Abstract
RIT1, (Ras-like without CAAX1), the founding member of a novel branch of the Ras subfamily, mediates a wide variety of cellular functions, including cell proliferation, survival, and differentiation, and it may play crucial oncogenic role in human cancer. The purpose of the current study was to characterize the expression pattern of RIT1 and assess the clinical significance of RIT1 expression in endometrial cancer patients. The mRNA and protein expression of RIT1 was significantly overexpressed in 7 endometrial cancer cell lines by qPCR and Western blot, respectively. In addition, RIT1 mRNA expression was elevated in 36 freshly frozen endometrial cancer tissues compared to 21 non-cancerous endometrial tissue samples. Similar results were observed by analyzing GEO datasets. Immunohistochemistry was used to examine the protein expression of RIT1 in two tissue microarrays containing 257 cases of tumor and 31 non-tumor tissues, which showed that elevated expression of RIT1 was significantly correlated with pathological type, clinical stage, grade and vascular invasion. Importantly, Kaplan-Meier survival analysis indicated that RIT1 expression was associated with overall survival of endometrial cancer patients. Multivariate Cox regression analysis revealed that RIT1 expression was one of the independent prognostic factors for endometrial cancer patients. Furthermore, RIT1 combined with other clinicopathological risk factors was a more significant model in ROC curve comparison. In conclusion, elevated expression of RIT1 may contribute to the progression of endometrial cancer and thus may serve as a novel prognostic marker and a promising molecular target for the treatment of endometrial cancer.
Keywords: RIT1, endometrial cancer, poor prognosis
Introduction
Endometrial cancer is the most common gynecologic malignancy of the female reproductive tract. Each year, more than 49,000 women were diagnosed with endometrial carcinoma in the United States. This accounts for about 6% of new cancer cases in woman annually and approximately 3% of all cancer deaths in women [1,2]. Early-stage patients have good prognosis. 5-year survival rates of patients with endometrial cancer are approximately 80% to 90%, mainly because the majority of women present with bleeding early on the disease course [3,4]. However, those women with advanced stage, poor differentiation, metastasis to regional nodes or high-risk histology may have poor prognosis, with 5-year survival rates of 57% for regional disease (stage III) and 19% for distant spread (stage IV), respectively [1,2]. Thus, there is an urgent need for identifying novel effective anticancer targets and agents with prognostic value to more accurately predict survival of endometrial cancer patients and to cure this disease efficiently.
Ras is the founding member of the Ras superfamily of small GTPases, it plays a crucial role in various cellular functions, including cell survival and death, which have captured much of the current research spotlight [5,6]. A newly Ras-related GTPases, RIT1, (Ras-like without CAAX 1), have been added to the family and it encodes a RAS-family small GTPase with significant domain and sequence homology to KRAS, HRAS, and NRAS. RIT has to be shown widely expressed and display distinct patterns that it is expressed throughout development and has diverse and complicated biological functions. For example, RIT1 has been shown to be critical in neuron stress-mediated survival and has been found to be involve in Elk1 transactivation through the Ras-MAPK signaling cascade that mediates a wide variety of cellular functions, including cell proliferation, survival, and differentiation [7-11]. Moreover, RIT1 has been reported to play an important role in many kinds of malignant tumors, such as lung adenocarcinoma, myeloid malignancies, hepatocellular carcinoma [12-14]. RIT1 was overexpressed in hepatocellular carcinoma (HCC), and the up-regulated expression of RIT1 was due to amplification in hepatocellular carcinoma [14,15]. Thereby it was speculated that RIT1 gene might be a candidate oncogene in human cancer. Although RIT1 has been implicated in cancer progression, its prognostic value in large numbers of endometrial cancer remains unclear.
In this study, we detected the expression pattern of RIT1 at both mRNA and protein level in 7 endometrial cancer cell lines. And then we tested the RIT1 mRNA expression level in 36 freshly frozen endometrial cancer tissues compared to 21 non-cancerous endometrial tissue samples and the protein expression of RIT1 in two tissue microarrays containing 257 cases of tumor and 31 non-tumor tissues, explored the relationship of RIT1 expression with clinicopathologic parameters, including overall survival.
