Abstract
The NF-E2 related factor 2 (Nrf2) could be activated in intracerebral hemorrhage (ICH), and trigger the expression of ARE regulated heme oxygenase 1 (HO-1) subsequently. This study aims to explore neuroprotection of HO-1 protein in regulating the Nrf2-ARE signaling pathway in ICH. In this study, the femoral artery injection method was used to establish the ICH model. The zinc porphyrin-9 (ZPP-IX) was used to inhibit the HO-1 expression in ICH rats. The ICH rats were randomly divided into 3 groups, ICH group, ZPP-IX (10 mg/kg) + ICH group and DMSO (10 mg/kg) + ICH group. Neurological scores were evaluated for the 3 groups. Double immunofluorescence staining method was employed to observe the co-expression of HO-1, Nrf2, NF-κB and TNF-α and CD11b in glia cells. Western blot and RT-PCR assay were used to detect the total Nrf2, binding Nrf2, HO-1, NF-κB and TNF-α expression. The results indicated that ZPP-IX could aggravate the neurological dyafunstions of ICH rats. The HO-1 level in ZPP-IX group was significantly decreased compared to the ICH group (P < 0.05). The binding-Nrf2 protein was significantly increased in ZPP-IX group compared to ICH group (P < 0.05). The NF-κB and TNF-α level were significantly increased in ZPP-IX group compared to ICH group (P < 0.05). The ZPP-IX significantly inhibited the HO-1 and Nrf2, and enhanced NF-κB and TNF-α co-expressing with the CD11b compared to the ICH group (P < 0.05). In conclusion, HO-1 protein regulates the Nrf2-ARE pathway in ICH model by inhibiting the Nrf2 entering nucleus and activating the NF-κB and TNF-α expression.
Keywords: Intracerebral hemorrhage, heme oxygenase 1, Nrf2-ARE signaling, zinc porphyrin-9
Introduction
Intracerebral hemorrhage (ICH) is an important kind of stroke, which with higher morbidity and mortality [1,2]. The previous studies have indicated that many inflammatory cytokine participates in the process of the pathogenesis of ICH [3]. Clinically, the occurrence of ICH may influence the brain function, especially for the cognitive abilities, and even cause the cognitive decline or cognitive impairment [4]. The previous reports have showed that the Nrf2-ARE could be activated in intracerebral hemorrhage [5,6]. The Nrf2 almost exists in all the types of the cells in the brain, such as microglia and neuron [7]. The activated Nrf2 could trigger the expression of ARE regulated heme oxygenase (HO-1), and play the role of anti-oxidative and anti-inflammatory, which could play the neuroprotective function in a further step [8]. Wang et al. [9] also indicated that when the Nrf2 gene was knocked out, the neurological function could be damaged after the ICH occurrence. The mechanism may relate with the apoptosis and reactive oxygen species accumulation in cells, which would damage the DNA. Therefore, we speculate that the neuron-protective functions of HO-1 post the ICH may associated with the anti-inflammatory response. NF-κB is an important transcriptional factor, which could mediate the inflammatory response. The former studies [10,11] illustrated that the HO-1 could inhibit the activation of the NF-κB.
In this study, we applied the ZPP-IX to inhibit the HO-1 expression, and observe the Nrf2 expression, entering nucleus and expressing of NF-κB. Furthermore, the relationship between microglia activation and the above protein expression was also explored to study the neuron-protective functions of HO-1 protein.
Materials and methods
Animals
Male SD rats were purchased from Slack Jiangda Laboratory Animal Co., Ltd. (Changsha, Hunan, China). The body weights range from 250 to 300 g.
Establishment of rat ICH model
The specific process and the establishing process were done due to the previous report [12]. The rats were subjected to fasting without water deprivation 8 h before surgery. At 3 mm lateral to the right side of the anterior fontanelle and a penetration depth of 5 mm, 50 μl autologous blood was slowly injected into the basal ganglia to establish a rat ICH model. The signa Excite HD 3.0 T MR scanner (GE, MI, USA) was used to examine the hematoma 3 h after the ICH had been established. When a notable round, oval or irregularly-shaped hematoma was discovered, the rat was considered as the model rat.
