Abstract
More and more evidence has confirmed that dysregulation of microRNAs (miRNAs) can conduce to the progression of human cancers. Previous studied have shown that dysregulation of miR-135b is in varieties of tumors. However, the roles of miR-135b in cervical cancer remain unknown. Therefore, our aim of this study was to explore the biological function and molecular mechanism of miR-135b in cervical cancer cell lines, discussing whether it could be a therapeutic biomarker of cervical cancer in the future. The MTT assay and ELISA-Brdu assay were used to assess cell proliferation. Cell cycle was detected by flow cytometry. Real-time quantitative polymerase chain reaction (PCR) and Western blot analyses were used to detect expressions of cyclin D1, p21, p27 and FOXO1. In our study, we found that miR-135b is up-regulated in cervical cancer cell lines. Down-regulation of miR-135b evidently inhibited proliferation and arrested cell cycle in cervical cancer cells. Bioinformatics analysis predicted that the FOXO1 was a potential target gene of miR-135b. Besides, miR-135b inhibition significantly increased expressions of the cyclin-dependent kinase inhibitors, p21/CIP1 and p27/KIP1, and decreased expression of cyclin D1. However, the high level of miR-135b was associated with increased expression of FOXO1 in cervical cancer cells. Further study by luciferase reporter assay demonstrated that miR-135b could directly target FOXO1. Down-regulation of FOXO1 in cervical cancer cells transfected with miR-135b inhibitor partially reversed its inhibitory effects. In conclusion, down-regulation of miR-135b inhibited cell growth in cervical cancer cells by up-regulation of FOXO1.
Keywords: Cervical cancer, miR-135b, FOXO1, proliferation, cell cycle
Introduction
In the past two decades, it has been reported that the most crucial cancer is cancer of the cervix among women [1]. Recent data from the National Cancer Registry Program (NCRP) also shows that the breasts and the cervix are the most common sites of cancer among women [1]. Moreover, in developing countries, the commonest cancer cause of death among women is cervical cancer (CC) [2]. Mortality due to cervical cancer is also an indicator of health inequities, because 86% of all deaths [3] caused by cervical cancer are in developing, low- and middle-income countries [4]. So far, surgery and radiotherapy are still the major treatment for CC. Besides, chemotherapy is used to treat patients with metastasis or recurrence at times [5]. In the recent decades, although some causes of CC have been revealed [6], its precise mechanisms are still largely unknown. Consequently, further researches on the molecular pathogenesis of CC and finding available biomarkers were useful to better forecast the cancer prognosis.
Accumulated studies have reported that microRNAs (miRNAs) are small (about 22 nucleotides in length), non-coding RNAs [7], and play important roles in regulation of the biological and pathologic processes [8]. They generally function as crucial gene regulators. Moreover, several reports have showed that miRNAs are involved in tumorigenesis and metastasis by targeting many types of molecules [9]. In recent years, it is reported that a wide variety of miRNAs are aberrantly expressed in multiple cancers such as cervical cancer. miR-491-5p is down-regulated in cervical cancer tissues and suppresses growth of cervical cancer cells by targeting human telomerase reverse transcriptase [10]. miR-142-3p is down-regulated in cervical cancer cells and inhibits cell proliferation and invasion by targeting Frizzled7 receptor (FZD7) [11]. miR-342-3p acts as a tumor suppressor and inhibits growth of cervical cancer cell by directly targeting FOXM1 [12]. These three miRNAs act as tumor suppressor. However, some oncogene miRNAs were also studied in cervical cancer. For example, miR-155 promotes cervical cancer cell proliferation via inhibition of its target gene LKB1 [13]. miR10a was significantly increased in primary tumor tissues in patients with positive lymph node metastasis, and markedly promotes migration and invasion abilities of cervical cancer cells by targeting phosphatase and tensin homologue (PTEN) [14]. miR-92a is involved in the regulation of F-box and WD repeat domain-containing 7 (FBXW7) to promote CC cell proliferation and invasion [15].
MiR-135b has been involved in regulators of many cellular processes such as cell growth and metastasis [16]. Recently, miR-135b was considered as oncogene and up-regulated in a variety of human tumors [17-19]. Li et al. reported that miR-135b promoted progression of colorectal cancer by targeting transforming growth factor beta receptor II [17]. Furthermore, miR-135b was up-regulated in cutaneous squamous cell carcinoma, and increased cancer cell motility and invasiveness by down-regulation of leucine zipper tumor suppressor 1 (LZTS1) [18]. Wu and his colleagues demonstrated that miR-135b acted as a oncogene through promoting migration and invasion in colorectal cancer by regulation of metastasis suppressor-1 (MTSS1) [19].
In this paper, we determined frequent up-regulation of miR-135b in cervical cancer cell lines. Suppression of miR-135b inhibited cell growth of cervical cancer cells. Moreover, we found that FOXO1 was the direct target of miR-135b in cervical cancer. Down-regulation of FOXO1 reversed the inhibitory effects of miR-135b inhibition. Therefore, our results showed important roles for miR-135b in the pathogenesis of cervical cancer and suggested its possible application in tumor treatment.