Materials and methods
Sample and clinical database
The expression profiling by array of GSE17025 deposited by Risinger JH et al. (Risinger JH et al., 2011 was downloaded from Gene Expression Omnibus database (GEO, http://www.ncbi.nlm.nih.gov/geo/) in the National Center for Biotechnology Information (NCBI). Ninety-one samples of pathologically reviewed stage I endometrial cancers (79 endometrioid and 12 serous) with a heterogeneous distribution of grade and depth of myometrial invasion were examined in relation to 12 samples of atrophic endometrium from postmenopausal women. Specimens were analyzed using oligonucleotide array analysis (Affymetrix Human Genome U133 Plus 2.0 Array). We subsequently performed integrative analyses on the Cancer Genome Atlas (TCGA) data for Corpus endometrioid carcinomas (TCGA, Nature 2013).
Two tissue microarrays (TMA) analyzed in this study contained 257 cases of tumor and 31 non-tumor tissues. The clinical tissue samples were recruited between April 2003 and March 2013 from Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, China, and the First People’s Hospital of Huai’an City, Jiangsu, China. The cases of endometrial cancer were selected in this study only if clinical data were available. All of them had complete clinicopathological and follow-up data, and completed informed consent and approval of ethics committee of each hospital for the use of samples. None of them had history of other solid tumors, underwent radical surgery treatment, radiotherapy, chemotherapy or other anticancer therapy prior to surgery. The histology and clinical stages were classified according to International Federation of Gynecology and Obstetrics (FIGO) staging system. An additional 36 freshly frozen endometrial cancer tissues and 21 non-cancerous endometrial tissue samples were also obtained during April 2008 and March 2014 from Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, China. All patients provided written informed consent, and the experiments were approved by the hospital Research Ethics Committees of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital.
TMA were constructed by ZhuoLi Biotech Co., Ltd, Shanghai, China. The diagnoses of the fixed points which displayed the most typical histological characteristics were confirmed by the pathologists. Cores (with a diameter of 1.1-mm) from per-donor block were diverted into a recipient block microarray. We cut four-micrometer-thick sections from the recipient block and pressed them to the glass slide with adhesive tape transfer system in order to ultraviolet cross linkage.
Cell culture
Human endometrial cancer cell lines AN3CA, ECC-1, HEC-1-A, HEC-1-B, ISHIKAWA, KLE and RL95-2 were all preserved in Shanghai Cancer Institute and a non-malignant cell line derived from the stromal cells, hTERT-HESCs, was purchased from American Type Culture Collection. All of these cells were cultured in specific medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% penicillin/streptomycin. The culture was maintained at 37°C in a humidified incubator containing 5% CO2.
RNA isolation and quantitative real-time PCR
Total RNA from frozen tissue samples or endometrial cancer cells were extracted with Trizol reagent (Invitrogen, Carlsbad, CA) according to the supplier’s protocols, and reverse transcription was performed using the PrimeScript RT-PCR kit (Takara, Japan). The mRNA levels of detected genes were quantified using an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Inc. USA) with SYBR Green Master Mix (Takara, Japan) and then normalized to β-actin. Primers used in this study were as follows: RIT1: forward: 5’-TTCATCAGCCACCGATTCCC-3’, reverse: 5’-GCAGGCTCATCATCAATACGGA-3’; β-actin: forward: 5’-CATGTACGTTGCTATCCAGGC-3’, reverse: 5’-CTCCTTAATGTCACGCACGAT-3’. All reactions were performed at least triplicate. The 2-∆Ct method was used to quantify the relative expression levels of RIT1.
Western blotting
Endometrial cancer cells were homogenized in a lysis buffer (Beyotime, Shanghai, China) containing proteinase inhibitors and phosphatase inhibitors. The protein concentration was determined by using a protein assay reagent kit (Beyotime) according to the manufacturer’s protocol. Equal amounts of total cellular proteins were separated on a 10% SDS-PAGE gel and transferred onto nitrocellulose membranes (Millipore, Billerica, MA). The membranes were then incubated with an anti-RIT1 (1:1000, Sigma) primary antibody. To ensure equal loading, membranes were probed with anti-β-actin antibody (1:3,000, Sigma). The proteins were detected using ECL Western Blotting Detection Reagents (Millipore) accordance with the supplier’s protocol.