Trial grouping
We selected 100 healthy SPF SD rats (weighing 250-300 g). There were a total of 90 successfully establised hemorrhage model rats for the experiment. All of the model rats were randomly divided into three groups, including ICH group, ZPP-IX (10 mg/kg) + ICH group, DMSO (10 mg/kg) + ICH group, respectively. For the above 3 groups, each group was divided into 5 subgroups, including 6 h, 12 h, 1 d, 2 d, 3 d and 7 d (after ICH) subgroups, and each subgroup contains 5 rats. Moreover, 5 health SPF rats were selected as the control group.
Rats treatment and tissues selection
The rats were anesthetized and the heart was rapidly exposed, a perfusion tube was inserted from the left ventricle to the root of the aorta, and a small cut was made on the right atrial appendage with scissors. Then, 200 ml 4°C saline was infused until the effluent fluid was clear, and 4% paraformaldehyde was used for perfusion until the limbs of the rat were rigid. The animal was then decapitated and its brain collected, placed in 4% paraformaldehyde for 24 h, and subsequently subjected to frozen sectioning at a slice thickness of 5 μm for immunofluorescence staining.
Neurological score
The Garcia 18-points test [13] was done to evaluate the neurological function of all of the experimental and normal rats. Detailly, the Garcia 18-points scores were evaluated at 24 h before surgery, 6 h, 12 h, 1 d, 2 d, 3 d and 7 d after surgery, respectively.
Western blot analysis
The brain tissues isolation, lysis and SDS page were done as the description of Xu et al.’s report [14]. The SDS page isolated proteins were incubated overnight at 4°C with the anti-Nrf2 (1:000, Abcam, U. K.), anti-HO-1 (1:1000, Abcam, UK), anti-NF-κB (1:1000, Cell Signaling Technology, U. S.), anti-TNF-α (1:3000, Santa Cruz, USA) and anti-β-actin (1:1000, Sigma, USA). Then, the washed membrane with the isolated proteins was incubated with HRP-conjugated rabbit anti-mouse antibody (1:2000, Santa Cruz, USA) for 1 hour at room temperature. The Tow-color Laser Odyssey Infrared Imaging System (Gene Company Limited, Hong Kong, China) was used to scan the membranes, and the images were analyzed using Odyessy Version 3.0 software. The β-actin protein acts as the internal control.
Immunofluorescence analysis
The immunofluorescence analysis processes in this study were done by using the following 7 steps: ① The brain tissues on the slices were fixed in pre-cooling paraformaldehyde for 15 min; ② The slices were ruptured using 0.25% triton for 15 min; ③ The slices were blocked using 10% BSA for 60 min; ④ The slices were incubated with primary antibody at 4°C overnight; ⑤ The slices were incubated with second antibody at room temperature for 2 hours in dark; ⑥ The slices were stained with DAPI for 15 min; ⑦ The sliced were mounted with glycerol, and observed with fluorescent microscope to take images. At 400 magnifications, images from 5 non-overlapping fields were analyzed for cell counts and the mean was calculated.
RT-PCR analysis
Fluorescent quantitative RT-PCR was performed to detect the transcription of Nrf2, HO-1, NF-κB and TNF-α. All primers were synthesized by Beijing Qing Ke New Industrial Biotechnology Co., Ltd. (Beijing, China), and the primer sequences are listed in Table 1. Trizol Reagent (Takara Co., Ltd., Japan) was used for total RNA extraction, and the RNA quality was tested at optical density (OD) 260/280. Then, 5.0 μg total RNA was used to synthesize the cDNA by using the third strand cDNA synthesis kit (Invitrogen, CA, USA). The cDNA was diluted to 150 ng/µl, and 2 µl cDNA (diluted) was used for polymerase chain reaction. The cycle threshold (Ct) was recorded. The results of the real-time quantitative PCR were automatically provided by the quantitative fluorescence analyzer, including the Ct values of the target genes and the reference gene, as well as -ΔΔCT. Experiments on each group of samples were repeated three times. The GADPH protein was used as the internal control.
