Abstract
Ets-1, the prototypical member of the family of Ets transcription factors, has been shown to participate in tissue fibrotic remodeling. However, its role in cardiac fibrosis has not been established. The aim of this study was to investigate the role of Ets-1 in profibrotic actions of angiotensin II (Ang II) in cardiac fibroblasts (CFs) and in the in vivo heart. In growth-arrested CFs, Ang II induced Ets-1 expression in a time- and concentration-dependent manner. Pretreatment with Ang II type 1 receptor blocker losartan, protein kinase C inhibitor bisindolylmaleimide I, extracellular signal-regulated kinase (ERK) inhibitor PD98059, or c-Jun NH(2)-terminal kinase (JNK) inhibitor SP600125 partly inhibited this induction accompanied with impaired cell proliferation and production of plasminogen activator inhibitor-1 (PAI-1) and connective tissue growth factor (CTGF) protein, the two downstream targets of Ets-1. Knockdown of Ets-1 by siRNA significantly inhibited the inductive effects of Ang II on cell proliferation and expression of CTGF and PAI-1. Moreover, the levels of Ets-1, PAI-1 and CTGF protein were simultaneously upregulated in left ventricle of Ang II-infused rats in parallel with an increase in the activation of ERK and JNK. Our data suggest that Ets-1 may mediate Ang II-induced cardiac fibrotic effects.
Keywords: Angiotensin, fibroblast, Ets-1, fibrosis
Introduction
Cardiac fibrosis, which implicates an increase in the volume of non-myocyte compartment of the heart, promotes the progression of left ventricular hypertrophy associated with hypertension, post-myocardial infarction remodeling and heart failure, and thus is a key determinant of clinical outcome in heart diseases [29]. The proximal effector cells in this process are cardiac fibroblasts (CFs). They can be activated to proliferate and produce extracellular matrix (ECM) proteins and proteinases, as well as growth factors and cytokines regulating remodeling process. These responses lead to ventricular wall stiffness and impaired diastolic function [29]. There is now convincing evidence that Angiotensin II (Ang II), which has been shown to be aberrant activated in hypertension and myocardial ischemia [13], is one of the most important humoral factors regulating the pathogenesis of cardiac fibrosis. Ang II controls the functions of cardiac fibroblast by directly activating the cell through its receptors. Various signaling pathways mediate these profibrotic effects of Ang II, including mitogen-activated protein kinases (MAPK), protein kinase C (PKC), reactive oxygen species and phosphate tyrosine kinase (PTK) [13,17].
Ets-1 is the prototypical member of the family of Ets transcription factors. It plays an important role in a wide variety of biological processes, including cellular proliferation, differentiation, apoptosis, metastasis and tissue remodeling. Ets-1 controls the expression of critical genes involved in these processes by binding to the core “GGAA/T” nucleotide sequence called the PEA3 element present in their transcriptional regulatory regions [10]. Ets-1 is induced during tissue remodeling. It participates in the processes by regulating the expression of genes encoding ECM, as well as enzymes involved in matrix degradation. For instance, Ets-1 regulates Ang II-induced fibroblast activation and kidney fibrosis [6]. Ets-1 has been shown to be involved in airway remodeling by being upregulated and regulating TNF-alpha-induced matrix metalloproteinase-9 and tenascin expression in bronchial CFs [21]. Ets-1 also regulates TGF-beta-induced type I collagen production in mesangial cells [18], stimulates collagen alpha 2 (I) promoter activity in dermal fibroblasts [4]. Moreover, Ets-1 is involved in hepatic stellate cells activation, suggesting its role in liver tissue repair [14].
More recently, Ets-1 has been shown to be rapidly induced by Ang II in vascular smooth muscle cells (VSMCs) and endothelial cells, and mediate vascular inflammation and remodeling [34]. It has also been shown in this report that Ang II-infusion-induced increase in heart size is significantly diminished in Ets1–/– mice, suggesting a potential role of Ets-1 in cardiac remodeling. However, the role of Ets-1 in regulating the functions of cardiac fibroblast and cardiac fibrosis has not been established. In this study, we first examined the expression of Ets-1 in Ang II activated CFs, then investigated the possible molecular mechanisms by which Ang II mediated Ets-1 expression and subsequent cell proliferation and production of downstream target genes of Ets-1. Finally, we researched the expression of Ets-1 and it target genes within the left ventricles of Ang II-infused rats in vivo.
Methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), FBS, penicillin, streptomycin were purchased from GIBCO BRL (Carlsbad, CA, U.S.A.). Ang II, PD123319, PD98059, SB203580, SP600125, anisomycin were from Sigma (St. Louis, MO, USA). Losartan was from Cayman (cayman Co., U.S.A.). Bisindolylmaleimide I (BIM), and genistein were from Alexis (Lausen, Switzerland). Rabbit polyclonal antibodies against Ets-1 and plasminogen activator inhibitor-1 (PAI-1) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal antibody against connective tissue growth factor (CTGF) was from ABCAM (Cambridge, UK). Rabbit polyclonal antibodies against c-Jun NH(2)-terminal kinase (JNK) and phospho-JNK (Thr183/Tyr185), ERK1/2, phospho-ERK1/2 (Ser217/221) were from Cell Signaling Technology (Beverly, MA, USA).