Immunohistochemistry
Tumor samples were fixed in 10% neutral formalin and embedded in paraffin. This tissue samples were deparaffinized and rehydrated using xylene and graded ethanol. After antigen retrieval and neutralization of endogenous peroxidase, slides were blocked with 5% bovine serum albumin for 1 h. Slides were then incubated overnight at 4°C with primary antibody (RIT1, Sigma-Aldrich, St. Louis, MO). After washing in phosphate-buffered saline (PBS) for three times, the section was labeled by HRP (rabbit) second antibody for 1 h and again washed three times with PBS. Visualization was performed by DAB and counterstained by hematoxylin. Scoring was conducted by the area of positive staining: 0-10% scored 0; 10-35% scored 1; 36-70% scored 2; more than 70% scored 3. The scoring by the pathologists was done in a blinded manner.
Statistical analysis
Statistic analysis was conducted using SPSS 19.0 software (SPSS Inc.; Chicago, IL., USA). Numerical variables were presented as means ± SD and analyzed by independent t-tests. Categorical variables were recorded as rates and evaluated by using the chi-square test or Fisher’s exact test. For survival analysis, the Kaplan-Meier method was used to analyze the correlation between OS and variables. Survival curves were analyzed by the log-rank test. The Cox proportional hazards regression model was established to identify factors that were independently associated with overall survival. Only significantly different variables in univariate analysis were entered into the next multivariate analysis. Receiver operating characteristic (ROC) curves were utilized to compare the prognostic accuracy of RIT1 with clinicopathological risk factors. P-values <0.05 were considered as statistically significant.
Results
RIT1 alteration in GEO database and TCGA
To identify the RIT1 alteration in endometrial cancer, we analyzed the GEO database and TCGA. In total, gene alteration of RIT1 were identified in 37/363 (11%), with 34 in amplification, 2 missense mutation, and 1 inframe mutation (Figure 1A). Additionally, we analyzed microarray datasets from GEO dataset for RIT expression (Figure 1B). Within the datasets, mRNA expression levels of RIT1 were upregulated in the majority of tumor tissues compared with non-tumors.
Figure 1.
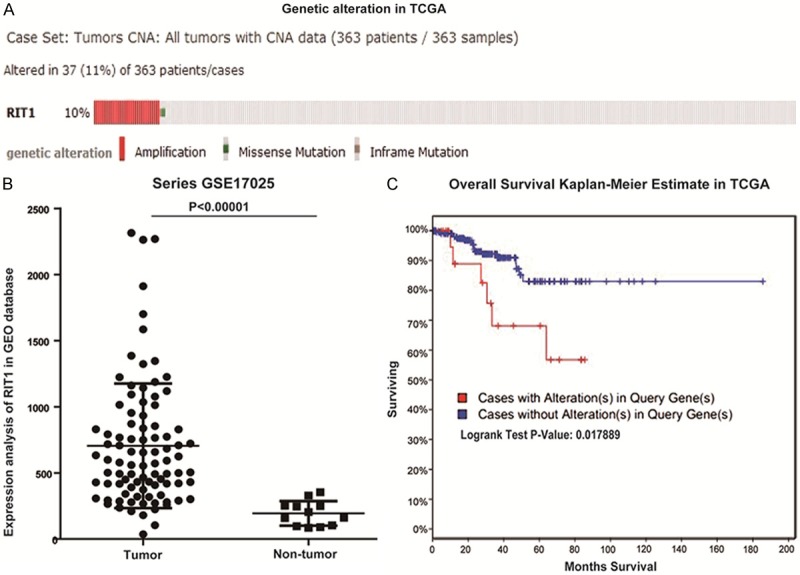
RIT1 alteration in GEO database and TCGA. A: RIT1 alteration in endometrial carcinoma in TCGA (TCGA, Nature 2013). B: RIT1 expression in 91 endometrial cancer tissues and 12 non-tumor tissues in GEO database. C: Overall survival Kaplan-Meier estimate of RIT1 alteration in TCGA (RIT1 alteration group: Red line, No RIT1 alteration group: Blue line).
RIT1 expression in endometrial cancer (EC) cell lines
RIT1 mRNA expression levels were examined using quantitative real-time PCR in the seven endometrial cell lines compared with the nonmalignant hTERT-HESCs (Figure 2). And RIT1 protein expression levels were performed using Western blot in the seven endometrial cell lines compared with the nonmalignant hTERT-HESCs (Figure 2). The results demonstrated that RIT1 mRNA expression was upregulated in EC cell lines compared to the nonmalignant hTERT-HESCs. Western blots show significantly elevated protein expression of RIT1 in EC cell lines.
