despite optimistic preclinical data, most of the antioxidants have failed to show significant neuroprotection in humans. Recently, a more targeted approach focusing on nuclear factor-E2-related factor-2 (Nrf2) has been increasingly recognized as a promising strategy to optimize neuroprotection in diabetic neuropathy [5]. Nrf2 is a master regulator of the cellular detoxification response and is considered as the axis of defense against oxidative stress. It has been shown that activation of Nrf2 results in the concerted upregulation of several antioxidant enzymes and cytoprotective genes, making it an attractive therapeutic target for diabetic complications [8]. Thus far, the protective effect of Nrf2 has been found in peripheral neuropathy [9], pancreatic β-cell damage [10], nephropathy [11] and cardiomyocytes damage [12] in diabetic mellitus. Therefore, antioxidants which are capable of manipulating transcriptional activity of Nrf2 to counteract oxidative damage hold more potential for diabetic neuropathy.
Luteolin (39, 49, 5, 7-tetrahydroxyflavone), is a naturally occurring flavonoid and exhibits antioxidant, anti-inflammatory and neuroprotective activities. Particularly, it has been found that the antioxidant and neuroprotective properties of luteolin are associated with Nrf2 pathway. In the middle cerebral artery occlusion-induced ischemia rat model, luteolin is capable of scavenging free radicals through upregulating Nrf2 protein level, which contribute to its neuroprotective effects by reducing infarct area and inhibiting neuronal cell death [13]. It has also been shown that luteolin is capable of protecting rat neural PC12 and glial C6 cells against N-methyl-4-phenyl-pyridinium-induced toxicity via activation of Nrf2 [14]. In addition, the antioxidant property of luteolin has been found to be related to its Nrf2 activating capability in previous studies [14,15]. All those evidences indicate that the antioxidant and neuroprotective properties of luteolin were closely associated with its Nrf-2 inducing activities, making it an attractive candidate for diabetic complications. Thus far, luteolin has been found to alleviate diabetic nephropathy [16] and encephalopathy [17] in rats. However, no information has been available on the potential effect of luteolin on diabetic neuropathy. The present study was designed to investigate the putative effect of luteolin on diabetic neuropathy in a rat model.
Materials and methods
Animal model of diabetic neuropathy
All procedures were conducted under a protocol approved by the Institutional Ethical Committee of Harbin Medical University. Adult male Sprague-Dawley rats were provided by the Laboratory Animal Center of the Harbin Medical University. Rats weighing from 180 g to 200 g were used for the present study. Diabetic neuropathy (n=40) was induced by intraperitoneal injection of streptozotocin (STZ) at 55 mg/kg. Normal control rats (n=10) were injected the same volume/kg of saline. Four weeks after STZ injection, plasma glucose was measured by a commercial kit and rats with plasma glucose levels above 13.89 mmol/L were considered as diabetic. Starting from the 28th day after STZ injection, the diabetic rats were randomly divided into 4 groups (n=10 in each group), including STZ control group, low-dose luteolin group (50 mg/kg), mid-dose luteolin group (100 mg/kg), high-dose luteolin group (200 mg/kg). The luteolin was daily administrated for 3 weeks. For the normal and diabetic control groups, the rats received daily same volume of saline for 3 weeks. At the end of luteolin treatment, behavioral, electrophysiological and biochemical analysis were performed. Then plasma glucose was assayed by an enzyme-linked immunosorbent assay method using commercial kits. Streptozotocin, luteolin and ELISA kits were purchased from Sigma Chemical Co., St. Louis, MO, USA.
Behavioral assessment of mechanical sensitivity
The mechanical sensation was evaluated after the final application of luteolin. The mechanical sensitivity was determined using a series of von Frey filaments [18]. Briefly, the animals were placed in a transparent cage with wire-mesh-bottom. Eight hairs with forces of 0.42 g, 2.5 g, 4.3 g, 6.8 g, 8.9 g, 10.8 g, 13.0 g and 15.5 g were applied 10 times to the plantar aspect of the hind paw in ascending order of force. The hair was applied for 3-5 s, with an inter-stimulus interval of 20 s. Abrupt paw withdrawal, licking, and shaking were taken to be positive responses. The threshold was the minimal force which elicited more than 5 positive responses. A significant decrease in mechanical threshold was interpreted as mechanical hyperalgesia.