Materials and methods
Cell culture
Cervical cancer cells such as C33A, HCC94, HeLa, HT-3, SiHa and CaSKi were grown in DMEM (Gibco, New York, USA) containing 10% fetal bovine serum (FBS; Gibco, New York, USA) and 1% streptomycin-penicillin (Invitrogen, Carlsbad, CA). A human normal cervical epithelium cell line End1/E6E7 was cultured in keratinocyte serum-free medium (Gibco, New York, USA) supplemented with 0.1 ng/ml human recombinant epithelial growth factor (PeproTech Inc, USA), 0.05 mg/ml bovine pituitary extract (Santa Cruz, USA), 1% streptomycin-penicillin at 37°C in 5% CO2.
miRNA transfection
To inhibit miR-135b expression in HT-3 and SiHa cells, HT-3 and SiHa cells were transfected with miR-135b inhibitor, which served as the anti-miR-135b group. HT-3 and SiHa cells transfected with anti-miR-negative control (anti-miR-NC) were used as anti-miR-NC group. After transfection for 24 h, cells at about 40 to 60% confluency were changed to the antibiotic-free medium. After 24 h, the cells were transfected with 50 nM miR-135b inhibitor using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol.
Quantitative real-time polymerase chain reaction (PCR)
The total RNA was isolated from human normal cervical epithelium cell and cervical cancer cells using Trizol reagent (Invitrogen). Next, Taqman miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) was used to perform reverse transcription according to the manufacturer’s protocols. The primers used for PCR were as follows: p21 forward primer: 5’-TGTCCGTCAGAACCCATGC-3’; reverse primer: 5’-AAAGTCGAAGTTCCATCGCTC-3’; p27 forward primer: 5’-TAATTGGGGCTCCGGCTAACT-3’; reverse primer: 5’-TGCAGGTCGCTTCCTTATTCC-3’; cyclin D1 forward primer: 5’-TGAGA-GAAAAAGGTCCTACG-3’; reverse primer: 5’-GTAGCAGCTACTGTAGACAG-3’; FOXO1 forward primer: 5’-TCGTCATAATCTGTCCCTACACA-3’; reverse primer: 5’-CGGCTTCGGCTCTTAGCAAA-3’; GAPDH forward primer: 5’-GAAGGTGAAGGTCGGAGT-3’; reverse primer: 5’-GAAGATGGTGATGGGATTTC-3’. PCR reactions were performed on Applied Biosystems 7500 real-time PCR system with the following conditions: 95°C, 10 min for 1 cycle; then 95°C, 25 sec; and 85°C for 30 sec, for 35 cycles. The mRNA expression of miR-135b was determined on Applied Biosystems 7500 real-time PCR system with the U6 small nuclear RNA used as an internal control. Each experiment was repeated three times.
Cell viability assay
HT-3 and SiHa cells (1×104 cells/100 μl) were seeded in 96-well plates for 24 h. After that, cells were transfected with miR-135b inhibitor and anti-miR-NC for 24 h. Then, the number of viable cells was determined using 3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide (MTT) reagent (Sigma Chemical Co., USA) following manufacturer’s instructions. Briefly, 10 μl MTT reagent (5 mg/ml) was added to the 100 μL medium, and incubated at 37°C for 4 h. The medium was removed and dimethyl sulfoxide (DMSO) was added (150 μl/well) to solubilize the formazan crystals. OD values of the medium at 490 nm were measured with Biotek Elx-800 plate reader.
Cell proliferation assay
To explore the effect of anti-miR-135b transfection on proliferation of HT-3 and SiHa cells, 1×104 cells were seeded in 96-well plate and allowed to grow overnight in complete DMEM medium. The medium was then removed and the cells were transfected with anti-miR-135b and anti-miR-NC for 24 h at 37°C. Cell Proliferation ELISA-BrdU (colorimetric) Kit (Roche Diagnostics, USA) was used to detect the cells proliferation following the manufacturer’s protocols.
Cell cycle analysis
The HT-3 and SiHa cells were transfected with miR-135b inhibitor or anti-miR-NC for 24 h. Then, HT-3 and SiHa cells were collected by trypsinization, washed with ice-cold PBS, and fixed in ice-cold 70% methanol by incubating them for 1 h at 4°C. After that, cells were centrifuged, resuspended in ice-cold PBS, and incubated with RNase (Sigma) for 30 min at 37°C, and then were incubated with propidium iodide (PI; Sigma Chemical Co., USA) at room temperature for 30 min. The cell cycle was analyzed by FACScan flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot analysis
To extract the proteins, cells were washed twice in cold PBS, and then lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China) with protease inhibitor cocktail (Merk, Germany). The protein concentration of cell lysates was measured using the BCA kit (Beyotime Institute of Biotechnology, Jiangsu, China), and protein samples (50 μg/samlpe) were separated by 10% SDS-PAGE and then transferred onto a PVDF membrane (Millipore, USA). The membranes were blocked in 5% skimmed milk diluted with TBST at room temperature for 1 h, and then probed overnight at 4°C with anti-mouse p21 (1:1000; cat. no. 2946; Cell Signaling Technology; USA), anti-mouse p27 (1:1000; cat. no. 3698; Cell Signaling Technology; USA), anti-rabbit cyclin D1 antibody (1:1000; cat. no. 2978; Cell Signaling Technology; USA), anti-rabbit FoxO1 antibody (1:1000; cat. no. 9454; Cell Signaling Technology; USA). The membranes were then incubated for 2 h with a goat anti-rabbit (1:1000; cat. no. 14708; Cell Signaling Technology; USA) or anti-mouse (1:1000; cat. no. 14709; Cell Signaling Technology; USA) IgG conjugated to horseradish peroxidase. Monoclonal mouse α-tubulin antibody (1:1000; cat. no. T5168; Sigma; USA) was used as an internal control. The proteins were visualized using ECL chemiluminescence reagents (Amersham Biosciences Corp., USA). The densitometry of the bands was determined using the Image J software (USA).