Figure 2.
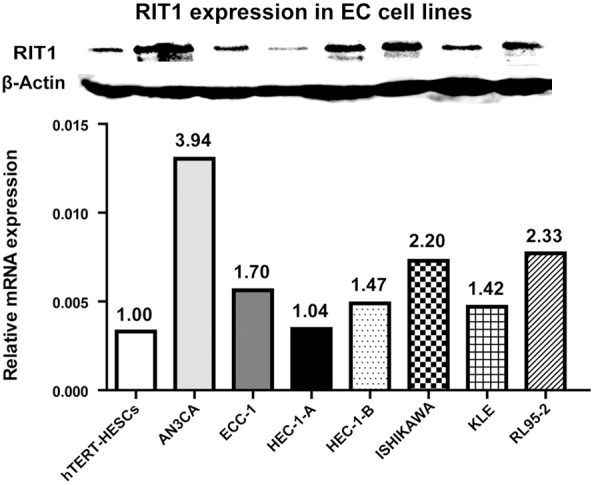
RIT1 expression in seven endometrial cancer cell lines. Upper group: Western blotting analysis of RIT1 protein expression in EC cell lines. Non-malignant cell line hTERT-HESCs were used as control. Lower group: Relative mRNA expression of RIT1 in EC cell lines. β-actin was used as a loading control.
RIT1 expression in endometrial caner patients
RIT1 mRNA expression levels were examined using real-time quantitative PCR in 36 endometrial cancer tissues compared to 21 non-tumor tissues (Figure 3A). Then RIT1 protein expression levels were evaluated by IHC using two tissue microarrays (TMA) containing 257 endometrial carcinoma cancers and 31 non-tumor samples (Figure 3B). This set of experiments showed that RIT1 was upregulated in EC patients. A statistically significant difference was detected between RIT1 expression in endometrial cancer tissues and non-tumor tissues (P<0.001). In the immunohistochemistry (IHC) analysis, RIT1 immunoreactivity was observed in plasma membrane. Representative stains of RIT1 scored as 0, 1, 2 and 3 were shown in Figure 3C. RIT1 expression was elevated in 51.7% (133/257) of endometrial cancer tissues and 19.3% (6/31) non-tumor tissues, respectively (Figure 3B).
Figure 3.
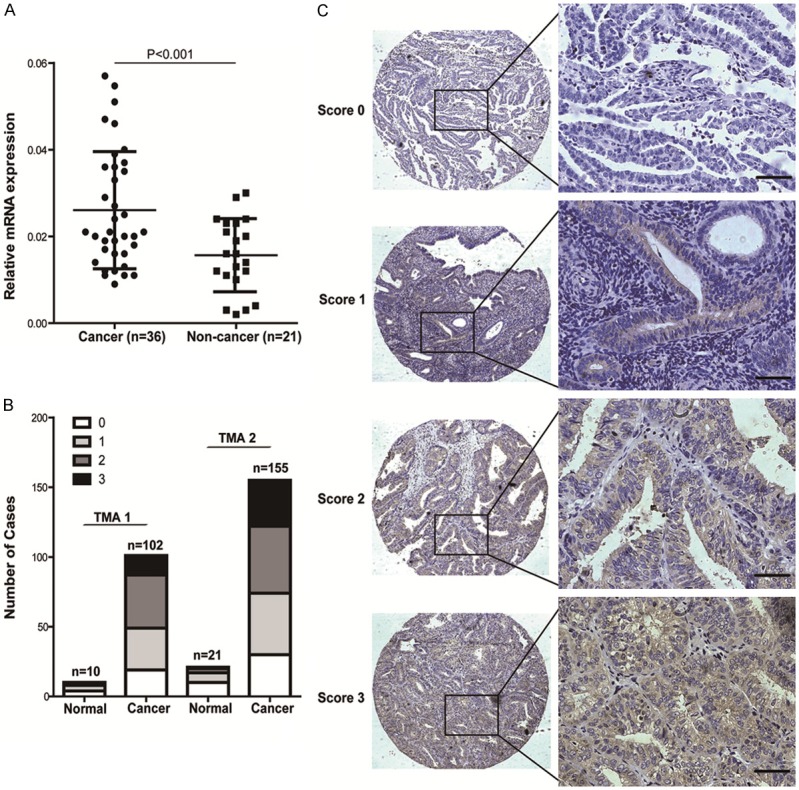
RIT1 expression in endometrial cancer samples. A: Relative mRNA expression in EC samples in 36 freshly frozen endometrial cancer tissues and 21 non-cancerous endometrial tissues. B: IHC scores for RIT1expression in two TMA containing 257 cases of tumor and 31 non-tumor tissues. C: Representative immunohistochemical staining of RIT1 expression in endometrial cancer samples. RIT1 immunostaining is observed primarily in the plasma membrane (Brown).