Cell culture
Our study followed the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). CFs were obtained from ventricles of one- to two-day-old Sprague-Dawley rats by collagenase and trypsin digestion methods as described previously [12] and were grown in DMEM with 10% (v/v) FBS, penicillin (100 U/ml) and streptomycin (100 U/ml). 95% of the cells displayed positive immunoreactivity towards vimentin. Cells within three passages were used for the studies. For subsequent experiments, cells at 80% confluence in culture dishes were growth-arrested by serum starvation for 48 h.
Real-time reverse-transcriptase polymerase chain reaction (real-time RT-PCR)
Total RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and DNA was removed by use of the DNA-free kit (Ambion, Austin TX, USA). The quality of the total mRNA was checked by denaturing agarose gel electrophoresis containing 1.5% formaldehyde. Total RNA concentration and purity was determined by using UV-Vis spectroscopy with the Bio-Rad SmartSpec 3000 system (California, USA). Reverse transcription and polymerase chain reaction of each sample were performed in triplicates by using the SYBR®ExScriptTM RT-PCR Kit (TaKaRa, Bio, Japan) and the ABI PRISM 7000 sequence detection PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Sequence-specific PCR primers designed with Beacon designer v4.0 (Premier Biosoft, USA) were as follows: GAPDH: sense (5’-GCC TTC TCC ATG GTG GTG AA-3’); anti-sense (5’-GGT CGG TGT GAA CGG ATT TG-3)’. Ets-1: sense (5’-TGA TGT GGG CTG TGA ATG AG-3’); anti-sense (5’-TGA AGT AAT CCG AGG TGT AAC G-3’). Total sample volume was 25 μl. PCR was performed under following conditions: 95°C for 2 min, 40 cycles of DNA amplification (95°C for 10 s, 60°C for 31 s) and a PCR-product dissociation stage for quality control. GAPDH was used as an internal control. Data were expressed as fold difference for Ets-1 against GAPDH by use of the 2-ΔΔCT method [26]. Results were validated by demonstrating generation of a single PCR product of an expected size by gel electrophoresis and by dissociation curve analysis. Serial 10-fold dilutions of the cDNA were used to confirm near-theoretical efficiencies of the assays.
Western blot analysis
Myocardial tissue and cell lysates were prepared with 200 µl ice-cold lysis buffer (50 mM HEPES, 5 mM EDTA, 100 mM NaCl, 1% Triton X-100, protease inhibitor cocktail [Roche; Germany]; pH 7.4). Protein concentrations were determined with the BCA protein assay kit (Pierce, Rockford, IL). Samples were subjected to electrophoresis in 8% SDS-PAGE gels, and were transferred onto a polyvinylidene difluoride (PVDF) membrane in a semi-dry system (Bio-Rad, Hercules, CA). the membranes were blocked with 10% fat-free milk in TBST buffer (20 mM Tris HCl, 137 mM NaCl, and 0.05% Tween 20) and subsequently incubated with specific antibodies against Ets-1 (1:200), PAI-1 (1:200), CTGF (1:1000), JNK (1:500), phospho-JNK (1:500), ERK1/2 (1:1000), phospho-ERK1/2 (1:1000) and β-actin (1:200) in TBST buffer overnight. The membranes were then washed and incubated with secondary antibody for 45 minutes. Antigen-antibody complexes were revealed by chemiluminescence and visualized by exposure to X-ray films. Optical densities of bands were scanned and quantified by using Gel Doc 2000 (Bio-Rad). β-actin was used as a loading control. Data are normalized against those of the corresponding β-actin. Results were expressed as fold increase over control.
Cell proliferation assay
Cell growth was determined by measuring 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl- tetrazolium bromide (MTT; Sigma) dye absorbance of living cells. Cells were seeded in 96-well plates at 1×104 per well. After indicated treatments, 20 μl of MTT solution at 5 mg/ml was added to each well, and plates were incubated for an additional 4 h at 37°C. The culture medium was removed. Then, 150 µl of dimethyl sulfoxide (DMSO) was added to each well. Absorbance was measured at 490 nm by using a microplate spectrophotometer (POLARstar, OPTIMA, Germany).
Small interfering RNA and transfection
The siRNA targeting rat Ets-1 mRNA was synthesized with the sense sequence 5’-GCU UCG ACU CCG AGG ACU ATT-3’. The double-strand RNAs were transfected into CFs using Lipofectamine 2000 (Invitrogen). A scrambled siRNA was used as a control. The FAM-labeled siRNA was transfected and visualized under fluorescence microscopy to confirm the transfection efficiency of siRNA.
Animal experiments
Male Sprague-Dawley rats (180±1.6 g) were divided into three groups (n = 6 each group): controls, Ang II-infusion for 3 days, Ang II-infusion for 7 days. Rats were anesthetized with pentobarbital (50 mg/kg) intraperitoneally and then osmotic minipumps (model 2001, Durect Corp. Cupertino, CA) were inserted subcutaneously to deliver Ang II (150 ng/kg/min) or saline alone (control) for indicated days. Systolic blood pressure (SBP) was measured by the tail-cuff method. At the end of the experiment, animals were sacrificed by injecting an excess amount of pentobarbital. Then the left ventricle was separated from atria and right ventricle and weighed. One portion of the ventricle was fixed in 4% formaldehyde solution and embedded in paraffin for collagen evaluation, the rest was snap-frozen in liquid nitrogen and stored at -70°C for subsequent biochemical assays.