Behavioral assessment of heat nociception
Heat nociception was evaluated by the latency to heat stimuli, which was recorded after final luteolin. The heat nociception was measured according to the procedure described previously [19]. In brief, A radiant heat source was used to stimulate the plantar side of the hind paw (46-48°C). Then the paw withdrawal latency was recorded in seconds by an automatic timer when the rats withdraw the paw. The heat stimulation would be stopped if the animals don’t withdraw the paw for 20 s to prevent thermal injury. All tests were repeated for 3 times with intervals of 5 min between each test.
Behavioral assessment of cold allodynia
Cold allodynia was evaluated by the latency to cold stimuli, which was recorded after final luteolin. The cold allodynia was assessed according to the procedure as previously described [20]. Briefly, rats were placed on a cold stainless steel plate (model 35100, Ugo Basile) maintained at 10°C. The latency(s) to the first lifting or shaking of the hind paws was recorded in seconds by an automatic timer. The cold stimulation would be stopped If the animals don’t withdraw the paw for 30 s to prevent tissue injury. The cold stimulation was repeated twice with intervals of 5 min between each test.
Nerve blood flow (NBF)
After behavioral tests, the animals were anesthetized by intraperitoneal injection of 1.0 wt% sodium pentobarbital solution (40 mg/kg body weight). The right sciatic nerve was exposed and laser probe was placed above the exposed nerve. A 10 minutes continuous NBF recording was performed using a laser Doppler flowmeter (Hpsonos 1500, USA). During the test, the body temperature of the rats was maintained at 37°C [21].
Nerve conduction velocity (NCV)
After NBF measurement, the stimulating and recording electrodes were placed under the exposed right sciatic nerve. The distance between the stimulating and recording electrodes was 8 mm. The sciatic nerve was stimulated with single pulse (1.2 V, 1.0 ms) using a PowerLab 4SP distal data acquisition system (Keypoint 3.02 Denmark). The motor NCV was then calculated. For sensory NCV, the locations of stimulating and recording electrodes were exchanged. Calculation of sensory NCV was the same as that of motor NCV [21].
Reactive oxygen species (ROS), malondialdehyde (MDA) and antioxidants activities
Immediately after electrophysiological analysis, the sciatic nerves and dorsal root ganglions (L4-L6) of the animals were rapidly removed and stored at -80°C until use. The ROS and MDA levels was measured in sciatic nerve tissue homogenates as described previously [22,23]. The antioxidants activities, including superoxide dismutase (SOD), glutathione-S-transferase (GST), glutathione peroxidase (GPx) and catalase (CAT), were measured by standard protocols for the standard sassy kits (Sigma-Aldrich, St. Louis, MO, USA). The results were expressed as Ug/ml, μmol/mg protein, U/mg protein, respectively.
Western blotting
The dorsal root ganglions were washed with PBS and lysed with lysis buffer containing protease inhibitors (Promega, US). Protein extracts were denatured by heat at 100°C for 5 min, electrophoretically separated on a 12% SDS-PAGE (Bio-Rad, CA), and transferred to a PVDF membrane (Bio-Rad, CA). The membrane was blocked with 5% nonfat dry milk in TBST buffer (50 mM Tris-HCl, 100 mM NaCl, and 0.1% Tween-20, pH 7.4) and incubated with rabbit anti-rat Nrf2 antibody (1:2000, Sigma, St. Louis, MO, USA), and mouse anti-rat HO-1 (1:1500, Sigma, St. Louis, MO, USA) in TBST buffer at 4°C overnight. The membrane was washed with TBST buffer (3×5 min), and incubated with HRP-conjugated goat anti-rabbit or goat anti-mouse IgG (1:3000, Sigma, St. Louis, MO, USA) at room temperature for 2 h. The membrane was then washed with PBS and the HRP activity was determined using an ECL kit (Millipore, US). The image was scanned with a GS 800 Densitometer Scanner (Bio-Rad, CA), and the optical density was determined using PDQuest 7.2.0 software (Bio-Rad, CA). Rabbit anti-rat DAPDH polyclonal antibody (1:10000; Sigma-Aldrich, US) was used as an internal control.