Luciferase reporter assay
HT-3 and SiHa cells (1×105/well) were seeded in 24-well plates and incubated for 24 h before transfection. Cells were cotransfected with pMir-FOXO1-3’UTR wild-type or mutant reporter plasmid, miR-135b inhibitor or anti-miR-NC, and pRL-TK Renilla plasmid (Promega, USA) using Lipofectamine 2000. At 24 h after transfection, both firefly and renilla luciferase activities were quantified using a dual luciferase reporter system (Promega, USA) according to the manufacturer’s instructions. All experiments were performed in triplicate.
Small interfering RNA (siRNA) transfection
The siRNA against the human FOXO1 gene (Dharmacon, USA) were transiently transfected with Lipofectamine 2000 Reagent (Invitrogen, USA) according to the manufacturer’s instructions. A negative siRNA was used as a negative control. The siRNA sequences were as follows: 5’-CUGGAUCACAGUUUUCCAAAUG-3’ (FOXO1) and 5’-GCAAGCUGACCCUGAAGUUCAU-3’ (negative). 48 hours later, cells were used for proliferation assay and Western blot.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, Inc., USA). Data for each study parameter from each group were presented as mean ± standard error of the mean (S.E.M.). Data from each group were statistically analyzed by a two-tailed Student’s t test or one-way analysis of variance (ANOVA). P<0.05 was considered to indicate a statistically significant difference.
Results
MiR-135b was up-regulated in cervical cancer cell lines
To detect levels of miR-135b expression in cervical cancer cells, we used real-time PCR to detect the miR-135b expression in a human normal cervical epithelium cell line (End1/E6E7) and six cervical cancer cell lines such as C33A, HCC94, HeLa, HT-3, SiHa and CaSKi. Our results showed that miR-135b was evidently up-regulated in all six cervical cell lines compared to that in End1/E6E7 (Figure 1). These results indicated that miR-135b was increased in cervical cancer.
Figure 1.
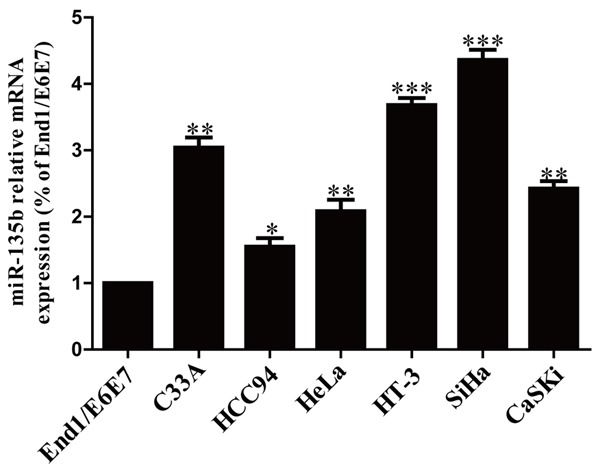
The mRNA level of miR-135b in cervical cancer (CC) cell lines. The relative expression of miR-135b in cervical cancer cell lines and human normal cervical epithelium cell line End1/E6E7 was determined by real-time PCR. U6 was detected as a loading control. All data are presented as mean ± SEM, n=6. *P<0.05, **P<0.01, ***P<0.001 vs. End1/E6E7.
Downregulation of miR-135b inhibited viability and proliferation in both HT-3 and SiHa cells
Among these cervical cancer cell lines, HT-3 and SiHa cells were used to study further. The results from real time-PCR analysis showed that the mRNA level of miR-135b was decreased in miR-135 inhibitor (anti-miR-135b) group compared to anti-miR-negative control (anti-miR-NC) group (Figure 2A). To determine the role of miR-135b in growth of cervical cancer cells, HT-3 and SiHa cells were transfected with anti-miR-135b or anti-miR-NC. The results from MTT assay demonstrated that suppression of miR-135b significantly reduced the viabilities of HT-3 and SiHa cells (Figure 2B). Besides, we also observed anti-proliferative effect in cells transfected with anti-miR-135b, as assessed by the Brdu-ELISA assay (Figure 2C). These data suggested that down-regulation of miR-135b had available anti-proliferative effect in both HT-3 and SiHa cells.
Figure 2.
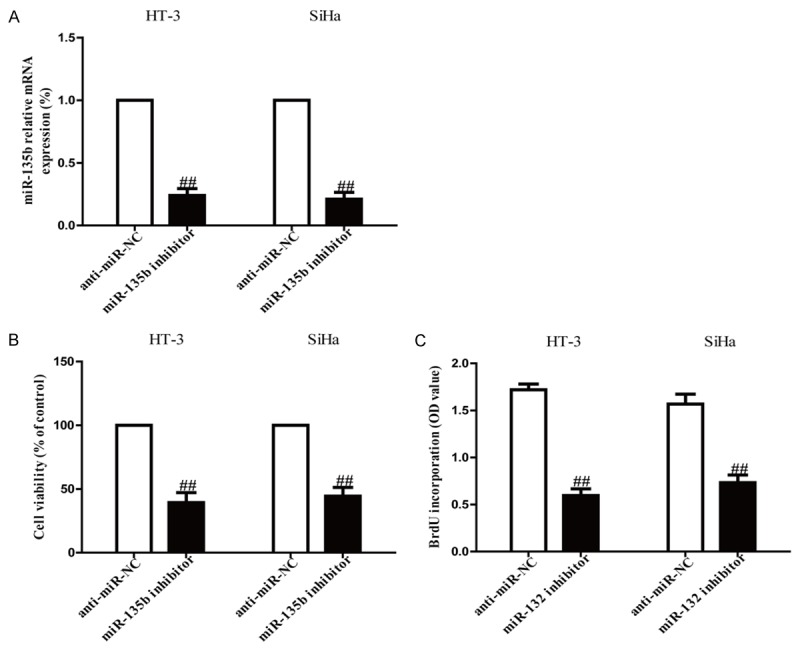
Effects of miR-135b inhibition on cell viability and proliferation in HT-3 and SiHa cells. HT-3 and SiHa cells were transfected with anti-miR-135b inhibitor or anti-miR-NC for 24 h. A. The mRNA level of miR-135b in HT-3 and SiHa cells was detect by real-time PCR. U6 was detected as a loading control. B. Cell viability was assessed by CCK-8 assay. C. Cell proliferation was assessed by BrdU-ELISA assay. All data are presented as mean ± SEM, n=6. ##P<0.01 vs. anti-miR-NC.