Table 1.
The primers of the genes
| Target gene | Forward primer (5’ to 3’) | Reverse primer (5’ to 3’) | Size (bp) |
|---|---|---|---|
| Nrf2 | GAATAAAGTTGCCGCTCAGAA | AAGGTTTCCCATCCTCATCAC | 209 |
| HO-1 | CTATCGTGCTCGCATGAAC | CAGCTCCTCAAACAGCTCAA | 118 |
| NF-κB | ACGATCTGTTTCCCCTCATCT | TGCTTCTCTCCCCAGGAATA | 150 |
| TNF-α | GACCCTCACACTCAGATCATC | GAACCTGGGAGTAGATAAGG | 197 |
| GAPDH | ACTCCCATTCCTCCACCTTT | TTACTCCTTGGAGGCCATGT | 143 |
Statistical analysis
The statistical analysis was performed by using the SPSS 19.0 statistical software package (Microsoft, CA, USA). Significant differences between groups were determined by Student’s t test. The data were represented as the mean ± standard deviations. P values less then 0.05 was taken as statistically significant.
Results
Neurological score
The significant difference between the ICH group and normal group appeared from the first day (1 d), and achieved the peak vlaue at second day (2 d) (Figure 1, P < 0.05). However, the neurological injury of the ICH group was also serious. The neurological scores in ZPP-IX group were significantly higher compared to the ICH group from the 12 h to 7 d post operation (Figure 1, P < 0.05). There were no differences between the DMSO group and ICH group (Figure 1, P > 0.05). The neurological results suggest that ZPP-IX could inhibit the expression of HO-1, and aggravated the neurological dyafunstions, which may be caused by the activation of the inflammatory response in Nrf2-ARE signaling pathway.
Figure 1.
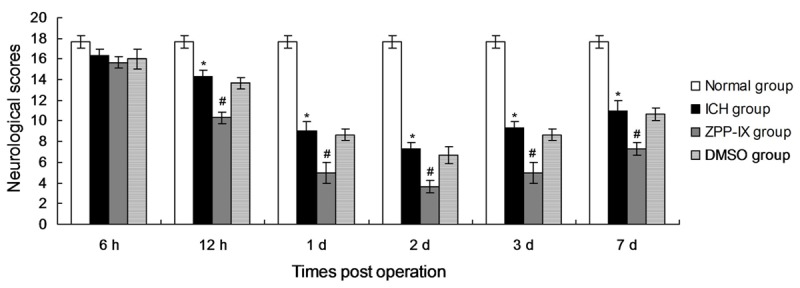
Neurological scores in every group at different time. *P < 0.05 represents the neurological scores in ICH group compared to the normal group. #P < 0.05 represents the neurological scores in ZPP-IX group compared to the ICH group.
ZPP-IX treatment decreases the HO-1 expression in ICH rats
In order to investigate the effects of the ZPP-IX treatment on the HO-1 expression, the HO-1 level was examined by using western blot assay. The results indicated that the HO-1 level in ICH group was significantly increased compared to the normal group (Figure 2, P < 0.05). The HO-1 level in ZPP-IX group was significantly decreased compared to the ICH group from 2 d post operation to 7 d post operation (Figure 2, P < 0.05), but also higher compared with the normal group.
Figure 2.
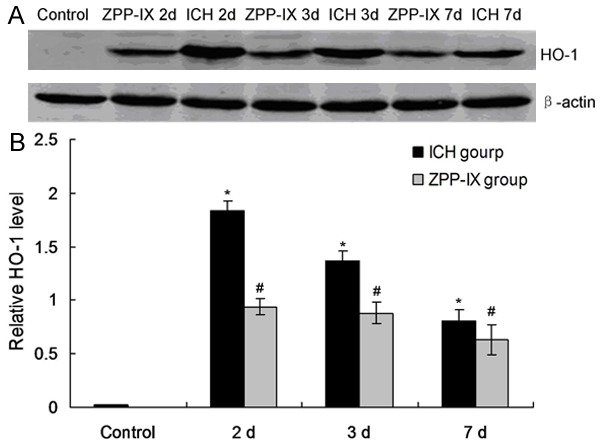
HO-1 expression in ICH and ZPP-IX group. A. Western blot assay for the HO-1 expression. B. Statistical analysis for the HO-1 protein. *P < 0.05 represents the HO-1 level in ICH group compared to the normal group. #P < 0.05 represents the HO-1 level in ZPP-XI group compared to the ICH group.