Evaluation of collagen deposition
The median part of the left ventricle sections (5 µm) were hydrated and stained with Sirius Red F3BA (0.5% in saturated aqueous picric acid; Sigma). Ten fields in each region of the heart were selected randomly from three nonconsecutive serial sections, and collagen contents were quantified as the Sirius Red-positive areas by use of a video system equipped with the image analysis system Quantimet 500/software QWin (Leica, Jena, Germany).
Statistical analysis
Results are expressed as mean ± s.e.m. Statistical significance between groups was assessed by one-way ANOVA, followed by Scheffé’s F test, with use of SPSS 11.5 (SPSS Inc., Chicago, IL). A value of P < 0.05 was considered statistically significant.
Results
Ets-1 expression in CFs and its upregulation by Ang II
To investigate the regulation of Ets-1 expression by Ang II in CFs, primary cultured cells were growth-arrested for 24 h. Quantitative Real-time RT-PCR (Figure 1A) revealed that Ets-1 mRNA expression was induced by 1 h of Ang II treatment, reached a peak at 4 h, and slightly decreased by 48 h, but was still significantly higher than the control value. Moreover, Ets-1 mRNA levels were shown to be concentration-dependent, with a maximal effect at 10-6 mol/l Ang II treatment for 4 h (Figure 1B). Ang II also induced Ets-1 protein expression in a time- (Figure 1C) and dose (Figure 1D) -dependent fashion. The induction of Ets-1 by Ang II indicates that Ets-1 may be a downstream effector of Ang II in CFs.
Figure 1.

Ang II induced Ets-1 expression in CFs. A, B. Real-time RT-PCR analysis of Ets-1 mRNA levels. A. Ang II (0.1 μM) treatment for different time point. B. Different concentration (0.01, 0.1 and 1 μM) of Ang II treatment for 4 h. C, D. Western bolt analysis of Ets-1 protein. C. Ang II (0.1 μM) treatment for different time point. D. Different concentration of Ang II treatment for 24 h. All data shown here are the mean ± s.e.m. of three independent experiments. *P < 0.05 versus control.
Ang II type 1 (AT1) receptor mediates Ang II-inducted Ets-1 expression
A selective AT2 receptor blocker PD123319 was ineffective in preventing Ang II-induced Ets-1 mRNA expression as assessed by real-time RT-PCR (Figure 2A). While, pretreatment with losartan, a selective AT1 receptor blocker, abrogated (by over 90%) Ang II-induced Ets-1 expression (P < 0.01 versus Ang II). These data suggest that Ang II-induced Ets-1 is mediated mainly through the AT1 receptor.
Figure 2.

The potential molecular mechanisms implicated in Ang II-induced Ets-1 expression in CFs. Cells were pretreated with or without AT1 receptor blocker losartan (1 μM), AT2 receptor blocker PD123319 (10 μM), BIM (a general PKC inhibitor; 5 μM), genistein (PTK inhibitor; 50 μM), SP600125 (a selective JNK inhibitor; 10 μM), PD98059 (a selective ERK inhibitor; 50 μM ) or SB203580 (a p38 inhibitor; 20 μM) for 1 h, and subsequently stimulated with Ang II (0.1 μM) or anisomycin (5 μM) for 24 h. Ets-1 mRNA levels were assessed by Real-time RT-PCR. Ang II increases Ets-1 expression via AT1 receptor (A) and signaling pathways of JNK, ERK and PKC (B). All the data shown here are the mean ± s.e.m. of three independent experiments. *P < 0.05 versus control; #P < 0.05 versus Ang II.
Effects of MAPKs, PKC and PTK inhibitors on Ang II-induced Ets-1 mRNA expression
As shown in Figure 2B, Ang II-induced Ets-1 expression was significantly inhibited by the selective ERK inhibitor PD98059 and JNK inhibitor SP600125, with a reduction by 59.6% and 82.8%, respectively (both P < 0.01 versus Ang II). In contrast, exogenous administration of anisomycin, a potent JNK agonist, significantly increased Ets-1 level (P < 0.05 versus control). A general PKC inhibitor BIM inhibited Ang II-induced Ets-1 production by 20.8% (P < 0.05 versus Ang II). Whereas, genistein, a PTK inhibitor, and SB203580, a p38MAPK inhibitor, unaffected Ets-1 level induced by Ang II.
PD98059 and SP600125 prevent Ang II-induced Ets-1 protein expression accompanied with a downregulation of CTGF and PAI-1 production in CFs
There is growing evidence that Ets-1 is involved in transcriptional control of CTGF [22,30] and PAI-1 [34], two inducing factors in cardiac fibrosis, thus we sought to investigate whether inhibitors that suppressed Ang II-induced Ets-1 expression could also regulate the stimulated CTGF and PAI-1 production. SP600125 and PD98059, which markedly inhibited Ets-1 protein expression in response to Ang II also resulted in abrogation of Ang II-induced CTGF and PAI-1 protein production (Figure 3A). In contrast, when exogenous given the specific JNK agonist anisomycin, the level of Ets-1, together with PAI-1 and CTGF, was significantly upregulated.
Figure 3.