Suppression of miR-135b markedly arrested cell cycle progression in cervical cancer cells
Because miR-135b inhibitor evidently restrained proliferation of HT-3 and SiHa cells, we speculated that inhibition of miR-135b could arrest the cell cycle of cervical cancer cells. We demonstrated this tentative by flow cytometry. We found that down-regulation of miR-135b significantly increased the percentage of cells in the G1 phase and decreased the percentage of cells in the S phase in both HT-3 and SiHa cells compared with cells transfected with anti-miR-NC (Figure 3A). Thus, inhibition of miR-135b might suppress the proliferation of cervical cancer cells by impeding the G1/S cell cycle transition. To confirm these results, we examined the effects of miR-135b inhibition on the expressions of p21, p27 and cyclin D1, which were known as critical molecules involved in cell cycle arrest. As shown in Figure 3B and 3C, the mRNA levels and protein expressions of the cyclin-dependent kinase inhibitors such as p21 and p27, were increased in HT-3 and SiHa cells transfected with miR-135b inhibitor. Besides, cyclin D1 is a nuclear protein required for cell cycle regulation in the G1 phase of proliferating cells. The mRNA level and protein expression of cyclin D1 were evidently decreased by down-regulation of miR-135b (Figure 3B and 3C). These results indicated that miR-135b inhibition caused growth arrest in HT-3 and SiHa cells by regulating expressions of cell cycle genes.
Figure 3.
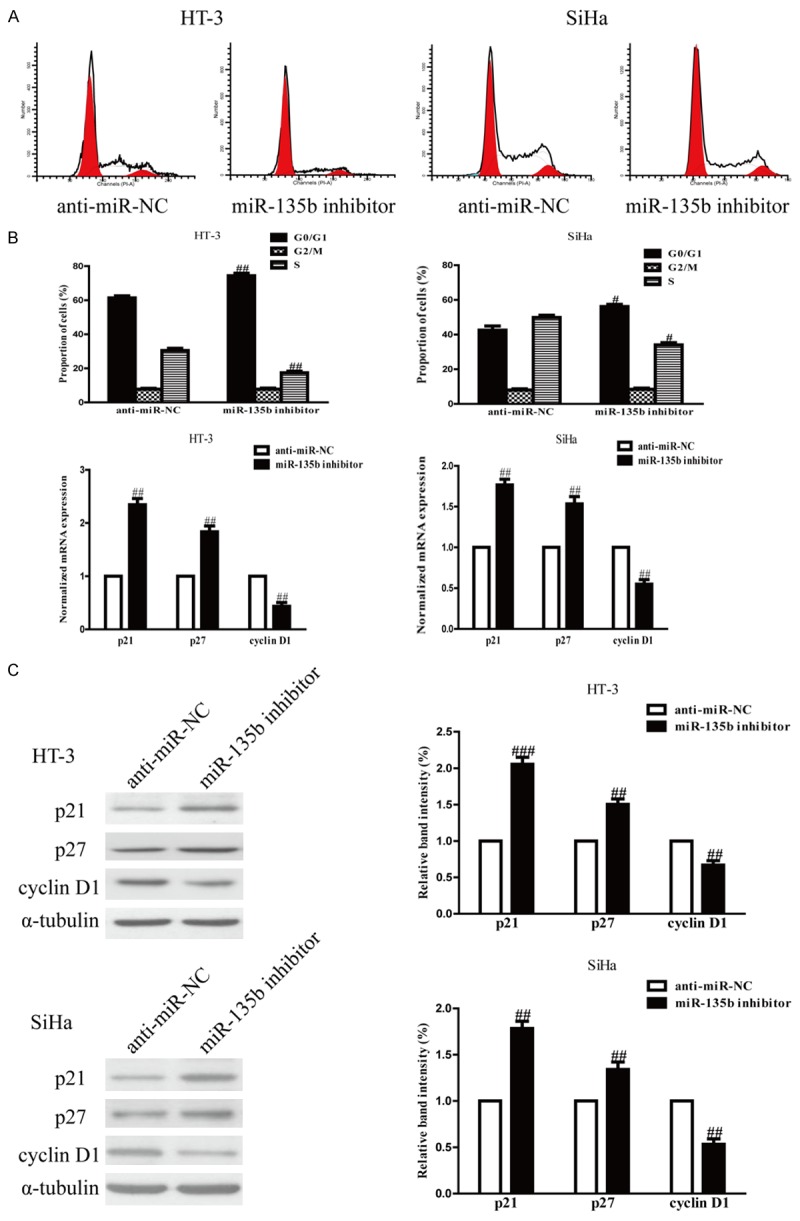
Effects of miR-135b inhibition on cell cycle and its related protein in HT-3 and SiHa cells. HT-3 and SiHa cells were transfected with miR-135b inhibitor or anti-miR-NC. A. Cell cycle was detected by flow cytometry. B. The mRNA levels of p21 and p27 and cyclin D1 were determined by real-time PCR. GAPDH was detected as a loading control. C. The protein expressions of p21 and p27 and cyclin D1 were determined by Western blot. α-tubulin was detected as a loading control. All data are presented as mean ± SEM, n=6. #P<0.05, ##P<0.01 vs. anti-miR-NC.