ZPP-IX enhances the level of binding-Nrf2
There are two kinds of Nrf2 proteins, inclusing total Nrf2 and binding Nrf2 protein. We also detected the total Nrf2 and binding Nrf2 protein by western blot. The results indicated that the total Nrf2 protein level was significantly decreased in ZPP-IX group compared to the ICH group (Figure 3A, 3B, P < 0.05). However, the binding-Nrf2 protein level was significantly increased in ZPP-IX group compared to the ICH group (Figure 3A, 3C, P < 0.05). Also, the ZPP-IX changes the Nrf2 and binding-Nrf2 proteins in ICH group compared to the normal group (Figure 3, P < 0.05).
Figure 3.
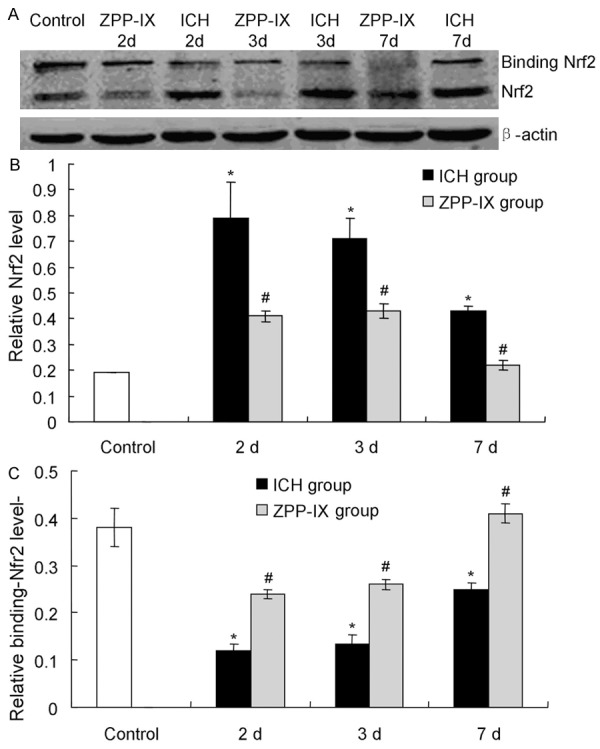
Examination of the total Nrf2 and binding Nrf2 protein in ICH and ZPPIX group at different time. A. Western blot assay for the total and binding Nrf2 expression. B. Statistical analysis of the total Nrf2 expression. C. Statistical analysis of the binding Nrf2 expression. *P < 0.05 represents the total or binding Nrf2 level in ICH group compared to the normal group. #P < 0.05 represents the total or binding Nrf2 level in ZPP-XI group compared to the ICH group.
ZPP-IX increases the inflammatory cytokines in ICH rats
In order to investigated the factors directly trigger the brain injury, the down-stream inflammatory cytokines of the Nrf2-ARE signaling pathway were detected. The resutls indicated that the NF-κB (Figure 4A) and TNF-α (Figure 4B) level were significantly increased in ZPP-IX group compared to the ICH group. Furthermore, the NF-κB and TNF-α level achieved the peak at the second day (2 d), and kept to the seventh day (7 d).
Figure 4.