Effects of PD98059 and SP600125 on Ang II-induced cell proliferation and production of Ets-1, CTGF and PAI-1 in CFs. Cells were pretreated with or without PD98059 (50 μM) or SP600125 (10 μM) for 1 h, and then were stimulated with Ang II (0.1 μM) or anisomycin (5 μM) for 24 h. A. Western blot analysis of Ets-1, PAI-1 and CTGF levels. Results are expressed as fold increase over control and mean ± s.e.m. data of three independent experiments are shown. B. MTT assays show the effects of PD98059 and SP600125 on Ang II-induced cell proliferation. Results are the mean ± s.e.m. (n=12) minus optical density of the vehicle control. *P < 0.05 versus control. #P < 0.05 versus Ang II.
PD98059 and SP600125 decrease Ang II-induced CFs proliferation
As Ets-1 has shown to be involved in cell proliferation [15,28], we then examined whether SP600125 and PD98059, that impaired Ang II-induced Ets-1 production, regulated proliferation of CFs. Both PD98059 and SP600125 potently decreased proliferation of CFs in response to Ang II as determined by MTT assays (Figure 3B, P < 0.05 versus Ang II), whereas anisomycin increased the proliferation (P < 0.05 versus control).
Effect of siRNA-mediated knockdown of Ets-1 on Ang II-induced expression of CTGF, PAI-1 and proliferation of CFs
To examine whether Ets-1 mediated the effects of Ang II on expression of CTGF and PAI-1 and on proliferation of CFs. CFs were transfected with the Ets-1 siRNA or control siRNA, The transfection efficiency of siRNA was confirmed with FAM-labeled siRNA, which showed that most cells uptake siRNA (Figure 4A). Ets-1 siRNA significantly decreased the mRNA level of endogenous Ets-1 by 64% (Figure 4A). The pre-incubation of cells with Ets-1 siRNA not only inhibited the inductive effects of Ang II on the expression of CTGF and PAI-1 protein (Figure 4B top and bottom panels), but also counteracted the induction of cell proliferation by Ang II (Figure 4C).
Figure 4.

Ets-1 siRNA inhibited AngII-induced expression of PAI-1, CTGF and cell proliferation. CFs were transfected with Ets-1 specific or control siRNA for 48 hours before treatment with Ang II for 24 h. Gene expressions were determined by real-time RT-PCR (A) and western blot analysis (B). (C) Cell proliferation was analyzed by MTT assays. The fluorescence photomicrograph shows uptake of FAM-labeled siRNA in CFs. *P < 0.05 versus control. #P < 0.05 versus Ang II.
SBP and left ventricle weight/body weight ratio in Ang II-infused rats
The change in SBP after Ang II-infusion for 3 days was similar to that of control group. After 7-day Ang II treatment, SBP was significantly increased (P < 0.05 versus control; Figure 5A). The ratio of left ventricle weight/body weight showed a slight increase in 3-day Ang II infusion group, but did not reach statistical significance in comparison with control. While Ang II-infusion for 7 days significantly increase the ratio (P < 0.05 versus control, Figure 5B).
Figure 5.
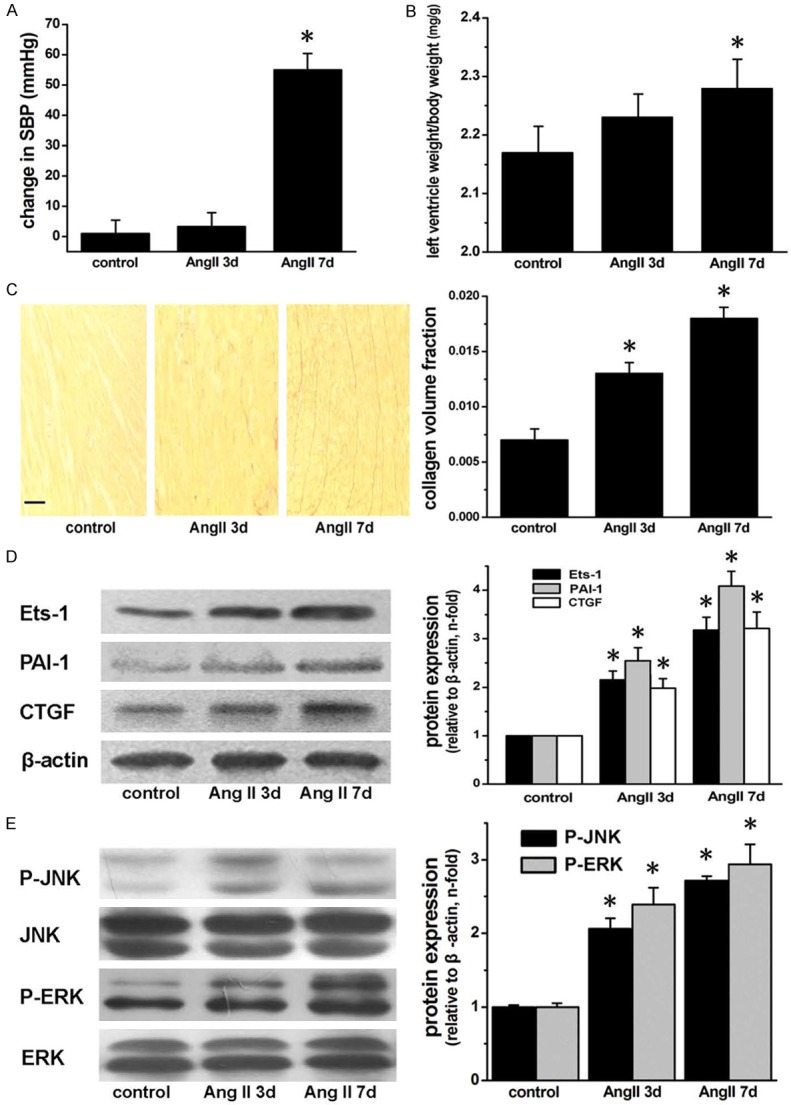
In vivo data performed on Ang II-infused rats. Ang II-infusion for 7 d increases SBP (A) and the ratio of left ventricle weight/body weight (B). (C) Left ventricular collagen deposition in Ang II-infused rats. Scale bar: 50 μm. (D) Western blot documents that Ang II-infusion upregulate levels of Ets-1, PAI-1 and CTGF protein in left ventricles. (E) Ang II-infusion increases phosphorylation of JNK and ERK. The quantitive values are showed as mean ± s.e.m. of six rats in each group. *P < 0.05 versus control.