FOXO1 is a direct target of miR-135b in cervical cancer cells. Since FOXO1 was a binding target of miR-135b predicted by the online database, TargetScan 6.2, we performed RT-PCR and Western blotting to detect the expression of FOXO1 on mRNA and protein levels in HT-3 and SiHa cells transfected with miR-135b inhibitor. Our outcomes indicated that the mRNA and protein levels of FOXO1 were remarkably increased after down-regulation of miR-135b (Figure 4A and 4B). To further confirm whether FOXO1 was a direct target of miR-135b, FOXO1 3’-UTR was cloned into a luciferase reporter vector and the putative miR-135b binding site in the FOXO1 3’-UTR was mutated (Figure 4C). The effect of miR-135b was detected by luciferase reporter assay. The results showed that down-regulation of miR-135b significantly enhanced the luciferase activity of pMir-FOXO1 3’-UTR WT (Figure 4D). Mutation of the miR-135b-binding site in the FOXO1 3’-UTR abolished the effect of miR-135b, which suggested that FOXO1 was directly and negatively regulated by miR-135b.
Figure 4.
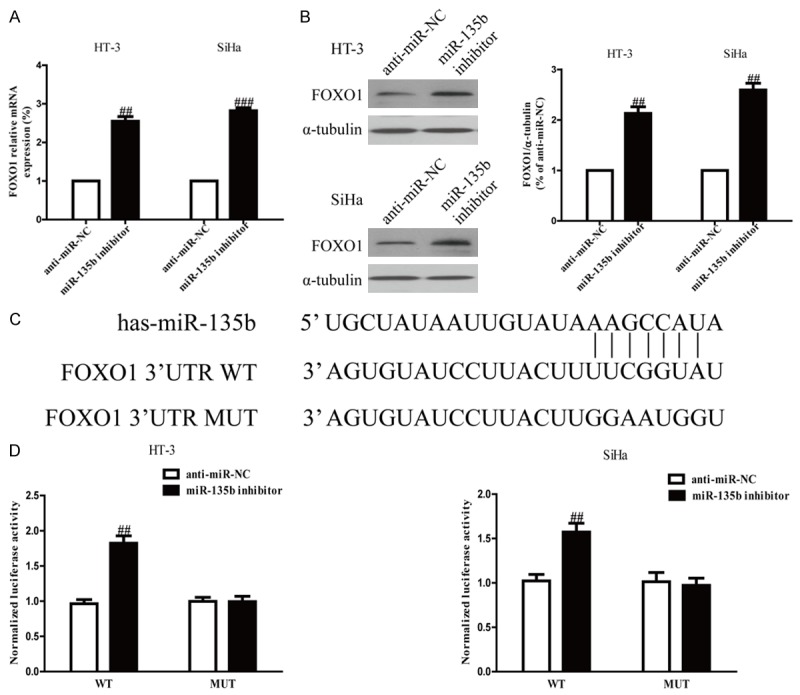
FOXO1 was a direct target of miR-135b. HT-3 and SiHa cells were transfected with miR-135b inhibitor or anti-miR-NC for 24 h. A. The mRNA level of FOXO1 was determined by real time-PCR. GAPDH was also detected as a loading control. B. The protein expression of FOXO1 was determined by Western blot. α-tubulin was detected as a loading control. C. Schematic representation of FOXO1 3’UTRs showing putative miRNA target site. D. The analysis of the relative luciferase activities of FOXO1-WT, FOXO1-MUT in CC cells. All data are presented as mean ± SEM, n=6. ##P<0.01 vs. anti-miR-NC.
Overexpression of FOXO1 is essential for inhibition of cell growth in cervical cancer cells by down-regulation of miR-135
To confirm whether down-regulation of miR-135b inhibited the growth of cervical cancer cells in a FOXO1-dependent manner, we cotransfected HT-3 and SiHa cells with miR-135b inhibitor and siRNA against FOXO1 (Figure 5A). Results from Brdu-ELISA assay indicated that down-regulation of FOXO1 in cells transfected with the miR-135b inhibitor promoted the growth of cervical cancer cells (Figure 5B). Furthermore, decreased FOXO1 expression down-regulated p21, p27 and up-regulated cyclin D1 at mRNA and protein levels in HT-3 and SiHa cells transfected with miR-135b inhibitor (Figure 5C and 5D). Therefore, down-regulation of FOXO1 could reverse anti-proliferative effect of miR-135b inhibition. Our findings clearly showed that down-regulation of miR-135b inhibited cell growth in cervical cancer cells by up-regulation of FOXO1.
Figure 5.