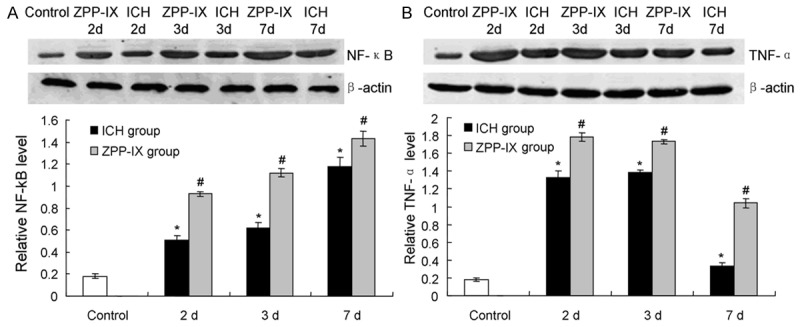
Inflammatory cytokines activation in ZPP-IX group by western blot assay detection. A. Western blot assay and statistical analysis for the NF-κB expression. B. Western blot assay and statistical analysis for the TNF-α expression. *P < 0.05 represents the NF-κB or TNF-α level in ICH group compared to the normal group. #P < 0.05 represents the NF-κB or TNF-α level in ZPP-XI group compared to the ICH group.
ZPP-IX affects mRNA expression of HO-1, Nrf2, NF-κB and TNF-α
The mRNA levels of HO-1, Nrf2, NF-κB and TNF-α were also examined by using the RT-PCR reaction. The results showed that the treatment of ZPP-XI significantly decreased the mRNA level of HO-1 (Figure 5A) and Nrf2 (Figure 5B) compared to the blank ICH rats (P < 0.05). However, the ZPP-IX significantly increased the levles of NF-κB (Figure 5C) and TNF-α (Figure 5D) gene expression compared to the blanl ICH rats (P < 0.05). Furthermore, the effects of ZPP-IX on the inflammatory cytokines achieved to the maximization at 3 d, and could retain to the 7 d (Figure 5C, 5D).
Figure 5.
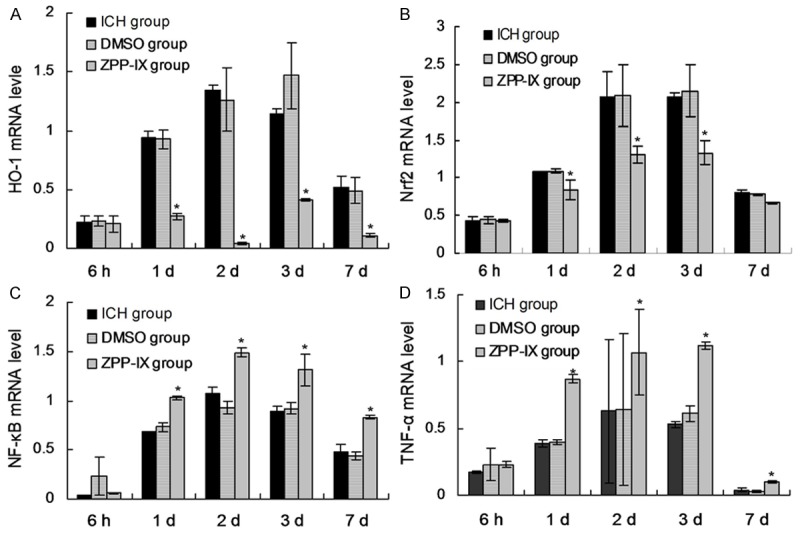
mRNA expression of HO-1, Nrf2, NF-κB and TNF-α in ICH and ZPP-IX group at different time. A. mRNA expression of HO-1 gene. B. mRNA expression of Nrf2 gene. C. mRNA expression of NF-κB gene. D. mRNA expression of TNF-α gene. *P < 0.05 represents the protein levels in ICH group compared to the normal group. #P < 0.05 represents the protein levels in ZPP-XI group compared to the ICH group.