Relationship between RIT1 expression and clinicopathologic factors of EC
In the IHC analysis of tissue microarrays (TMA), the correlations between RIT1 protein expression and clinicopathologic factors were analyzed. The clinicopathologic parameters included age, menopause, pregnancy, family history, pathological type, clinical stage, grade, vascular invasion and lymphatic metastasis. The results demonstrated that RIT1 expression in EC tissues is closely correlated with pathological type (P = 0.017), clinical stage (P = 0.001), grade (P = 0.0.042), and vascular invasion (P = 0.003). No significant correlation was observed between RIT1 expression and age, menopause, pregnancy, family history and lymphatic metastasis (Table 1, P>0.05).
Table 1.
Correlation between RIT1 expression and clinicopathologic features in patients with endometrial cancer (EC)
| Expression of RIT1 | ||||
|---|---|---|---|---|
|
|
||||
| Total | Low (%) | High (%) | P value | |
| Age | ||||
| <45 | 24 | 14 (58.3) | 10 (41.7) | 0.299 |
| ≥45 | 233 | 110 (47.2) | 123 (52.8) | |
| Menopause | ||||
| Yes | 169 | 81 (47.9) | 88 (52.1) | 0.887 |
| No | 88 | 43 (48.9) | 45 (51.1) | |
| Pregnancy | ||||
| Yes | 13 | 8 (61.5) | 5 (38.5) | 0.325 |
| No | 224 | 116 (51.8) | 128 (58.2) | |
| Family history | ||||
| Yes | 17 | 5 (29.4) | 12 (70.6) | 0.108 |
| No | 240 | 119 (49.6) | 121 (50.4) | |
| Pathological type | ||||
| Adenocarcinoma | 233 | 118 (50.6) | 115 (49.4) | 0.017 |
| Squamous cell carcinoma, papillary serous carcinoma, and clear cell carcinoma | 24 | 6 (25.0) | 18 (75.0) | |
| Clinical Stage (FIGO) | ||||
| I | 216 | 114 (52.8) | 102 (47.2) | 0.001 |
| II | 19 | 7 (36.8) | 12 (63.2) | |
| III and IV | 22 | 3 (13.6) | 19 (86.4) | |
| Grade | ||||
| G1 | 144 | 79 (54.9) | 65 (45.1) | 0.042 |
| G2 | 78 | 33 (42.3) | 45 (57.7) | |
| G3 | 35 | 12 (34.3) | 23 (65.7) | |
| Vascular invasion | ||||
| Yes | 22 | 4 (18.2) | 18 (81.8) | 0.003 |
| No | 235 | 120 (51.1) | 115 (48.9) | |
| Lymphatic metastasis | ||||
| Yes | 14 | 3 (21.4) | 11 (78.6) | 0.053 |
| No | 243 | 121 (49.8) | 122 (50.2) | |
Note: Values in parentheses demonstrate percentage values. FIGO: International Federation of Gynecology and Obstertrics. The bold numbers present the P-values with significant differences.
Prognostic implications of RIT1 expression in EC patients
To determine the prognostic value of RIT1 in EC patients, the correlation between RIT1 expression and clinical follow-up information was assessed by Kaplan-Meier analysis and log-rank test. As shown in Figure 4A, high RIT1 expression was associated with poor overall survival (P = 0.014). Similar results were observed in TCGA (Figure 1C). In addition, we analyzed the relationship between RIT1 expression and overall survival in EC patients with advanced endometrial cancer patients who in stage II, III and IV or in grade 2 and grade 3 (Figure 4B and 4C). These results showed that overall survival was shorter in advanced EC patients with higher RIT1 expression. Similar results were also found in EC patients with vascular invasion (Figure 4D). Furthermore, univariate and multivariate analyses were performed to analyze the possibility that RIT1 could be used as an independent risk factor for poor prognosis in EC patients. These results showed that RIT1 expression, pathological type, clinical stage, grade, vascular invasion, and lymphatic metastasis were hazardous prognostic factors for the overall survival of endometrial cancer patients (Table 2). Importantly, multivariate analyses indicated that RIT1 expression was an independent prognostic factor for endometrial cancer (Table 2).