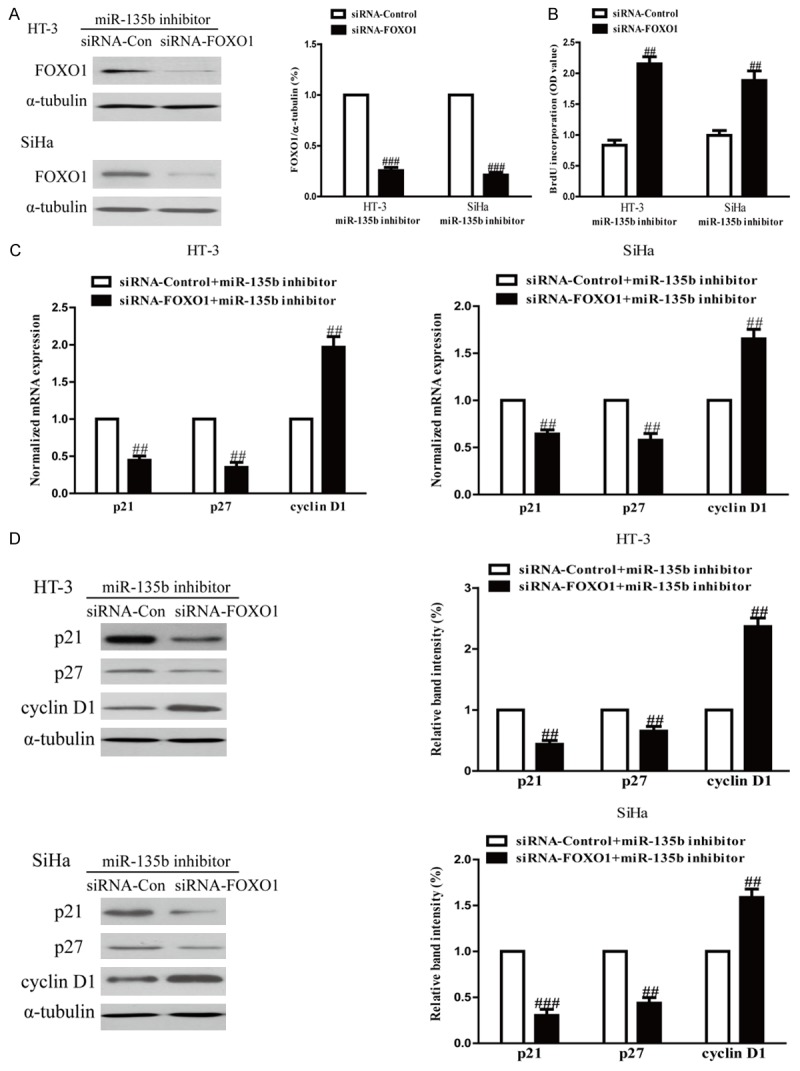
Down-regulation of FOXO1 partially rescued cell proliferation inhibited by down-regulation of miR-135b in CC cells. HT-3 and SiHa cells were transfected with either miR-135 inhibitor with or without siRNA against FOXO1. A. The protein expression of FOXO1 was determined by Western blot. α-tubulin was detected as a loading control. B. Cell proliferation was assessed by BrdU-ELISA assay. C. The mRNA levels of p21 and p27 and cyclin D1 were determined by real-time PCR in HT-3 and SiHa cells, respectively. GAPDH was detected as a loading control. D. The expressions of p21 and p27 and cyclin D1 were determined by Western blotting in HT-3 and SiHa cells, respectively. α-tubulin was detected as a loading control. All data are presented as mean ± SEM, n=6. #P<0.05, ##P<0.01, ###P<0.001 vs. anti-miR-135b + FOXO1 siRNA.
Discussion
Recently, miRNAs have become key regulators involved in different biological processes such as cell proliferation, apoptosis, metastasis, transcriptional regulation and tumorigenesis [20]. miRNA dysregulation of cancers have provided crucial insights into the molecular pathogenesis of cancers [21]. Recently, several studies have reported that the dysregulation of miR-135b is associated with various human cancers [17-19]. More and more reports have shown that miR-135b is up-regulated and acts as an oncogene in various types of human cancers, such as in colon, colorectal, breast, prostate and lung cancers [22-25]. Zhang et al. found miR-135b acted as a tumor promoter and promoted cell proliferation, colony formation, survival and angiogenesis by targeting the hypoxia-inducible factor pathway in human head and neck squamous cell carcinoma [26]. Study from Aakula et al. showed miR-135b significantly decreased cell growth in breast and prostate cancer cells by regulation of estrogen receptor α (ERα), androgen receptor (AR) and hypoxia inducible factor 1 alpha (HIF1AN) [24]. Lin et al. demonstrated that miR-135b was up-regulated in highly invasive non-small-cell lung cancer cells and promoted cancer cell invasion and metastasis in vitro and in vivo [25].
In this paper, we found that the expression of miR-135b was up-regulated in cervical cancer cells (C33A, HCC94, HeLa, HT-3, SiHa and CaSKi) compared to human normal cervical epithelium cell line End1/E6E7. The expression model of miR-135b in cervical cancer cell lines in this study was consistent with that in osteosarcoma cell lines [26], which indicated that miR-135b might act as an oncogene. Hence, we believed that miR-135b could negatively regulate cancer cell proliferation. In order to investigate the role of miR-135b in cervical cancer cells, the expression of miR-135b was decreased by transfection with miR-135b inhibitor in HT-3 and SiHa cells. The MTT assay showed that the viability of HT-3 and SiHa cells was remarkably lower in anti-miR-135b group than that in anti-miR-NC group, which suggested that miR-135b inhibition could suppress the viability of HT-3 and SiHa cells. Analysis of cell cycle indicated that down-regulation of miR-135b could induce cell cycle arrest at G1 phase in HT-3 and SiHa cells, which was potentially due to an increase in growth inhibitory factors in the downstream of the miR-135b target genes. WAF1/p21 and KIP1/p27 proteins, members of the Cip/Kip family, are key regulators of the cell growth, both of which induce cell cycle arrest primarily at the G1/S transition of the cell cycle [27]. Therefore, we determined the expressions of p21 and p27 in this study. Our findings showed that the mRNA and protein levels of p21 and p27 were significantly increased in anti-miR-135b group, which suggested that miR-135b inhibition might be an important event in prohibiting progression of CC, partly due to its ability to enhance the expressions of p21 and p27, resulting in arresting cell cycle progression. Besides, cyclin D1 is a growth-promoting factor required for cell cycle regulation in the G1 phase of proliferating cells. Our results showed that down-regulation of miR-135b significantly decreased the expression of cyclin D1 in HT-3 and SiHa cells, which further proved miR-135b inhibition caused cell cycle arrest in G1 phase.