Co-expression of Nrf2, HO-1, NF-κB, TNF-α and glia cells
To investigate the effects ofHO-1, Nrf2, NF-κB, TNF-α on ICH perifocal tissues, co-expression of Nrf2, HO-1, NF-κB, TNF-α were examined by double immunofluorescence staining. The resutls showed that all of the HO-1, Nrf2, NF-κB, TNF-α could co-express with the CD11b positively in glia cells, and which reached the peak on day 32 (Figure 6). Thus, we selected the 2 d as the study time point to investigate the effects of ZPP-IX on the co-expression of the factors. The results indicatd that there were more co-expression positive cells in ZPP-IX group compared to ICH group for the HO-1 and Nrf2 protein (Figure 6A). Furthermore, there were fewer co-expression positive cells in ZPP-IX group compared to ICH group for the NF-κB, TNF-α protein (Figure 6A). The statistical analysis results also showed that the ZPP-IX significantly inhibited the HO-1 and Nrf2 co-expressing with the CD11b compared to the ICH group (Figure 6B, P < 0.05). The ZPP-IX also enhanced the NF-κB and TNF-α co-expressing with the CD11b compared to the ICH group (Figure 6B, P < 0.05).
Figure 6.
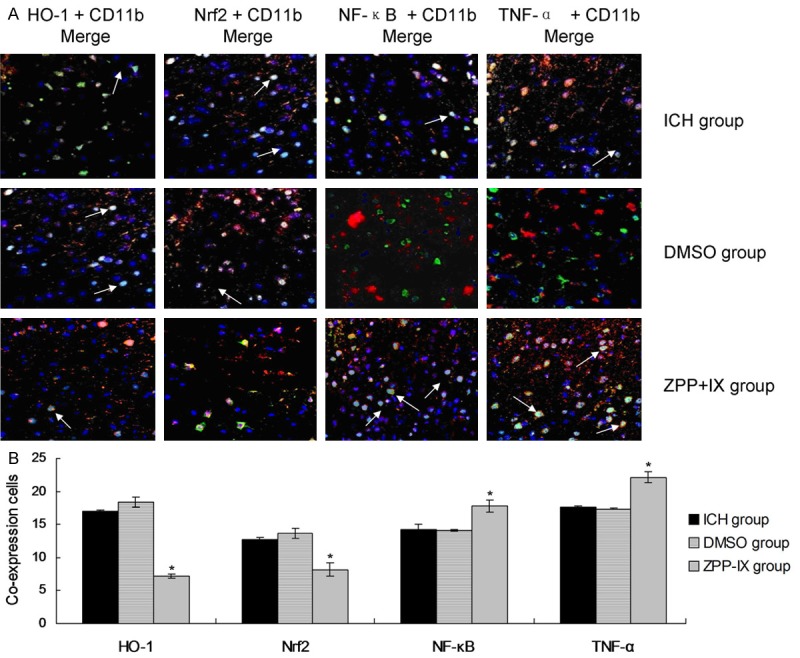
Double immunofluorescence staining assay for the co-expression in glia cells. A. Immunofluorescence merge images for co-expressing of HO-1, Nrf2, NF-κB and TNF-α with the CD11b in glia cells. B. Statistical analysis for the co-expression cytokines. *P < 0.05 represents the co-expression cells amounts in ZPP-XI group compared to the ICH group.
Discussion
As all is known, the Nrf2-ARE pathway is an important signaling pathway for the endogenously antioxidant stress and detoxication. When the Nrf2 was activated, it would be dissociated from the complex with Keap1, and entered into the nucleus. The Nrf2 protein in nucleus could regulate the antioxidant and detoxication related enzymes expression, which process was mediated by the p38 MAPK [15]. Park et al. [16] found that the HO-1 expression was significantly decreased when the Nrf2 was silenced or using the MAPK inhibitor, while significantly increased when using the Nrf2 activator. The previous studies [17,18] indicated that the activation of Nrf2 protein was associated with NF-κB inflammatory transcriptional pathway. Shang et al. [19] also proved that the HO-1 protein is an important protein in the downstream of the Nrf2 protein. The above studies suggest that the neuroprotection of Nrf2 protein activation may associated with the HO-1 expression or regulation.
