Abstract
Limbal stem cell (LSC) on the basal layer of cornea plays an important role in the epithelial repair after corneal injury as it can proliferate, differentiate and migrate into injury sites under the direction of cytokines. This study explored the signaling pathway and cellular mechanism between corneal epithelial cells LSC, on a mouse model with mechanic corneal injury. Ipsilateral corneal mechanic injury model was prepared on mice using the contralateral eye as the control. Tissues from both central and peripheral regions of cornea were collected, cultured and quantified for expression of various cytokines including epidermal growth factor (EGF), fibroblast growth factor-β (FGF-β), heparin-like growth factor (HGF), keratinocyte growth factor (KGF), transforming growth factor-β1 (TGF-β1), IGF-1 and IGF-2. The effects of these factors on the differentiation of LSC and fibroblasts were also studied. Most of those cytokines had elevated gene expressions after the corneal injury. Among those IGF-2 had significantly increased expression, along with the high expression of IGF-2 receptor in corneal peripheral cells. IGF-2 also induced the differentiation of LSC into keratin-12-positive cells. Further studies showed the prominent expression of α-actin in injured tissues, suggesting the potential transformation of fibroblasts into myofibroblasts. Both IGF-2 and its receptor had elevated expressions after corneal injury. They may facilitate the transformation of LSC into epithelial cells, in addition to the role in transformation from fibroblasts to myofibroblasts.
Keywords: Corneal injury and repair, insulin-like growth factor-2, fibroblasts, myofibroblasts
Introduction
Corneal epithelial cells have been reported to have certain self-renewal and repair abilities, due to the existence of limbal stem cell (LSC), which can assist repairing injured corneal tissues via cell proliferation, differentiation and migration [1-5]. The repair ability of cornea is severely compromised when LSC is absent, causing conjunctivalization, chronic inflammation and persistent epithelial damages, or even leading to vision lost. The transplantation of LSC and peripheral corneal tissues, therefore, can ameliorate corneal injury via its proliferation, differentiation and migration into injury sites, where it induces the expression of epithelial markers [6-9]. The molecular mechanism and signaling pathway between LSC and peripheral tissues, however, remained unknown. One previous study on corneal tissues after mechanical or chemical injury revealed potentiated expression of growth and differentiation related cytokines, including transforming growth factor-α (TGF-α), TGF-β, epidermal growth factor (EGF), heparin-like growth factor (HGF), fibroblast growth factor-β (FGF-β), all of which are synthesized by corneal epithelial cells for regulating cell proliferation and growth [10]. Besides, insulin-like growth factor-2 (IGF-2) has been demonstrated to facilitate the proliferation of keratinocytes and synthesis of protein N-cadherin, both of which contribute to the generation of extracellular matrix and keratin collagen [11-14]. IGF related signaling pathway has been known to be involved in cell proliferation and differentiation, thus playing a crucial role in mediating growth at both cellular and organ levels. Therefore IGF and other cytokines may also be related to the proliferation, differentiation and migration of LSC, which can be identified from other corneal cells by cell surface markers such as keratin K3, K12 or connexin 43. Those abovementioned factors are also related with the proliferation and differentiation of peripheral corneal cells. There were studies reporting the ability of supernatants of corneal cell culture to facilitate the differentiation of multiple stem cells including saccular stem cells, mesenchymal stem cells (MSCs) and embryonic stem cells into K12-positive cells, although the molecular mechanism is still unclear [3,4,15-19]. This study thus investigated the molecular mechanism between corneal epithelial cells and LSC, using a mechanical corneal injury model on mice.