Statistical analysis
All data in the study were expressed as the mean ± standard error of mean. One-way analysis of variance (ANOVA) was applied to compare the data using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Tukey post hoc test was applied for comparisons between groups. Values of P<0.05 were considered as statistically significant.
Results
Luteolin lowers plasma glucose in diabetic rats
The plasma glucose was significantly decreased by luteolin in diabetic rats. The glucose level in STZ-treated rats was 23.17±1.82 mmol/L, which was significantly higher than that in normal control rats (3.95±0.27 mmol/L), confirming the establishment of diabetes in rats. At the end of luteolin treatment, The glucose level in diabetic rats decreased to 18.59±0.63 mmol/L (50 mg/kg), 11.26±0.37 mmol/L (100 mg/kg) and 12.45±0.48 mmol/L (200 mg/kg). Additionally, the glucose level in diabetic rats receiving luteolin at 100 mg/kg and 200 mg/kg were significantly lower than that receiving luteolin at 50 mg/kg (P<0.05).
Luteolin ameliorates abnormal sensation in diabetic rats
Luteolin ameliorated abnormal sensation in diabetic rats. The mechanical threshold in luteolin-treated diabetic rats were significantly higher than that in control diabetic rats (P<0.05, Figure 1A). In addition, the mechanical threshold in diabetic rats which received luteolin at 100 mg/kg and 200 mg/kg were significantly higher than that received luteolin at 50 mg/kg (P<0.05, Figure 1A), indicating a dose dependent beneficial effect of luteolin on diabetic mechanical hyperalgesia. For heat and cold sensation, the beneficial effect of luteolin was also observed in the current study. The heat and cold paw withdrawal latencies in luteolin-treated diabetic rats were significantly higher than those in control diabetic rats (P<0.05, Figure 1B, 1C). Further studies showed that the heat and cold paw withdrawal latencies in luteolin groups at 100 mg/kg and 200 mg/kg were significantly higher than that in luteolin group at 50 mg/kg (P<0.05, Figure 1B, 1C), suggesting that luteolin ameliorates heat hyperalgesia and cold allodynia in a dose dependent manner in diabetic rats.
Figure 1.
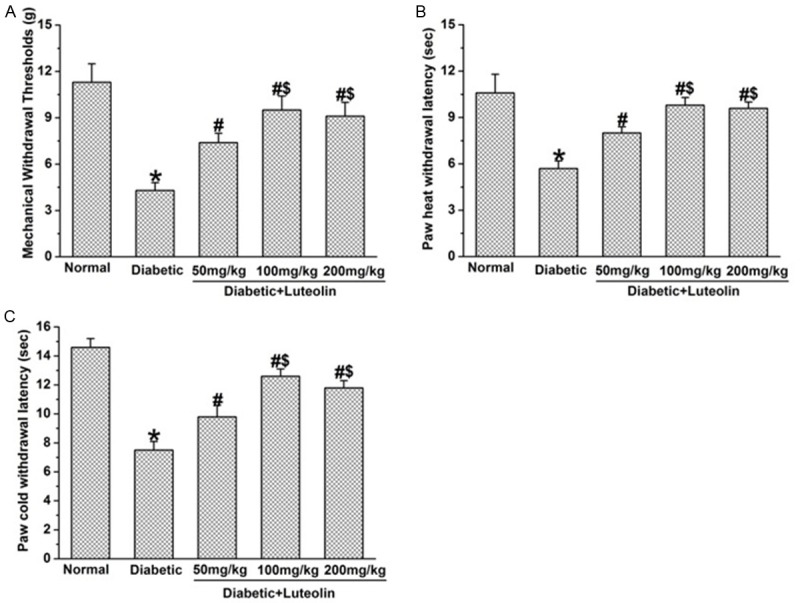
Luteolin improves diabetes-induced deficit in mechanical, heat and cold sensation (n=10 in each group). Luteolin significantly increased mechanical withdrawal threshold (A), the paw withdrawal latency to heat (B) and cold stimuli (C) in diabetic rats. *P<0.05 for the comparison to the normal rats, #P<0.05 for the comparison to the saline-treated diabetic rats, $P<0.05 for the comparison to the diabetic rats treated with luteolin at 50 mg/kg.