Collagen deposition
As shown in Figure 5C, Collagen content of the left ventricle was higher in the group of Ang II-infusion for 3 days compared with the control group (P < 0.05), and continued to be elevated in 7-day Ang II infusion group (P < 0.05 versus control).
Expression of Ets-1, CTGF and PAI-1 in the left ventricle of Ang II-infused rats
Ets-1 level was significantly higher in the left ventricle of rats treated with Ang II for 3 days, and remained elevating in group of Ang II-infusion for 7 days (both P < 0.05 versus control; Figure 5D). The changes of CTGF and PAI-1 levels were similar to that of Ets-1 as assessed by Western bolt analysis (all P < 0.05 versus control).
Phosphorylation of ERK and JNK in left ventricle of Ang II-infused rats
The level of phospho-ERK expression were significantly increased in the left ventricle of 3-day Ang II-infusion rats in comparison with control (P < 0.05), and remained elevated in the group of 7-day Ang II-infusion (Figure 5E). Similar results were found with phospho-JNK levels (both P < 0.05 versus control).
Discussion
ECM remodeling of the heart plays an important role in determining cardiac diastolic function [7,13]. Despite considerable scientific data on the biochemical characteristics of remodeling, the critical transcriptional factors mediating these structural alterations still remain incompletely understood. In the present study, we examined the regulation of Ets-1 expression by Ang II in CFs and the in vivo heart and the molecular mechanisms underlying this action. The main findings of the present study are that in growth-arrested CFs, Ang II induces Ets-1 expression in a time- and concentration-dependent manner. Pretreatment with AT1 receptor blocker losartan, PKC inhibitor BIM, ERK inhibitor PD98059, or JNK inhibitor SP600125 partly inhibited this induction accompanied with impaired cell proliferation and the production of PAI-1 and CTGF protein, which are the downstream targets of Ets-1. Knockdown of Ets-1 by siRNA significantly inhibited the inductive effects of Ang II on cell proliferation and expression of CTGF and PAI-1. In vivo, the levels of Ets-1, PAI-1 and CTGF protein are simultaneously upregulated in left ventricle of Ang II-infused rats accompanied with an increase in the activation of ERK and JNK. These findings for the first time point to a potential role of Ets-1 in cardiac fibrosis both in vitro and in vivo, and thus may advance our basic understanding of the molecular mechanisms of this pathological process.
As a transcription factor, Ets-1 is originally described for its role in the development of malignancies. It has subsequently been shown to be regulated by multiple stimuli, such as proinflammatory cytokines, growth factors, and vasoactive peptides, and regulate a wide variety of biological processes, including tissue fibrosis [3,19,31]. However, the expression of Ets-1 and its induction in CFs has not been investigated. Our findings showed that treatment with Ang II resulted in increased levels of Ets-1 mRNA and protein in CFs. Ang II has been found previously to induce Ets-1 expression in many cell types, including aortic endothelial cells and VSMCs [23,24,34]. Our results indicate that Ang II may regulate Ets-1 expression via transcriptional levels. Furthermore, being a transcriptional factor, Ets-1 may also be implicated in the effects of Ang II on CFs functions.
Ang II acts via receptors that are members of the G protein–coupled receptor (GPCR) superfamily. There are two types of Ang II receptors: AT1 and AT2 receptor. It is well established that by binding to AT1 receptor, Ang II exerts multiple profibrotic effects [13], including promotion of fibroblast growth and proliferation, as well as synthesis and secretion of ECM and multiple profibrotic factors, such as TGF-beta, PAI-1 and CTGF [2]. However, this classical Ang II-AT1 axis in the induction of Ets-1 has not been investigated yet. Our data showed that a usual dose of AT1 receptor inhibitor losartan, but not AT2 receptor inhibitor PD123319, significantly lowered Ang II-induced Ets-1 expression, suggesting that the induction of Ets-1 by Ang II is most likely to be mediated via AT1 receptor in CFs.