Figure 4.
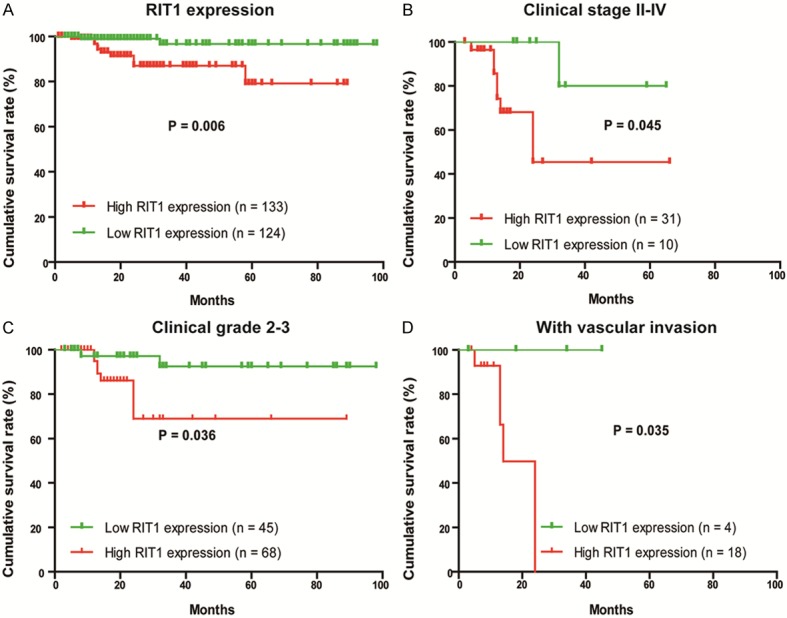
Correlation between RIT1 expression and overall survival in EC patients. A: Prognostic significance of RIT1 expression was assessed for EC patients by Kaplan-Meier method and log-rank test. B: Comparisons of overall survival between RIT1 low expression and RIT1 high expression groups in clinical stage II-IV. C: Comparisons of overall survival between RIT1 low expression and RIT1 high expression groups in clinical Grade 2-3. D: Comparisons of overall survival between RIT1 low expression and RIT1 high expression groups with or without vascular invasion. (P-values were calculated by log-rank test).
Table 2.
Univariate and multivariate analysis of prognostic factors for survival in patients with endometrial cancer (EC)
| Prognostic parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Expression of RIT1 (low vs. high) | ||||||
| 6.327 | 1.381-28.979 | 0.017 | 6.208 | 1.050-36.685 | 0.044 | |
| Age | ||||||
| 0.44 | 0.096-2.025 | 0.292 | - | - | - | |
| Menopause | ||||||
| 2.397 | 0.525-10.947 | 0.259 | - | - | - | |
| Pregnancy | ||||||
| 0.564 | 0.073-4.371 | 0.583 | - | - | - | |
| Family history | ||||||
| 2.01 | 0.436-9.260 | 0.37 | - | - | - | |
| Pathological type | ||||||
| 7.77 | 2.437-24.775 | 0.001 | 0.081 | 0.006-1.107 | 0.058 | |
| Clinical Stage (FIGO) | ||||||
| 5.113 | 2.698-9.692 | <0.001 | 2.141 | 0.063-7.189 | 0.217 | |
| Grade | ||||||
| 2.923 | 1.448-5.904 | 0.003 | 1.717 | 0.596-5.381 | 0.336 | |
| Vascular invasion | ||||||
| 23.122 | 6.976-76.635 | <0.001 | 1.610 | 0.231-10.816 | 0.627 | |
| Lymphatic metastasis | ||||||
| 59.696 | 15.153-235.168 | <0.001 | 37.295 | 4.107-359.241 | 0.001 | |
Note: HR: Hazard ratio; CI: confidence interval. The bold number presents the P-values with significant differences. FIGO: International Federation of Gynecology and Obstertrics.