Materials and methods
Animal model
A total of 20 male BALB/C mice (10 weeks old) were purchased from Shandong University and were kept in standard cages with food and water provided ad libitum. After anesthesia by xylazine and ketamine, surgical eye and peripheral skins were sterilized by 0.5% iodine. A 1.5 mm-diameter circle was made in the center of cornea using a trephine. 23-gauge needle was used to break the epithelial cornea in the center but leaving corneal matrix or peripheral tissues intact. The other eye of the mouse was kept intact as the control. HE staining was used to detect the injury site and severity on the cornea. After different post-injury time points including 3 hr, 6 hr, 12 hr and 24 hr. Central and peripheral corneal tissues were extracted and separated into different layers under the microscope. All experimental protocols have been approved by the ethical committee in Provincial Hospital Affiliated to Shandong University, in accordance with related codes for animal experiments.
Single-cell suspension of corneal tissues and supernatants of culture medium
The peripheral corneal tissues were separated and digested by pepsin (10 min, ×10 times). Cells were collected by centrifugation (250 g, 8 min) and were transferred into DMEM medium (Sigma, US) containing 10% fetal bovine serum (FBS, Sigma, US), antibiotics (penicillin 100 U/mL plus streptomycin 100 μg/mL) and HEPES.
Twenty-four hours after corneal injury, samples (~1 mm diameter, without peripheral tissues) in the central corneal region were collected from both experimental and control eyes and cultured in 48-well plate containing DMEM medium. After 24 hour incubation, supernatants were centrifuged at 400 g for 10 min, filtered using 0.22 μm mesh, and were stored at -80°C for further use.
Real-time PCR
Real-time PCR was used to describe the expression levels of cytokines (including EGF, FGF-β, KGF, HGF, TGF-β1, IGF-1 and IGF-2) in injured corneal tissues. Samples from both central and peripheral cornea were transferred into tubes containing 500 μL Trizol (Molecular Research Center, US) for total RNA extraction following the manual instruction. In vitro reverse transcription was performed using M-MLV reverse transcriptase (Promega, US) to synthesize first strand cDNA. Real-time PCR was performed using the parameters: pre-denature for 3 min (95°C), followed by 40 cycles (95°C for 20 sec, 60°C annealing for 30 sec, 72°C for 30 sec). Each experiment was performed in triplicates. Target gene expression levels were determined with reference to GADPH gene.
Immunofluorescent staining
Both LSC and corneal epithelial cells (25,000 per mL) were cultured in 24-well plate containing DMEM for 48 hours. Apoptotic cells were labelled by Hoechst 33258 (Invitrogen, US) and separated by BD Influx (BD Bioscience, US). Remaining living cells were fixed in 4% paraformaldehyde for 20 min and rinsed in 0.1% Triton X-100. Fixed cells were then incubated with goat anti-K2 polyclonal antibody (Santa Cruz, US) or mouse anti-α-actin antibody for 1-hour incubation, followed by anti-IgG antibody conjugated with Alexa Flour 594/488 (Invitrogen, US). Cell nucleus was visualized by DAPI. Staining images were captured by a fluorescent microscope (Leica, Germany).
Analysis of corneal culture supernatants
Goat anti-IGF-2 antibody (Peprotech, US) was mixed with supernatants of cultured corneal tissues and then was added into freshly separated LSC (25,000 cells per mL). After 48 hours, real-time PCR was carried out for quantification of K12 gene expression. Further Immunofluorescent was used in the same way as described above to stain cells (A5528 plus AlexaFluor 488). The expression levels of α-actin were compared between those cells with or without IGF-2 antibody.
Statistical analysis
Student t-test was used to compare means across groups using SPSS 16.0 software. A statistical significance was defined when P<0.05.
Results
Mechanical injury of cornea
In a pilot study, HE staining was used to describe the morphology of injury cornea tissues. As shown in Figure 1, the needle only penetrated the epithelial layer, leaving basal layer intact, suggesting the validity of our surgical procedures.
Figure 1.