On binding to the AT1 receptor, Ang II activates multiple intracellular signaling systems, including MAPKs, PKC and PTK, which control cell growth, cell death and gene expression involved in fibrosis processes [13,17]. Use of the inhibitors of these signals, we found that Ang II-induced Ets-1 expression might depend on ERK, JNK and PKC pathways in CFs. These results are consistent with previous studies showing that blockage of ERK or PKC activity prevent Ets-1 expression induced by many stimuli, such as TGF-beta1, vascular endothelial growth factor, TNF-alpha, Platelet-derived growth factor-BB and endothelin-1 in glomerular podocytes [16], endothelial cells [32] and VSMCs [8,20]. These findings suggest that Ang II, via AT1, activates several intracellular signaling systems, such as JNK, ERK and PKC, which contribute to Ets-1 regulation.
CFs proliferation is the basic function in response to profibrotic stimuli and can lead to excessive ECM production and subsequent cardiac fibrosis. Ets-1 plays an important role in proliferation of many cancer cells [6,9,28]. We then sought to investigate whether Ets-1 was also involved in Ang II-induced CFs proliferation. Our data, which were in line with a previous reports, showed that Ang II stimulated CFs proliferation, and more importantly, with the concurrence of Ets-1 upregulation. The two inhibitors PD98059 and SP600125 and an agonist anisomycin, which regulated Ets-1 expression, also modulated CFs proliferation. By the use of siRNA targeting Ets-1, we further showed that cell proliferation induced by Ang II was mostly inhibited by siRNA-mediated knockdown of Ets-1. It has been shown in previous study that Ets-1 stimulates the proliferation of VSMCs [25]. Moreover, VSMCs isolated from Ets-/- deficient mice exhibit decreased proliferative responses to Ang II [34]. So, it is highly likely that Ang II-induced proliferation of CFs may be mediated, at least in part, via Ets-1.
PAI-1 is a member of serine protease inhibitor family and strongly implicated as a determinant of fibrosis in diverse organs and tissue [27]. Induction of PAI-1 has also been suggested as a possible mechanism mediating the effects of Ang II on promoting cardiovascular remodeling by preventing degradation of the ECM [27]. Furthermore, the induction of PAI-1 is Ets-1-dependent [34]. CTGF belongs to the CCN family of growth factors. It is highly expressed in fibroblasts and triggers many of the cellular processes underlying fibrosis. Recent studies have demonstrated that CTGF is upregulated in CFs, and is a mediator in Ang II-induced myocardial remodeling [5]. More importantly, Ets-1 can induce CTGF expression by binding to an Ets-1-binding sequence in CTGF promoter [22,30], suggesting that CTGF is the down target of Ets-1. In our experiments, the upregulation of PAI-1 and CTGF by Ang II was coincident with the induction of Ets-1 in CFs. Both ERK and JNK inhibitors decreased Ets-1 level with a concurrent of decreased PAI-1 and CTGF production. More importantly, siRNA-mediated knockdown of Ets-1 significantly blocked Ang II-induced production of PAI-1 and CTGF, further demonstrating the role of Ets-1 in regulating these two target genes involved in the pathological process of cardiac fibrosis. Collectively, our experiments suggest that Ets-1 may either promote CFs growth or induce the expression of genes that regulate the development of fibrosis, such as PAI-1 and CTGF.
Finally, we used Ang II-infused rats as the animal model to further elucidate the role of Ets-1 in Ang II-induced cardiac fibrosis in vivo. Previous study has described the elevation of vascular Ets-1 and its role in vascular inflammation and remodeling in this model [34], but its expression status and its functions in the heart have not been well investigated yet. Our data demonstrated that systemic infusion of Ang II into rats, which did not affect SBP in 3 days, caused a marked induction of left ventricular Ets-1, and lasted at least 7 days, when SBP was significant increased. The inconsistent changes between SBP and Ets-1 indicate that the induced level of Ets-1 is most likely due to the direct cellular effects of Ang II and may be independent of pressurization. This notion is supported by our in vitro studies on CFs, which are not affected by pressurization or hemodynamic-induced changes, and by the result of the previous study demonstrating that Ets-1 mediated vascular remodeling is not dependent on alteration of blood pressure in response to Ang II [34]. Previous studies have shown that Ang II infusion lead to an increase in cardiac PAI-1 [1] and CTGF [11]. Our experiments further demonstrated that this increase was concurrent with the induction of Ets-1. These results are similar with our in vitro study, suggesting that the increase in PAI-1 and CTGF by Ang II may be partly due to the induction of Ets-1. Because PAI-1 and CTGF favor ECM deposition in tissue fibrosis, the induction of these two parameters may contribute the increase in collagen content and the ratio of left ventricle weight/body weight. More importantly, in consistent with our in vitro studies, we also found that the upregulation of Ets-1, PAI-1 and CTGF was accompanied with an increase in phosphorylation of JNK and ERK. It has been shown previously that Ang II-infusion cause activation of left ventricular JNK and ERK [33]. These results suggest that Ang II-induced Ets-1 may be mediated by activation of JNK and ERK. Thus, our in vivo findings indicate that Ets-1 may participate in the pathological possess of cardiac fibrosis by being regulated by Ang II via several signaling pathways such as JNK and ERK.