Luteolin improves NCV and NBF in diabetic rats
Luteolin significantly increased NCV and NBF in diabetic rats. The NCV and NBF was significantly decreased in diabetic rats (P<0.05, Figures 2, 3). Luteolin significantly increased NCV and NBF in diabetic rats in a dose dependent manner. The motor and sensory NCV, as well as NBF in luteolin groups at 100 mg/kg and 200 mg/kg were significantly higher than those in luteolin group at 50 mg/kg (P<0.05, Figures 2, 3). The findings suggest that luteolin is capable of improving nerve functions in a dose dependent manner in diabetic rats.
Figure 2.
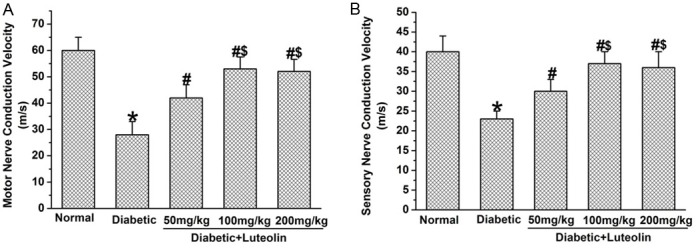
The benificial effect of luteolin on nerve conduction velocities in diabetic rats (n=10 in each group). Luteolin significantly increased the amplitude of motor (A) and sensory (B) conduction velocities in diabetic rats. *P<0.05 for the comparison to the normal rats, #P<0.05 for the comparison to the saline-treated diabetic rats, $P<0.05 for the comparison to the diabetic rats treated with luteolin at 50 mg/kg.
Figure 3.
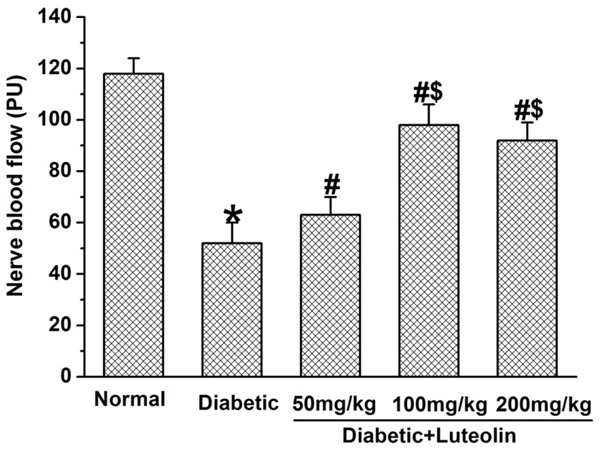
Luteolin significantly increased nerve blood flow in diabetic rats. *P<0.05 for the comparison to the normal rats, #P<0.05 for the comparison to the saline-treated diabetic rats, $P<0.05 for the comparison to the diabetic rats treated with luteolin at 50 mg/kg.
Luteolin decreases ROS and MDA in diabetic rats
Luteolin is capable of attenuating oxidative stress in diabetic rats, which was a key contributor to the pathogenesis of diabetic neuropathy. The ROS and MDA levels in diabetic rats was significantly higher than that in normal rats (P<0.05, Figure 4). Luteolin dose dependently decreased the elevated levels of ROS and MDA in diabetic rats. The levels of ROS and MDA were significantly lower in luteolin groups at 100 mg/kg and 200 mg/kg than those in luteolin group at 50 mg/kg (P<0.05, Figure 4). The findings suggest that luteolin dose dependently decreases oxidative stress in diabetic rats.