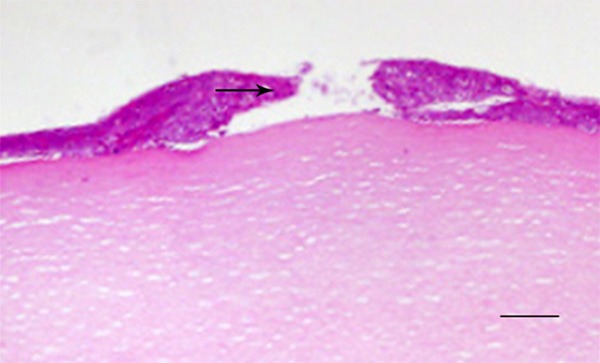
Morphology of cornea after mechanical injury. The arrow indicate breakage of epithelial layer of cornea. Scale bar, 100 μm.
Cytokines and related receptor gene expression
Real-time PCR was used to describe cytokine expressions in various corneal tissues at different time points (0, 3, 6, 12, 24 hours after surgery). As shown in Figure 2, expressions of EGF, FGF-β, KGF, HGF, TGF-β1, IGF-1 and IGF-2 were significantly elevated (P<0.05), especially for IGF-2 at 3 hours after surgery. Thus we further compared IGF-1 and IGF-2 receptor gene expressions in central and peripheral corneal tissues. Results found significantly elevated expressions of those two receptor genes in peripheral tissues when compared to epithelial cells at 3 hours post-surgery (P<0.05, Figure 3).
Figure 2.
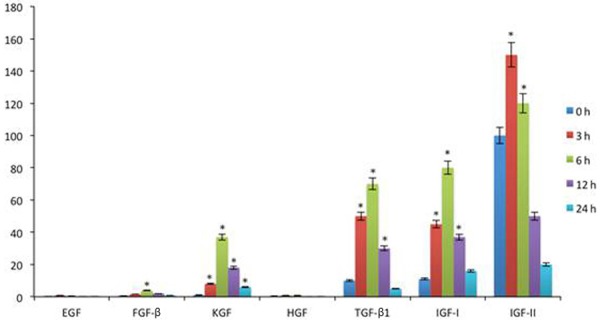
Gene expressions of cytokines. Data were presented as mean ± standard deviation (SD). *P<0.05 compared to those at 0 hour.
Figure 3.
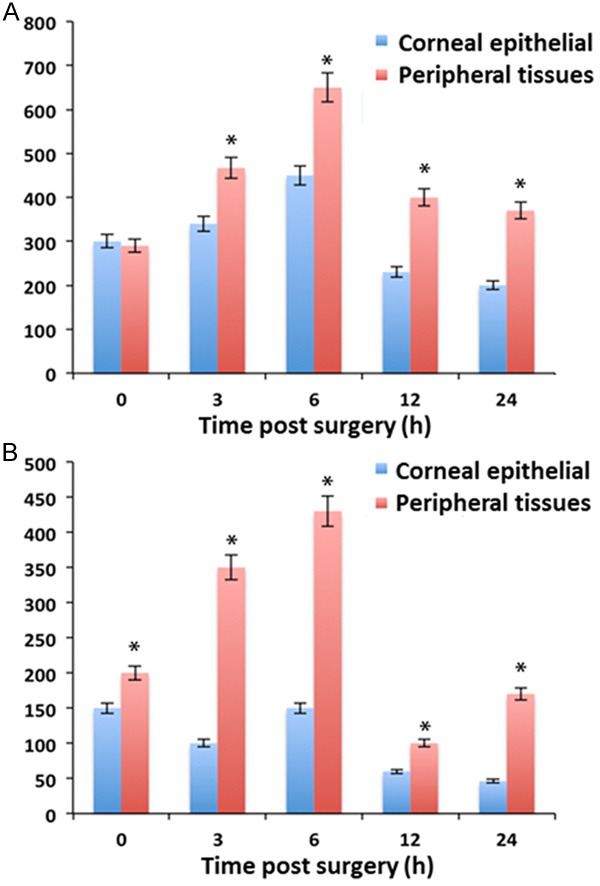
mRNA levels in corneal epithelial and peripheral tissues. A. IGF-1 receptor; B. IGF-2 receptor. Data were presented as mean ± standard deviation (SD). *P<0.05 compared to corneal epithelial tissues.