In conclusion, the present study demonstrates that Ang II induces Ets-1 expression in CFs via AT1 and activation of PKC, ERK and JNK signaling pathways, and that Ets-1 may be a vital mediator in Ang II-induced cardiac fibrosis by regulating CFs proliferation, PAI-1 and CTGF production. These findings point to a potential role of Ets-1 in cardiac fibrotic remodeling, and may advance our basic understanding of the molecular mechanisms of this pathological process.
Acknowledgements
This study was supported by the grants from National Science Foundation of China (No. 30900693 to GHH) and the grants from Science and Technology Developing Project of Shaanxi (No. 2015SF021 to ZHH).
Disclosure of conflict of interest
None.
References
- 1.Abrahamsen CT, Pullen MA, Schnackenberg CG, Grygielko ET, Edwards RM, Laping NJ, Brooks DP. Effects of angiotensins II and IV on blood pressure, renal function, and PAI-1 expression in the heart and kidney of the rat. Pharmacology. 2002;66:26–30. doi: 10.1159/000063252. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed MS, Oie E, Vinge LE, Yndestad A, Oystein Andersen G, Andersson Y, Attramadal T, Attramadal H. Connective tissue growth factor--a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. J Mol Cell Cardiol. 2004;36:393–404. doi: 10.1016/j.yjmcc.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Chen CC, Ferreri NR. Epithelium-specific ETS-1: a counter-regulatory factor against vascular dysfunction and inflammation. Am J Hypertens. 2010;23:1252. doi: 10.1038/ajh.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czuwara-Ladykowska J, Sementchenko VI, Watson DK, Trojanowska M. Ets1 is an effector of the transforming growth factor beta (TGF-beta ) signaling pathway and an antagonist of the profibrotic effects of TGF-beta. J Biol Chem. 2002;277:20399–408. doi: 10.1074/jbc.M200206200. [DOI] [PubMed] [Google Scholar]
- 5.Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA. Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf) 2009;195:321–38. doi: 10.1111/j.1748-1716.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 6.Di J, Jiang L, Zhou Y, Cao H, Fang L, Wen P, Li X, Dai C, Yang J. Ets-1 targeted by microrna-221 regulates angiotensin II-induced renal fibroblast activation and fibrosis. Cell Physiol Biochem. 2014;34:1063–74. doi: 10.1159/000366321. [DOI] [PubMed] [Google Scholar]
- 7.Fujiu K, Nagai R. Fibroblast-mediated pathways in cardiac hypertrophy. J Mol Cell Cardiol. 2014;70:64–73. doi: 10.1016/j.yjmcc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Goetze S, Kintscher U, Kaneshiro K, Meehan WP, Collins A, Fleck E, Hsueh WA, Law RE. TNFalpha induces expression of transcription factors c-fos, Egr-1, and Ets-1 in vascular lesions through extracellular signal-regulated kinases 1/2. Atherosclerosis. 2001;159:93–101. doi: 10.1016/s0021-9150(01)00497-x. [DOI] [PubMed] [Google Scholar]
- 9.Hahne JC, Okuducu AF, Kaminski A, Florin A, Soncin F, Wernert N. Ets-1 expression promotes epithelial cell transformation by inducing migration, invasion and anchorage-independent growth. Oncogene. 2005;24:5384–8. doi: 10.1038/sj.onc.1208761. [DOI] [PubMed] [Google Scholar]
- 10.Hahne JC, Okuducu AF, Sahin A, Fafeur V, Kiriakidis S, Wernert N. The transcription factor ETS-1: its role in tumour development and strategies for its inhibition. Mini Rev Med Chem. 2008;8:1095–105. doi: 10.2174/138955708785909934. [DOI] [PubMed] [Google Scholar]
- 11.He Z, Way KJ, Arikawa E, Chou E, Opland DM, Clermont A, Isshiki K, Ma RC, Scott JA, Schoen FJ, Feener EP, King GL. Differential regulation of angiotensin II-induced expression of connective tissue growth factor by protein kinase C isoforms in the myocardium. J Biol Chem. 2005;280:15719–26. doi: 10.1074/jbc.M413493200. [DOI] [PubMed] [Google Scholar]
- 12.Kim NN, Villarreal FJ, Printz MP, Lee AA, Dillmann WH. Trophic effects of angiotensin II on neonatal rat cardiac myocytes are mediated by cardiac fibroblasts. Am J Physiol. 1995;269:E426–37. doi: 10.1152/ajpendo.1995.269.3.E426. [DOI] [PubMed] [Google Scholar]
- 13.Kurdi M, Booz GW. New take on the role of angiotensin II in cardiac hypertrophy and fibrosis. Hypertension. 2011;57:1034–8. doi: 10.1161/HYPERTENSIONAHA.111.172700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leask A, Chen S, Pala D, Brigstock DR. Regulation of CCN2 mRNA expression and promoter activity in activated hepatic stellate cells. J Cell Commun Signal. 2008;2:49–56. doi: 10.1007/s12079-008-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Zang W, Liu P, Wang Y, Du Y, Chen X, Deng M, Sun W, Wang L, Zhao G, Zhai B. MicroRNA-124 inhibits cellular proliferation and invasion by targeting Ets-1 in breast cancer. Tumour Biol. 2014;35:10897–904. doi: 10.1007/s13277-014-2402-2. [DOI] [PubMed] [Google Scholar]