ROC analysis was carried out to further confirm the sensitivity and specificity of prediction of RIT1 for endometrial cancer by logistic regression. Eight models were constructed including single clinicopathological risk factor, combinations of clinicopathological factors, RIT1, and RIT1 combined with other clinicopahological risk factors. The ROC curve compared the prognostic accuracy of RIT1 with that of other clinicopathological risk factors was shown in Figure 5. The area under the curve (AUC) was 0.670 for pathological type, 0.818 for clinical stage, 0.705 for grade, 0.717 for vascular invasion, 0.777 for lymphatic metastasis, 0.666 for RIT1, 0.824 for combined clinical prognostic factors, and 0.893 for RIT1 and combined clinical prognostic factors. These results displayed that AUC for RIT1 combined with other clinicopathological prognostic factors was higher than any individual factors or other clinicopathological prognostic factors combination, which showed that RIT1 combined with other clinicopathological prognostic factors had more sensitivity and specificity and was a more significant prognostic model than the single risk factor or their combination.
Figure 5.
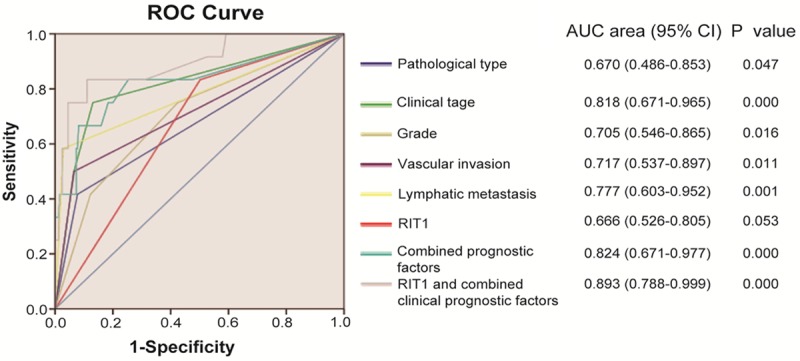
ROC curve compared the prognostic accuracy of RIT1 with clinicopathological risk factors (pathological type, clinical stage, grade, vascular invasion, and lymphatic metastasis) in all 257 endometrial cancer patients by logistic regression. ROC: Receiver operator characteristic. AUC: area under curve.
Discussion
The molecular mechanisms underlying carcinogenesis and progression of endometrial cancer have not yet been fully elucidated. Therefore, endometrial cancer will remain a serious women health problem for decades to come, and novel effective anticancer targets and agents with prognostic value is urgently needed.
RIT1, (Ras-like without CAAX 1) is a member of a subfamily of Ras-related GTPases, and mediates a wide variety of cellular functions, including cell proliferation, survival, and differentiation. Previous studies have shown that RIT1 played an important role in many kinds of cancer, like lung adenocarcinoma, myeloid malignancies, hepatocellular carcinoma [12-14]. RIT1 has been shown to increase phosphorylation of AKT and thereby inhibit apoptosis and also activate proliferation through mitogen-activated protein kinase [6-8]. Furthermore, ectopic expression of active Rit stimulated CREB-Ser133 phosphorylation to induce expression for the anti-apoptotic Bcl-2 and Bcl (XL) proteins, and promote cell survival. RNAi-mediated Rit silencing was found to increase cell death [16]. This indicates that inhibition of RIT1 might be a novel potential therapy target for endometrial cancer. In this study, we have shown the RIT1 expression and its correlation with clinicopathological features and clinical prognosis. RIT1 expression was significantly elevated both in endometrial cancer cell lines and endometrial cancer tissues at mRNA and protein level. The data in GEO database collectively confirmed that RIT1 expression was increased in EC patients. Together, these observations indicated that RIT1 may have an important role in endometrial cancer. Furthermore, we also investigated the correlation between RIT1 expression and clinicopathologic factors in detail. In our present study, RIT1 expression was significantly correlated with pathological type, clinical stage, grade and vascular invasion in endometrial carcinomas patients. Importantly, patients with a high level of RIT1 expression had significantly shorter survival times compared to those with a low level of RIT1 expression. Univariate analysis showed that elevated RIT1 expression was significantly associated with the overall survival rate in EC patients. Multivariate analysis indicated that RIT1 expression was an independent risk factor for poor prognosis of EC patients. These findings were consistent with previous studies which demonstrated the roles of RIT1 in the carcinogenesis and progression of cancer [14]. Moreover, RIT1 combined with other clinicalpathological risk factors was a more significant model when we compared the prognosic accuracy of RIT1 with other clinicopathological risk factors. Collectively, these results suggest that RIT1 may play a critical role in progression of endometrial cancer and might serve as a target for patient prognosis and therapy for endometrial cancer patients.