Forkhead box protein O1 (FOXO1), a member of the FOXO family of transcription factors, was characterized by the presence of a winged-helix DNA binding domain called a forkhead box [28]. Numerous studies have reported that FOXO1 plays important roles in different biological processes, including cell proliferation; cell cycle, apoptosis, and DNA damage repair [29-32]. For example, FOXO1 can induce cell cycle arrest by increasing the expression of p27 and p21, and decreasing the expression of cyclin D1 [33,34]. Growing evidence suggested the involvement of FOXO1 in many types of cancer and the regulation of FOXO1 by miRNAs has been reported elsewhere. For example, Wu et al. found that FOXO1 is a direct target of miR-223 and cell proliferation is greatly inhibited by overexpression of miR-223 in colorectal cancer, cervical cancer and hepatoma cells [35]. Besides, three microRNAs such as miR-153, miR-96, and miR-370 have all been found to directly target FOXO1 and regulate endogenous FOXO1 protein expression in prostate cancer cells, while down-regulation of these microRNAs leads to up-regulation of FOXO1 protein and inhibition of cell growth [36-38]. It is also known that mir-196a promotes cervical cancer proliferation through directly targeting FOXO1 and p27 (Kip1) [39], and upregulation of miR-107 induces proliferation of human gastric cancer cells by negatively regulating the expression of FOXO1 [40]. In this study, we found that FOXO1 showed a converse expression pattern of miR-135b. FOXO1 was subsequently identified as a direct target of miR-135b in CC cells using bioinformatics analysis. Furthermore, down-regulation of FOXO1 significantly promoted cervical cancer cells proliferation inhibited by down-regulation of miR-135b. Therefore, miR-135b exerted its role by inhibiting FOXO1 expression in CC cells.
In conclusion, we found that down-regulation of miR-135b was associated with up-regulation of FOXO1 in CC cells. Moreover, miR-135b acted as an oncogene. Down-regulation of miR-135b inhibited cell growth in CC cells by directly targeting FOXO1. These results collectively suggested that miR-135b-FOXO1 axis was considered as a promising prognostic and therapeutic target for future CC treatment.
Disclosure of conflict of interest
None.
References
- 1.Nandakumar A, Ramnath T, Chaturvedi M. The magnitude of cancer cervix in India. Indian J Med Res. 2009;130:219–221. [PubMed] [Google Scholar]
- 2.Denny L. Cervical cancer: prevention and treatment. Discov Med. 2012;14:125–131. [PubMed] [Google Scholar]
- 3.Arbyn M, Castellsague X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 4.Yeole BB, Kumar AV, Kurkureet A, Sunny L. Population-based survival from cancers of breast, cervix and ovary in women in Mumbai. Asian Pac J Cancer Prev. 2004;5:308–315. [PubMed] [Google Scholar]
- 5.Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C ESMO Guidelines Working Group. Cervical cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii27–32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 6.Castellsague X, Diaz M, de Sanjose S, Munoz N, Herrero R, Franceschi S, Peeling RW, Ashley R, Smith JS, Snijders PJ, Meijer CJ, Bosch FX. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98:303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. Micrornas: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T, Parenti AR, Daidone MG, Bicciato S, Piccolo S. A microRNA targeting diacer for metastasis control. Cell. 2010;141:1195–1207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM. Activation of the Wnt pathway in non small cell lung cancer: evidence of disheveled overexpression. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Q, Zhai YX, Liu HQ, Shi YA, Li XB. MicroRNA-491-5p suppresses cervical cancer cell growth by targeting hTERT. Oncol Rep. 2015;34:979–86. doi: 10.3892/or.2015.4013. [DOI] [PubMed] [Google Scholar]
- 11.Deng B, Zhang Y, Zhang S, Wen F, Miao Y, Guo K. MicroRNA-142-3p inhibits cell proliferation and invasion of cervical cancer cells by targeting FZD7. Tumour Biol. 2015;36:8065–73. doi: 10.1007/s13277-015-3483-2. [DOI] [PubMed] [Google Scholar]
- 12.Li XR, Chu HJ, Lv T, Wang L, Kong SF, Dai SZ. miR-342-3p suppresses proliferation, migration and invasion by targeting FOXM1 in human cervical cancer. FEBS Lett. 2014;588:3298–307. doi: 10.1016/j.febslet.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Lao G, Liu P, Wu Q, Zhang W, Liu Y, Yang L, Ma C. Mir-155 promotes cervical cancer cell proliferation through suppression of its target gene LKB1. Tumour Biol. 2014;35:11933–11938. doi: 10.1007/s13277-014-2479-7. [DOI] [PubMed] [Google Scholar]
- 14.Zeng T, Li G. MicroRNA-10a enhances the metastatic potential of cervical cancer cells by targeting phosphatase and tensin homologue. Mol Med Rep. 2014;10:1377–1382. doi: 10.3892/mmr.2014.2370. [DOI] [PubMed] [Google Scholar]
- 15.Zhou C, Shen L, Mao L, Wang B, Li Y, Yu H. miR-92a is upregulated in cervical cancer and promotes cell proliferation and invasion by targeting FBXW7. Biochem Biophys Res Commun. 2015;458:63–69. doi: 10.1016/j.bbrc.2015.01.066. [DOI] [PubMed] [Google Scholar]