K12 expression in LSC
To evaluate the potency of IGF-2 on the differentiation of LSC, we used Immunofluorescent method to detect the expression of keratin 12 (K12) proteins in LSC. During the culture of LSC, IGF-2 was added. Results showed the prominent expression of K12 in IGF-2 added LSC (Figure 4). This was consistent with corneal epithelial cells, suggesting the role of IGF-2 in facilitating differentiation of LSC into corneal epithelial cells for injury recovery.
Figure 4.
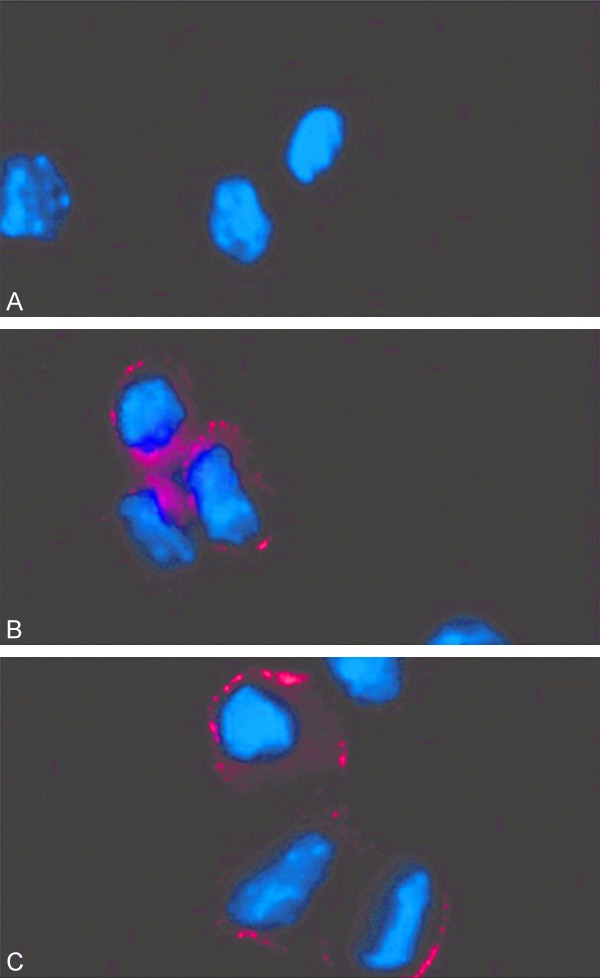
Effects of IGF-2 on LSC differentiation. A. Controlled LSC without IGF-2 addition; B. LSC added with IGF-2 had keratin 12 (K12) expression; C. Normal corneal epithelial cells also expressed K12.
Effects of IGF-2 on LSC differentiation
To further substantiate the effect of IGF-2 in LSC differentiation, we mixed the cultured supernatants with anti-IGF-2 antibody. Results showed the significantly elevated K12 gene expression of LSC after treating with culture supernatants (Figure 5). The pre-incubation with IGF-2 antibody, however, blocked such potentiation effect. These results further confirmed the role of IGF-2 on LSC differentiation.
Figure 5.
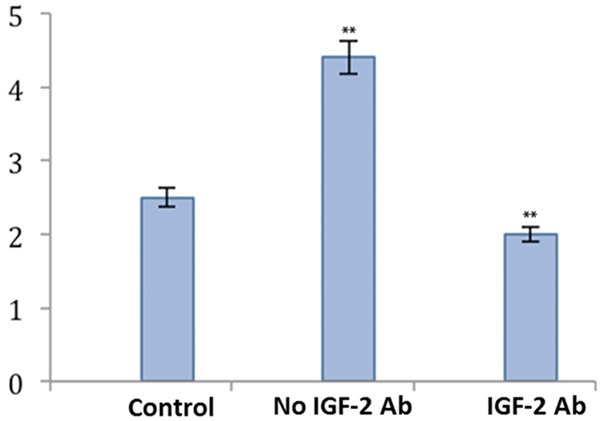
IGF-2 antibody and LSC differentiation. K12 gene expression was significantly suppressed after the co-incubation with IGF-2 antibody. **P<0.01 compared to the control group.