Figure 4.
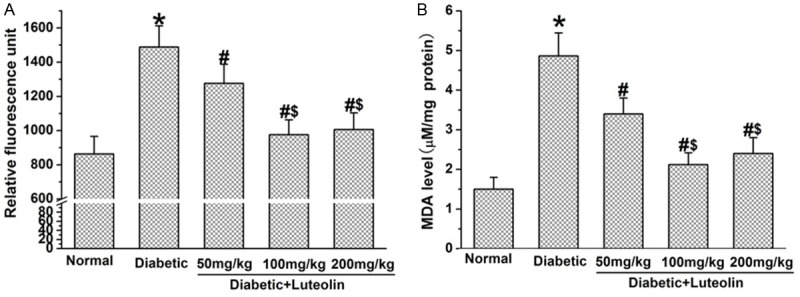
The effect of luteolin on oxidative stress in diabetic rats. Luteolin significantly lowered the production of ROS (A) and MDA (B) in diabetic nerves. *P<0.05 for the comparison to the normal rats, #P<0.05 for the comparison to the saline-treated diabetic rats, $P<0.05 for the comparison to the diabetic rats treated with luteolin at 50 mg/kg.
Luteolin increases the antioxidant activities in diabetic rats
Luteolin partially recovered the activities of antioxidant in nerve tissues in diabetic rats. The activities of SOD, GST, GPx and CAT in nerve tissues were significantly lower in control diabetic rat than those in normal control rats (P<0.05, Figure 5). Luteolin increased the activities of SOD, GST, GPx and CAT in nerve tissues in diabetic rats in a dose dependent manner. The activities of SOD, GST, GPx and CAT in nerve tissues were significantly higher in luteolin groups at 100 mg/kg and 200 mg/kg than those in luteolin group at 50 mg/kg (P<0.05, Figure 5). The findings indicate that luteolin is capable of restoring the impaired antioxidant defense system in diabetic rats.
Figure 5.
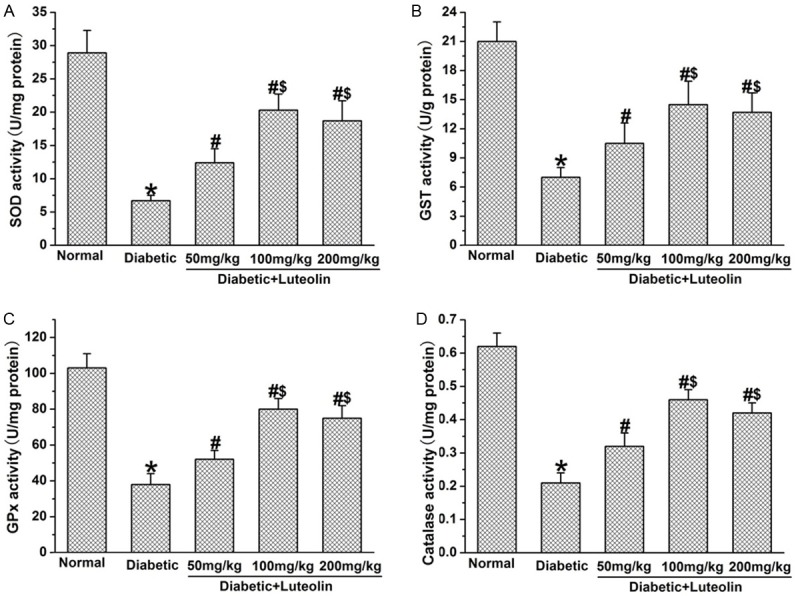
The effect of luteolin on antioxidants activities in diabetic rats. Luteolin significantly increased the activities of SOD (A), GST (B), GPx (C) and CAT (D) in diabetic nerves. *P<0.05 for the comparison to the normal rats, #P<0.05 for the comparison to the saline-treated diabetic rats, $P<0.05 for the comparison to the diabetic rats treated with luteolin at 50 mg/kg.