In the process of intracerebral hemorrhage, the up-regulation of HO-1 is the main marker for the occurrence of oxygen stress, which also plays the important role of anti-oxidant. Especially for the glia cells, the HO-1 may also strengthen the anti-oxidant stress and anti-injury functions of neurons [20]. HO-1 may interfere the NF-κB nuclear localization signal (NLS) directly [21]. HO-1 also inhibits the NF-κB activation by regulating the GSK-3β signaling pathway [22]. Furthermore, the HO-1 protein could also inhibit the inflammatory cytokines, such as TNF-α and IL-1β expression [23], which could induce the activation of NF-κB. Therefore, the present study mainly discussed the effects of HO-1 expression on the NF-κB expression and Nrf2 entering nucleus.
In the present study, the neuroprotection of HO-1 protein initiated from 12 hours to 7 days after ICH. Actually, the HO-1 expression has been increased at the 6 hour after ICH, which was consistent with the Nrf2 activation time. Our results suggest that the neuroprotection of HO-1 was triggered at the early time of the ICH. Furthermore, the neuroprotection of HO-1 in the early period may related with the Nrf2 and NF-κB entering nucleus.
The HO-1 inhibitor, ZPP-IX, was used to inhibit the HO-1 epxression and observe the function of HO-1 in the ICH indirectly. The resutls indicated that the ZPP-IX decreased the HO-1 expression, and inhibited the Nrf2 entering nucleus (double immunofluorescence staining results), triggered the NF-κB entering nucleus (double immunofluorescence staining results), and resulted in the over-expression of NF-κB and TNF-α. The RT-PCR reaction was also proved the above results.
In conclusion, HO-1 protein regulates Nrf2-ARE pathway in ICH model by inhibiting Nrf2 entering nucleus and activating NF-κB and TNF-α expression.
Acknowledgements
This study was granted by the National Natural Science Fund of China (81260183).
Disclosure of conflict of interest
None.
References
- 1.Carhuapoma JR, Wang PY, Beauchamp NJ, Keyl PM, Hanley DF, Barker PB. Diffusion-weighted MRI and proton MR spectroscopic imaging in the study of secondary neuronal injury after intracerebral hemorrhage. Stroke. 2000;3:726–32. doi: 10.1161/01.str.31.3.726. [DOI] [PubMed] [Google Scholar]
- 2.Zheng M, Zhu H, Gong Y, Wang D, Xie Q, Tang H, Yang Z, Lu B, Chen X, Wang X. Involvement of GMRP1, a novel mediator of Akt pathway, in brain damage after intracerebral hemorrhage. Int J Clin Exp Pathol. 2013;6:224–229. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Garcia PY, Roussel M, Bugnicourt JM, Lamy C, Canaple S, Peltier J, Loas G, Deramond H, Godefroy O. Cognitive impairment and dementia after intracerebral hemorrhage: a cross-sectional study of a hospital-based series. J Stroke Cerebrovasc Dis. 2013;22:80–86. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhao F, Li K, Zhao L, Liu J, Suo Q, Zhao J, Wang H, Zhao S. Effect of Nrf2 on rat ovarian tissues against atrazine-induced anti-oxidative response. Int J Clin Exp Pathol. 2014;7:2780–2789. [PMC free article] [PubMed] [Google Scholar]
- 6.Aliaghaei A, Khodagholi F, Ahmadiani A. Conditioned media of plexus epithelial cells induces Nrf-2 activated phase II antioxidant response proteins and suppresses oxidiative stress-induced apoptosis in PC-12 cells. J Mol Neurosci. 2014;53:617–625. doi: 10.1007/s12031-014-0228-4. [DOI] [PubMed] [Google Scholar]