IGF-2 receptor and fibroblasts
We further added the supernatants from cultured injury corneal tissues. IGF-2 receptor antibody was also added in the experimental group. Immunofluorescent staining showed significantly decreased α-actin levels in the experimental group, suggesting the increased myofibroblasts in controlled animals (Figure 6). These results thus suggest the certain role of IGF-2 receptor in the transformation from fibroblasts to myofibroblasts.
Figure 6.
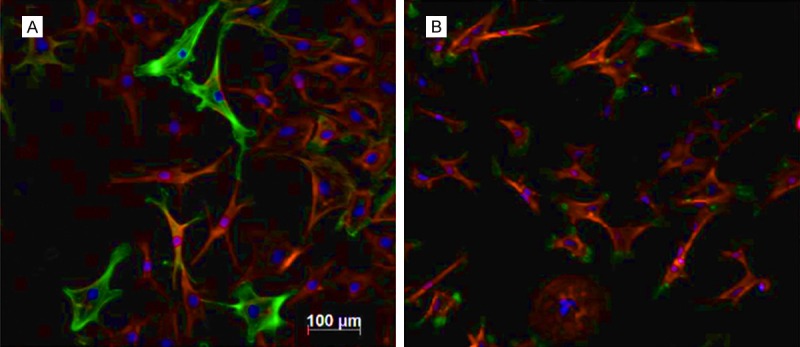
IGF-2 affects fibroblasts growth. A. Controlled cells with no IGF-2 receptor antibody; B. Experimental groups with the addition of IGF-2 receptor antibody. Green, α-actin; Red, F-actin; Blue, DAPI. Scale bar, 100 μm.
Discussion
The slow recovery of corneal epithelial layers under the absence of LSC suggest the origin of epithelial cells from LSC’s differentiation, as supported by further studies showing the transplantation of peripheral corneal tissues or LSC improved regeneration of cornea [20]. LSC can be recognized from epithelial cells by the surface markers and morphological features. Under normal circumstances, LSC is protected within tissue clefts and migrates into injury sites following cytokines release, cell proliferation and differentiation induction under corneal damage. The related mechanisms and induction of LSC differentiation, however, still remained unknown yet. Studies showed certain induction effects on the differentiation of stem cells into corneal epithelial cells by using embryonic stem cells, MSCs, human tissue-specific stem cells or cultured corneal cells. These studies all suggested the critical role of extracellular matrix, including the containing cytokines in such differentiation [21-23]. Moreover, supernatants of corneal cells culture also induced the expression of corneal cell-specific marker K12 in stem cells, but leaving related cytokines unknown.
This study found prominent expression of cell proliferation and differentiation related cytokines after corneal injury. Those cytokines may penetrate lacrimal membrane, aqueous humor and epithelial layer to reach the peripheral region, where it can induce the proliferation of LSC. We collected LSC 24 hours after primary corneal injury and found significantly elevated cell proliferation when compared to the healthy eye. To confirm the related cytokine, we mixed all cytokines produced by both LSC and epithelial cells after injury. Of all those factors only IGF-2 elevated the gene expression of K12, which cannot be produced by LSC under normal physiological conditions. Previous studies have used IGF-2 to differentiate between pleiotropic stems cells and corneal stem cells [24,25]. As IGF-2 expression level was potentiated in corneal epithelial cells after primary injury, it may exert a major role in inducing LSC proliferation and differentiation. The secretion of IGF by corneal cells has been reported to exert multiple effects on corneal cells. This study suggested the major role of IGF-2 for stimulating epithelial cell regeneration after corneal damage as it exerts a critical function in the proliferation and differentiation of LSC. As one family of IGF signaling pathway, IGF-2 is responsible for multiple cellular functions and pathways covering peptide hormones (IGF-1 and 2), cell surface receptor (insulin-like receptor I/II) and IGF binding protein 1-6 via the specific binding onto relevant receptors for signaling cascades.