Luteolin increases the expression of Nrf2 and HO-1 in diabetic rats
Nrf2 has been considered as the axis of defense against oxidative stress, and has been recently surfaced as an important target in diabetes and related complications. We further examined the effect of luteolin on the expression of Nrf2 and HO-1 in diabetic rats. It was found that the expression of Nrf2 and HO-1 was significantly lower in diabetic rats than that in normal control rats (P<0.05, Figure 6). Luteolin significantly increased expression of Nrf2 and HO-1 in nerve tissues in diabetic rats in a dose dependent manner. The expression of Nrf2 and HO-1 in luteolin groups at 100 mg/kg and 200 mg/kg were significantly higher than that in luteolin group at 50 mg/kg (P<0.05, Figure 6).
Figure 6.
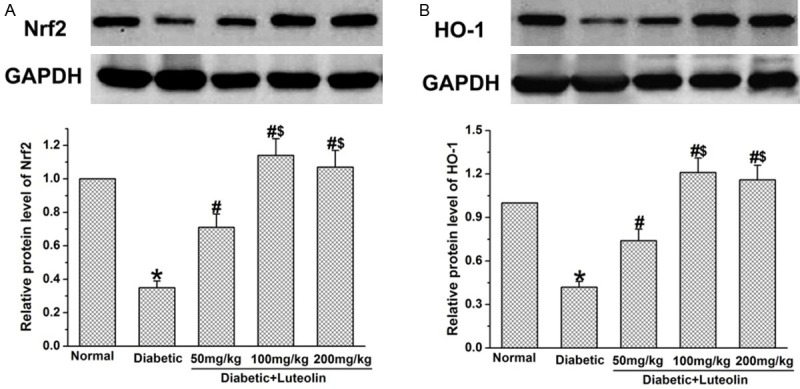
The effect of Luteolin on Nrf2 and HO-1 in diabetic rats. STZ significantly lowered the levels of Nrf2 (A) and HO-1 (B). Administration of Luteolin significantly decreased the levels of Nrf2 (A) and HO-1 (B) in diabetic rats. *P<0.05 for the comparison to the normal rats, #P<0.05 for the comparison to the saline-treated diabetic rats. $P<0.05 for the comparison to the diabetic rats treated with luteolin at 50 mg/kg.
Discussion
The present study provided behavioral and biochemical evidences for the beneficial effect of luteolin on diabetic neuropathy. Firstly, it was found that luteolin was capable of attenuating diabetes-induced mechanical hyperalgesia, heat hyperalgesia and cold allodynia. In addition, luteolin was found to improve NCV and NBF in diabetic rats. Further studies showed that luteolin was able to reduce the production of ROS and MDA levels, as well as increased antioxidants activities in nerves, indicating that luteolin holds the ability to protect peripheral nerves against oxidative damage in diabetic rats. Furthermore, it was found that luteolin significantly increased the expression of Nrf2 and HO-1 in dorsal root ganglions in diabetic rats, suggesting the involvement of Nrf2 dependent endogenous antioxidant defense in the beneficial effect of luteolin on diabetic neuropathy.
Luteolin is capable of alleviating diabetes-induced abnormal sensation and increasing nerve conduction velocities. In previous study, the anti-nociceptive and neuroprotective effects of luteolin has been found [24-27]. In a rat model of neuropathic pain induced by constriction injury to sciatic nerve, luteolin dose-dependently attenuated mechanical and cold hyperalgesia, which was partially attributable to activating GABAA receptors and μ-opioid receptors in the spinal cord [24]. In addition, luteolin exhibits neuroprotection in models of traumatic brain injury [25], Alzheimer’s disease [26] and neuroinflammation in the spinal cord [27]. All those findings highlights the possibility that luteolin might directly protect neurons and nerve fibers in the peripheral nervous system against damage induced by diabetes, which might be partially responsible for the beneficial effect of luteolin on impaired nerve functions in diabetic condition.