- 16.Liu MY, Eyries M, Zhang C, Santiago FS, Khachigian LM. Inducible platelet-derived growth factor D-chain expression by angiotensin II and hydrogen peroxide involves transcriptional regulation by Ets-1 and Sp1. Blood. 2006;107:2322–9. doi: 10.1182/blood-2005-06-2377. [DOI] [PubMed] [Google Scholar]
- 17.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–13. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 18.Mizui M, Isaka Y, Takabatake Y, Sato Y, Kawachi H, Shimizu F, Takahara S, Ito T, Imai E. Transcription factor Ets-1 is essential for mesangial matrix remodeling. Kidney Int. 2006;70:298–305. doi: 10.1038/sj.ki.5001541. [DOI] [PubMed] [Google Scholar]
- 19.Mouly E, Chemin K, Nguyen HV, Chopin M, Mesnard L, Leite-de-Moraes M, Burlen-defranoux O, Bandeira A, Bories JC. The Ets-1 transcription factor controls the development and function of natural regulatory T cells. J Exp Med. 2010;207:2113–25. doi: 10.1084/jem.20092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naito S, Shimizu S, Maeda S, Wang J, Paul R, Fagin JA. Ets-1 is an early response gene activated by ET-1 and PDGF-BB in vascular smooth muscle cells. Am J Physiol. 1998;274:C472–80. doi: 10.1152/ajpcell.1998.274.2.C472. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura Y, Esnault S, Maeda T, Kelly EA, Malter JS, Jarjour NN. Ets-1 regulates TNF-alpha-induced matrix metalloproteinase-9 and tenascin expression in primary bronchial fibroblasts. J Immunol. 2004;172:1945–52. doi: 10.4049/jimmunol.172.3.1945. [DOI] [PubMed] [Google Scholar]
- 22.Nakerakanti SS, Kapanadze B, Yamasaki M, Markiewicz M, Trojanowska M. Fli1 and Ets1 have distinct roles in connective tissue growth factor/CCN2 gene regulation and induction of the profibrotic gene program. J Biol Chem. 2006;281:25259–69. doi: 10.1074/jbc.M600466200. [DOI] [PubMed] [Google Scholar]
- 23.Ni W, Zhan Y, He H, Maynard E, Balschi JA, Oettgen P. Ets-1 is a critical transcriptional regulator of reactive oxygen species and p47 (phox) gene expression in response to angiotensin II. Circ Res. 2007;101:985–94. doi: 10.1161/CIRCRESAHA.107.152439. [DOI] [PubMed] [Google Scholar]
- 24.Pearse DD, Tian RX, Nigro J, Iorgulescu JB, Puzis L, Jaimes EA. Angiotensin II increases the expression of the transcription factor ETS-1 in mesangial cells. Am J Physiol Renal Physiol. 2008;294:F1094–100. doi: 10.1152/ajprenal.00458.2007. [DOI] [PubMed] [Google Scholar]
- 25.Santiago FS, Khachigian LM. Ets-1 stimulates platelet-derived growth factor A-chain gene transcription and vascular smooth muscle cell growth via cooperative interactions with Sp1. Circ Res. 2004;95:479–87. doi: 10.1161/01.RES.0000141135.36279.67. [DOI] [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 27.Simon DI, Simon NM. Plasminogen activator inhibitor-1: a novel therapeutic target for hypertension? Circulation. 2013;128:2286–8. doi: 10.1161/CIRCULATIONAHA.113.006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taniguchi H, Fujiwara Y, Doki Y, Sugita Y, Sohma I, Miyata H, Takiguchi S, Yasuda T, Tomita N, Morishita R, Monden M. Gene therapy using ets-1 transcription factor decoy for peritoneal dissemination of gastric cancer. Int J Cancer. 2007;121:1609–17. doi: 10.1002/ijc.22870. [DOI] [PubMed] [Google Scholar]
- 29.Tao H, Yang JJ, Shi KH, Deng ZY, Li J. DNA methylation in cardiac fibrosis: new advances and perspectives. Toxicology. 2014;323:125–9. doi: 10.1016/j.tox.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Van Beek JP, Kennedy L, Rockel JS, Bernier SM, Leask A. The induction of CCN2 by TGF-beta1 involves Ets-1. Arthritis Res Ther. 2006;8:R36. doi: 10.1186/ar1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verschoor ML, Wilson LA, Verschoor CP, Singh G. Ets-1 regulates energy metabolism in cancer cells. PLoS One. 2010;5:e13565. doi: 10.1371/journal.pone.0013565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe D, Takagi H, Suzuma K, Suzuma I, Oh H, H Ohashi, Kemmochi S, Uemura A, Ojima T, Suganami E, Miyamoto N, Sato Y, Honda Y. Transcription factor Ets-1 mediates ischemia- and vascular endothelial growth factor-dependent retinal neovascularization. Am J Pathol. 2004;164:1827–35. doi: 10.1016/S0002-9440(10)63741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yano M, Kim S, Izumi Y, Yamanaka S, Iwao H. Differential activation of cardiac c-jun amino-terminal kinase and extracellular signal-regulated kinase in angiotensin II-mediated hypertension. Circ Res. 1998;83:752–60. doi: 10.1161/01.res.83.7.752. [DOI] [PubMed] [Google Scholar]
- 34.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508–16. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]