It has been reported that IGF receptor expression was suppressed after corneal damage, possibly due to the focal suppression of IGF binding onto corneal cells, thus facilitating the penetration of IGF into peripheral regions for IGF receptor expression and LSC differentiation [26]. We confirmed the ability of supernatants from corneal cell culture to stimulate IGF-2 expression of LSC. As one cytokine, IGF-2 directly stimulated the expression of its receptors in peripheral cells. Meantime IGF-2 from supernatants also induces cells to expression K12 protein. The addition of IGF-2 antibody, however, may damage the potency for proliferation/differentiation. IGF-1, on the other hand, may induce cell proliferation and had no significant effect on LSC in mice. Previous reports mentioned the potentiated proliferation on keratin by IGF-1/2 via the binding onto substances P to stimulate growth of rabbit corneal epithelial cells. IGF may also have certain trophic effects as it had certain facilitating functions on KGF and HGF in rabbit epithelial layer. Our study found the potential role of IGF-2 and its receptor in facilitating transformation of myofibroblasts, thus causing the scar formation after corneal injury [27,28]. Currently it is known that TGF-β family relates with fibrosis and scar formation of cornea. Therefore it is necessary to elucidate the interaction between IGF cytokines and corneal injury.
Recent studies showed the ability of IGF in inducing MSC differentiation into hepatocytes, in addition to the differentiation of myoblasts, inducing insulin-like differentiation of human adipocyte stem cells, facilitating activation and differentiation of human cardiac stem cells in various organs [29,30]. The differentiation of LSC and related cytokines had clinical significance for stem cell treatment, as reported by recent studies in which cultured LSC and transplantation had satisfactory treatment efficacy for severe corneal damage and LSC loss. The addition of cytokines in LSC culture medium should enrich the proliferation of target cell types, benefiting its application in regenerative medicine.
Disclosure of conflict of interest
None.
References
- 1.Majo F, Rochat A, Nicolas M, Jaoudé GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250–4. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- 2.Chang CY, Green CR, McGhee CN, Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008;49:5279–86. doi: 10.1167/iovs.07-1260. [DOI] [PubMed] [Google Scholar]
- 3.Dua HS, Miri A, Alomar T, Yeung AM, Said DG. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116:856–63. doi: 10.1016/j.ophtha.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Gomes JA, dos Santos MS, Cunha MC, Mascaro VL, Barros Jde N, de Sousa LB. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003;110:466–73. doi: 10.1016/s0161-6420(02)01888-2. [DOI] [PubMed] [Google Scholar]
- 5.Du Y, Chen J, Funderburgh JL, Zhu X, Li L. Functional reconstruction of rabbit corneal epithelium by human limbal cells cultured on amniotic membrane. Mol Vis. 2003;9:635–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–55. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 7.Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998;111:2867–75. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- 8.Jia C, Zhu W, Ren S, Xi H, Li S, Wang Y. Comparison of genome-wide gene expression in suture-and alkali burn-induced murine corneal neovascularization. Mol Vis. 2011;17:2386–99. [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng CC, Wang DY, Kao MH, Chen JK. The growth-promoting effect of KGF on limbal epithelial cells is mediated by upregulation of DeltaNp63alpha through the p38 pathway. J Cell Sci. 2009;122:4473–80. doi: 10.1242/jcs.054791. [DOI] [PubMed] [Google Scholar]