Luteolin significantly improved the decreased NBF, which has been proposed as one contributing factor to decreased nerve conduction velocities in diabetic rats. It has been shown that luteolin is capable of protecting against high glucose-induced endothelial dysfunction [28]. Therefore, the potential beneficial effect of luteolin on vascular endothelial cells in nerve microvessels might be partially involved in restoring the impaired NBF, which might contribute to the improved nerve function in diabetic rats. Further studies were needed to identify the effect of luteolin on endothelial cells in nerve micro-vessels, providing more evidences for the beneficial effect of luteolin on diabetic neuropathy.
Excessive production of ROS is a key component in the development and progression of diabetic neuropathy. Since the antioxidant property of luteolin has been well established in previous studies [5,6], we examined the effect of luteolin on oxidative stress in diabetic nerves. It was found that luteolin significantly lowered the production of ROS, as well as MDA levels, indicating the luteolin is capable of attenuating oxidative damage and lipid peroxidation in diabetic peripheral nerves. Further studies showed that luteolin holds the ability to increase the antioxidant activities (SOD, CAT, GPx and GST), suggesting that luteolin could restore the endogenous antioxidant defenses in diabetic nerves. Those evidences indicate that the antioxidant property of luteolin is responsible for its beneficial effect on impaired nerve functions in diabetic rats. It has been recognized that the antioxidant properties of luteolin was partially dependent on Nrf2 pathway, which is considered as the axis of defense against oxidative stress [14,15]. We further investigated the effect of luteolin on Nrf2 expression in diabetic nerves. We found that significantly up-regulated Nrf2 expression, as well as HO-1 (a phase II detoxifying enzyme) in diabetic nerves. Those findings suggest that luteolin might activate an array of antioxidant defense machinery through Nrf2 pathway to counterbalance the damage caused by reactive radicals, which might be involved in its beneficial effect on diabetic nerve functions.
Conclusion
Luteolin is capable of improving diabetes-induced deficit in motor and sensory functions, which could be attributed, at least in part, to its Nrf2-dependent antioxidant capacity. The findings in the present study highlight the potential therapeutic value of luteolin for diabetic neuropathy.
Disclosure of conflict of interest
None.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Didangelos T, Doupis J, Veves A. Painful diabetic neuropathy: clinical aspects. Handb Clin Neurol. 2014;126:53–61. doi: 10.1016/B978-0-444-53480-4.00005-9. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: Physiopathology and treatment. World J Diabetes. 2015;6:432–444. doi: 10.4239/wjd.v6.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer S, Tanenberg RJ. Pharmacologic management of diabetic peripheral neuropathic pain. Expert Opin Pharmacother. 2013;14:1765–1775. doi: 10.1517/14656566.2013.811490. [DOI] [PubMed] [Google Scholar]
- 5.Negi G, Kumar A, Joshi RP, Sharma SS. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun. 2011;408:1–5. doi: 10.1016/j.bbrc.2011.03.087. [DOI] [PubMed] [Google Scholar]
- 6.Hosseini A, Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid Med Cell Longev. 2013;2013:168039. doi: 10.1155/2013/168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galuppo M, Giacoppo S, Bramanti P, Mazzon E. Use of natural compounds in the management of diabetic peripheral neuropathy. Molecules. 2014;19:2877–2895. doi: 10.3390/molecules19032877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan SM, de Haan JB. Combating oxidative stress in diabetic complications with Nrf2 activators: how much is too much? Redox Rep. 2014;19:107–117. doi: 10.1179/1351000214Y.0000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Negi G, Kumar A, Sharma SS. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-κB and Nrf2 cascades. J Pineal Res. 2011;50:124–131. doi: 10.1111/j.1600-079X.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 10.Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS, Park R, Kwon KB, Park BH. Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol Appl Pharmacol. 2009;235:57–67. doi: 10.1016/j.taap.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:850–860. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46:47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YC, Gan FF, Shelar SB, Ng KY, Chew EH. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem Toxicol. 2013;59:272–280. doi: 10.1016/j.fct.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 14.Wruck CJ, Claussen M, Fuhrmann G, Römer L, Schulz A, Pufe T, Waetzig V, Peipp M, Herdegen T, Götz ME. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J Neural Transm Suppl. 2007;72:57–67. doi: 10.1007/978-3-211-73574-9_9. [DOI] [PubMed] [Google Scholar]
- 15.Huang CS, Lii CK, Lin AH, Yeh YW, Yao HT, Li CC, Wang TS, Chen HW. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxicol. 2013;87:167–178. doi: 10.1007/s00204-012-0913-4. [DOI] [PubMed] [Google Scholar]
- 16.Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective Effects of Luteolin on Diabetic Nephropathy in STZ-Induced Diabetic Rats. Evid Based Complement Alternat Med. 2011;2011:323171. doi: 10.1155/2011/323171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren G, Kong J, Jia N, Shang X. Luteolin attenuates neuronal apoptosis in the hippocampi of diabetic encephalopathy rats. Neural Regen Res. 2013;8:1071–1080. doi: 10.3969/j.issn.1673-5374.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogelaar CF, Vrinten DH, Hoekman MF, Brakkee JH, Burbach JP, Hamers FP. Sciatic nerve regeneration in mice and rats: recovery of sensory innervation is followed by a slowly retreating neuropathic pain-like syndrome. Brain Res. 2004;1027:67–72. doi: 10.1016/j.brainres.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 19.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Chen W, Qian X, Fang Y, Zhu N. Liquiritigenin alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Sci Rep. 2014;4:5676. doi: 10.1038/srep05676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Wang L, Li X, Lv C, Feng D, Luo Z. Tanshinone IIA improves impaired nerve functions in experimental diabetic rats. Biochem Biophys Res Commun. 2010;399:49–54. doi: 10.1016/j.bbrc.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Padiya R, Chowdhury D, Borkar R, Srinivas R, Pal Bhadra M, Banerjee SK. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS One. 2014;9:e94228. doi: 10.1371/journal.pone.0094228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J, Liu J, Wang Q, Yu H, Chen Y, Xiang L. The beneficial effect of ginsenoside Rg1 on Schwann cells subjected to hydrogen peroxide induced oxidative injury. Int J Biol Sci. 2013;9:624–636. doi: 10.7150/ijbs.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara K, Haranishi Y, Terada T, Takahashi Y, Nakamura M, Sata T. Effects of intrathecal and intracerebroventricular administration of luteolin in a rat neuropathic pain model. Pharmacol Biochem Behav. 2014;125:78–84. doi: 10.1016/j.pbb.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Wang H, Ding K, Zhang L, Wang C, Li T, Wei W, Lu X. Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE pathway. Free Radic Biol Med. 2014;71:186–195. doi: 10.1016/j.freeradbiomed.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Paterniti I, Cordaro M, Campolo M, Siracusa R, Cornelius C, Navarra M, Cuzzocrea S, Esposito E. Neuroprotection by association of palmitoylethanolamide with luteolin in experimental Alzheimer’s disease models: the control of neuroinflammation. CNS Neurol Disord Drug Targets. 2014;13:1530–1541. doi: 10.2174/1871527313666140806124322. [DOI] [PubMed] [Google Scholar]
- 27.Paterniti I, Impellizzeri D, Di Paola R, Navarra M, Cuzzocrea S, Esposito E. A new co-ultramicronized composite including palmitoylethanolamide and luteolin to prevent neuroinflammation in spinal cord injury. J Neuroinflammation. 2013;10:91. doi: 10.1186/1742-2094-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian LB, Wang HP, Chen Y, Chen FX, Ma YY, Bruce IC, Xia Q. Luteolin reduces high glucose-mediated impairment of endothelium-dependent relaxation in rat aorta by reducing oxidative stress. Pharmacol Res. 2010;61:281–287. doi: 10.1016/j.phrs.2009.10.004. [DOI] [PubMed] [Google Scholar]