- 16.Wu W, Wang Z, Yang P, Yang J, Liang J, Chen Y, Wang H, Wei G, Ye S, Zhou Y. MicroRNA-135b regulates metastasis suppressor 1 expression and promotes migration and invasion in colorectalcancer. Mol Cell Biochem. 2014;388:249–259. doi: 10.1007/s11010-013-1916-z. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Liang H, Bai M, Ning T, Wang C, Fan Q, Wang Y, Fu Z, Wang N, Liu R, Zen K, Zhang CY, Chen X, Ba Y. miR-135b Promotes Cancer Progression by Targeting Transforming Growth Factor Beta Receptor II (TGFBR2) in Colorectal Cancer. PLoS One. 2015;10:e0130194. doi: 10.1371/journal.pone.0130194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olasz EB, Seline LN, Schock AM, Duncan NE, Lopez A, Lazar J, Flister MJ, Lu Y, Liu P, Sokumbi O, Harwood CA, Proby CM, Neuburg M, Lazarova Z. MicroRNA-135b Regulates Leucine Zipper Tumor Suppressor 1 in Cutaneous Squamous Cell Carcinoma. PLoS One. 2015;10:e0125412. doi: 10.1371/journal.pone.0125412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Xu D, Bao C, Zhang Y, Chen D, Zhao F, Ding J, Liang L, Wang Q, Liu L, Li J, Yao M, Huang S, He X. MicroRNA-135b, a HSF1 target, promotes tumor invasion and metastasis by regulating RECK and EVI5 in hepatocellular carcinoma. Oncotarget. 2015;6:2421–2433. doi: 10.18632/oncotarget.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J, Sung JJ. MicroRNA dysregulation in gastric cancer: a new player enters the game. Oncogene. 2010;29:5761–5771. doi: 10.1038/onc.2010.352. [DOI] [PubMed] [Google Scholar]
- 21.Miao J, Wu S, Peng Z, Tania M, Zhang C. MicroRNAs in osteosarcoma: diagnostic and therapeutic aspects. Tumour Biol. 2013;34:2093–2098. doi: 10.1007/s13277-013-0940-7. [DOI] [PubMed] [Google Scholar]
- 22.Cascione L, Sumani KM, Veronese A, Fabbri M, Carasi S, Alder H, Lanza G, Gafa’ R, Moyer MP, Ridgway RA, Cordero J, Nuovo GJ, Frankel WL, Rugge M, Fassan M, Groden J, Vogt PK, Karin M, Sansom OJ, Croce CM. MicroRNA-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways incolon cancer. Cancer Cell. 2014;25:469–483. doi: 10.1016/j.ccr.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu XM, Qian JC, Deng ZL, Cai Z, Tang T, Wang P, Zhang KH, Cai JP. Expression of miR-21, miR-31, miR-96 and miR-135b is correlated with the clinical parameters of colorectal cancer. Oncol Lett. 2012;4:339–345. doi: 10.3892/ol.2012.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aakula A, Leivonen SK, Hintsanen P, Aittokallio T, Ceder Y, Børresen-Dale AL, Perälä M, Östling P, Kallioniemi O. MicroRNA-135b regulates ERα, AR and HIF1AN and affects breast and prostate cancer cell growth. Mol Oncol. 2015;9:1287–300. doi: 10.1016/j.molonc.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Sun ZJ, Bian Y, Kulkarni AB. MicroRNA-135b acts as a tumor promoter by targeting the hypoxia-inducible factor pathway in genetically defined mouse model of head and neck squamous cell carcinoma. Cancer Lett. 2013;331:230–238. doi: 10.1016/j.canlet.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pei H, Jin Z, Chen S, Sun X, Yu J, Guo W. MiR-135b promotes proliferation and invasion of osteosarcoma cells via targeting FOXO1. Mol Cell Biochem. 2015;400:245–252. doi: 10.1007/s11010-014-2281-2. [DOI] [PubMed] [Google Scholar]
- 27.Narla G, Friedman SL, Martignetti JA. Kruppel cripples prostate cancer: KLF6 progress and prospects. Am J Pathol. 2003;162:1047–1052. doi: 10.1016/S0002-9440(10)63901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 29.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, De Pinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 31.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 32.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 33.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 34.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Li H, Jia CY, Cheng W, Yu M, Peng M, Zhu Y, Zhao Q, Dong YW, Shao K, Wu A, Wu XZ. MicroRNA-223 regulates FOXO1 expression and cell proliferation. FEBS Lett. 2012;586:1038–1043. doi: 10.1016/j.febslet.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, He B, He J, Mao X. Upregulation of miR-153 promotes cell proliferation via downregulation of the PTEN tumor suppressor gene in human prostate cancer. Prostate. 2013;73:596–604. doi: 10.1002/pros.22600. [DOI] [PubMed] [Google Scholar]
- 37.Fendler A, Jung M, Stephan C, Erbersdobler A, Jung K, Yousef GM. The antiapoptotic function of miR-96 in prostate cancer by inhibition of FOXO1. PLoS One. 2013;8:e80807. doi: 10.1371/journal.pone.0080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Sun H, Zeng W, He J, Mao X. Upregulation of MircoRNA-370 induces proliferation in human prostate cancer cells by downregulating the transcription factor FOXO1. PLoS One. 2012;7:e45825. doi: 10.1371/journal.pone.0045825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou T, Ou J, Zhao X, Huang X, Huang Y, Zhang Y. MicroRNA-196a promotes cervical cancer proliferation through the regulation of FOXO1 and p27Kip1. Br J Cancer. 2014;110:1260–1268. doi: 10.1038/bjc.2013.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, Liu B, Gao Y, Liu Y, Xu Y, Tong W, Zhang A. Upregulation of microRNA-107 induces proliferation in human gastric cancer cells by targeting the transcription factor FOXO1. FEBS Lett. 2014;588:538–544. doi: 10.1016/j.febslet.2013.12.009. [DOI] [PubMed] [Google Scholar]