- 7.Bao LJ, Jaramillo MC, Zhang ZB, Zheng XY, Yao M, Zhang DD, Yi XF. Nrf2 induces cisplatin resistance through activation of autophagy in ovarian carcinoma. Int J Clin Exp Pathol. 2014;7:1502–1513. [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Nel AE. Role of the nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8:88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Field J, Zhao C. Role of Nrf2 in protein against intracerebral hemorrhage injury in mice. Free Raci Biol Med. 2007;43:408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai PS, Chen CC, Tsai PS, Yang LC, Huang WY, Huang CJ. Heme oxygenase1, nuclear factor E2-related factor 2, and nuclear factor kappaB are involved in hemin inhibition of type 2 cationic amino acid transporter expression and L-Arginine transport in stimulated macrophages. Anesthesiology. 2006;105:1201–1210. doi: 10.1097/00000542-200612000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Lee JE, Cho SM, Park E, Lee SM, Kim Y, Auh JH, Choi HK, Lim S, Lee SC, Kim JH. Anti-inflammatory effects of rubus coreanus miquel through inhibition of NF-kB and MAP kinase. Nutr Res Pract. 2014;8:501–508. doi: 10.4162/nrp.2014.8.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deinsberger W, Lang C, Hornig C, Boeker DK. Stereotactic aspiration and fibrinolysis of spontaneous supratentorial intracerebral hematomas versus conservative treatment: a matched-pair study. Zentralbl Neurochir. 2003;64:145–150. doi: 10.1055/s-2003-44617. [DOI] [PubMed] [Google Scholar]
- 13.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. statistical validation. Stroke. 1995;26:627–634. 635. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 14.Xu K, Wang X, Shi Q, Chen C, Tian C, Li XL, Zhou RM, Chu YL, Dong XP. Human prion protein mutants with deleted and inserted octarepeats undergo different pathways to trigger cell apoptosis. J Mol Neurosci. 2011;43:225–234. doi: 10.1007/s12031-010-9387-0. [DOI] [PubMed] [Google Scholar]
- 15.Luo Z, Dong X, Ke Q, Duan Q, Shen L. Chitooligosaccharides inhibit ethanol-induced oxidative stress via activation of Nrf2 and reduction of MAPK phosphorylation. Oncol Rep. 2014;32:2215–2222. doi: 10.3892/or.2014.3463. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Kim JH, Lee SJ. Involvement of PKA and HO-1 signaling in anti-inflamatory effects of surfactin in BV-2 microglial cells. Toxicol Appl Pharmacol. 2013;368:68–78. doi: 10.1016/j.taap.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Kim SW, Lee HK, Shin JH, Lee JK. Up-down regulation of HO-1 and Inos gene expression by ethylpyruvate via recruiting p300 to Nrf2 and depriving it from p65. Free Radic Biol Med. 2013;65:468–76. doi: 10.1016/j.freeradbiomed.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Shu XL, Yu TT, Kang K, Xu H, Lei T. Protective effects of bifidobacterial adhesion on intestinal mucosa of stressed male rats via modulation of inflammation. Int J Clin Exp Pathol. 2014;7:3324–3331. [PMC free article] [PubMed] [Google Scholar]
- 19.Shang H, Yang D, Zhang W, Li T, Ren X, Wang X, Zhao W. Time course of Keap1-Nrf2 pathway expression after experimental intracerebral haemorrhage: correlation with brain oedema and neurological deficit. Free Radic Res. 2013;47:368–375. doi: 10.3109/10715762.2013.778403. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Wang S, Zhang M. Pharmacological induction of heme oxygenase-1 by a trigerpenoid protects neurons against ischemic injury. Stroke. 2012;43:1390–1397. doi: 10.1161/STROKEAHA.111.647420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho YJ, Kim SJ. Effect of quercetin on the production of nitric oxide in murine macrophages stimulated with lipopolysaccharide from prevotella intermedia. J Periodontal Implant Sci. 2013;43:191–197. doi: 10.5051/jpis.2013.43.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uddin MJ, Jesong SO, Zheng M. Carbon monoxide attenuates dextran sulfate sodium-induced colitis of GSK-3-beta signaling. Oxid Med Cell Longev. 2013;2013:210563. doi: 10.1155/2013/210563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JW, Choi YJ, Park JH, Sim JY, Kwon YS, Lee HJ, Kim SS, Chun W. 3,4,5-Trihydroxycinnamic acid inhibits lipopolysaccharide-induced inflammatory response through the activation of Nrf2 pathway in BV2 microglial cells. Biomol Ther (Seoul) 2013;21:60–65. doi: 10.4062/biomolther.2012.091. [DOI] [PMC free article] [PubMed] [Google Scholar]