- 10.Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 11.Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–92. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 12.Ko JA, Yanai R, Nishida T. IGF-1 released by corneal epithelial cells induces up-regulation of N-cadherin in corneal fibroblasts. J Cell Physiol. 2009;221:254–61. doi: 10.1002/jcp.21850. [DOI] [PubMed] [Google Scholar]
- 13.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–35. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honma Y, Nishida K, Sotozono C, Kinoshita S. Effect of transforming growth factor-beta1 and -beta2 on in vitro rabbit corneal epithelial cell proliferation promoted by epidermal growth factor, keratinocyte growth factor, or hepatocyte growth factor. Exp Eye Res. 1997;65:391–6. doi: 10.1006/exer.1997.0338. [DOI] [PubMed] [Google Scholar]
- 15.Etheredge L, Kane BP, Hassell JR. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest Ophthalmol Vis Sci. 2009;50:3128–36. doi: 10.1167/iovs.08-3077. [DOI] [PubMed] [Google Scholar]
- 16.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 17.Liu CY, Zhu G, Converse R, Kao CW, Nakamura H, Tseng SC, Mui MM, Seyer J, Justice MJ, Stech ME, et al. Characterization and chromosomal localization of the cornea-specific murine keratin gene Krt1.12. J Biol Chem. 1994;269:24627–36. [PubMed] [Google Scholar]
- 18.Matic M, Petrov IN, Chen S, Wang C, Dimitrijevich SD, Wolosin JM. Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation. 1997;61:251–60. doi: 10.1046/j.1432-0436.1997.6140251.x. [DOI] [PubMed] [Google Scholar]
- 19.Blazejewska EA, Schlötzer-Schrehardt U, Zenkel M, Bachmann B, Chankiewitz E, Jacobi C, Kruse FE. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27:642–52. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu S, Xing C, Han J, Tso MO, Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis. 2009;15:99–107. [PMC free article] [PubMed] [Google Scholar]
- 21.Homma R, Yoshikawa H, Takeno M, Kurokawa MS, Masuda C, Takada E, Tsubota K, Ueno S, Suzuki N. Induction of epithelial progenitors in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Invest Ophthalmol Vis Sci. 2004;45:4320–6. doi: 10.1167/iovs.04-0044. [DOI] [PubMed] [Google Scholar]
- 22.Jiang TS, Cai L, Ji WY, Hui YN, Wang YS, Hu D, Zhu J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis. 2010;16:1304–16. [PMC free article] [PubMed] [Google Scholar]
- 23.Krulova M, Pokorna K, Lencova A, Fric J, Zajicova A, Filipec M, Forrester JV, Holan V. A rapid separation of two distinct populations of mouse corneal epithelial cells with limbal stem cell characteristics by centrifugation on percoll gradient. Invest Ophthalmol Vis Sci. 2008;49:3903–8. doi: 10.1167/iovs.08-1987. [DOI] [PubMed] [Google Scholar]
- 24.Holan V, Pokorna K, Prochazkova J, Krulova M, Zajicova A. Immunoregulatory properties of mouse limbal stem cells. J Immunol. 2010;184:2124–9. doi: 10.4049/jimmunol.0903049. [DOI] [PubMed] [Google Scholar]
- 25.Svobodova E, Krulova M, Zajicova A, Pokorna K, Prochazkova J, Trosan P, Holan V. The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-cell or proinflammatory helper T-cell 17 population. Stem Cells Dev. 2012;21:901–10. doi: 10.1089/scd.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi K, Kurosaka D, Iwata T, Oguchi Y, Tanaka Y, Mashima Y, Tsubota K. Involvement of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in corneal fibroblasts during corneal wound healing. Invest Ophthalmol Vis Sci. 2006;47:591–8. doi: 10.1167/iovs.05-0097. [DOI] [PubMed] [Google Scholar]
- 29.Matheny RW Jr, Nindl BC. Loss of IGF-IEa or IGF-IEb impairs myogenic differentiation. Endocrinology. 2011;152:1923–34. doi: 10.1210/en.2010-1279. [DOI] [PubMed] [Google Scholar]
- 30.Kang HM, Park S, Kim H. Insulin-like growth factor 2 enhances insulinogenic differentiation of human eyelid adipose stem cells via the insulin receptor. Cell Prolif. 2011;44:254–63. doi: 10.1111/j.1365-2184.2011.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