We first suggested that RIT1 may be a potential and promising therapeutic target for the treatment of endometrial cancer. However, there are some limitations. Further randomized study of larger sample size will be required to confirm the prognostic role of RIT1 in endometrial cancer. Moreover, the molecular mechanisms of RIT1 in endometrial cancer need to be elucidated by further studies.
In conclusion, this study demonstrated that elevated RIT1 expression is associated with poor survival in endometrial cancer patients. Thus, RIT1 expression might be a novel prognostic marker and may represent a promising molecular target for the treatment of endometrial cancer.
Acknowledgements
Supported by Shanghai Science and Technology Commission Project, 13JC1404500.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Bradford LS, Rauh-Hain JA, Schorge J, Birrer MJ, Dizon DS. Advances in the management of recurrent endometrial cancer. Am J Clin Oncol. 2015;38:206–12. doi: 10.1097/COC.0b013e31829a2974. [DOI] [PubMed] [Google Scholar]
- 3.Amant F, Mansoor RM, Creutzberg CL. Carcinoma of the corpus uteri. Int J Gynaecol Obstet. 2012;119(Suppl 2):S110–S117. doi: 10.1016/S0020-7292(12)60024-1. [DOI] [PubMed] [Google Scholar]
- 4.Lewin SN, Herzog TJ, Barrena Medel NI, Deutsch I, Burke WM, Sun X, Wright JD. Comparative performance of the 2009 international Federation of gynecology and obstetrics’ staging system for uterine corpus cancer. Obstet Gynecol. 2010;116:1141–1149. doi: 10.1097/AOG.0b013e3181f39849. [DOI] [PubMed] [Google Scholar]
- 5.Reuther GW, Der CJ. The Ras branch of small GTPases: Ras family members don’t fall far from the tree. Curr Opin Cell Biol. 2000;12:157–65. doi: 10.1016/s0955-0674(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 6.Shi GX, Jin L, Andres DA. A rit GTPase-p38 mitogen-activated proteinkinase survival pathway confers resistance to cellular stress. Mol Cell Biol. 2011;31:1938–1948. doi: 10.1128/MCB.01380-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rusyn EV, Reynolds ER, Shao H, Grana TM, Chan TO, Andres DA, Cox AD. Rit, a non-lipid-modified Ras-related protein, transforms NIH3T3 cells without activating the ERK, JNK, p38 MAPK or PI3K/Akt pathways. Oncogene. 2000;19:4685–4694. doi: 10.1038/sj.onc.1203836. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Rudolph JL, Harrison SM, Jin L, Frantz AL, Harrison DA, Andres DA. An evolutionarily conserved Rit GTPase-p38 MAPK signaling pathway mediates oxidative stress resistance. Mol Biol Cell. 2011;22:3231–3241. doi: 10.1091/mbc.E11-05-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi GX, Han J, Andres DA. Rin GTPase couples nerve growth factor signaling to p38 and b-Raf/ERK pathways to promote neuronal differentiation. J Biol Chem. 2005;280:37599–37609. doi: 10.1074/jbc.M507364200. [DOI] [PubMed] [Google Scholar]
- 10.Shi GX, Andres DA. Rit contributes to nerve growth factor-induced neuronal differentiation via activation of B-Raf-extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades. Mol Cell Biol. 2005;25:830–846. doi: 10.1128/MCB.25.2.830-846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lein PJ, Guo X, Shi GX, Moholt-Siebert M, Bruun D, Andres DA. The novel GTPase Rit differentially regulates axonal and dendritic growth. J Neurosci. 2007;27:4725–4736. doi: 10.1523/JNEUROSCI.5633-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger AH, Imielinski M, Duke F, Wala J, Kaplan N, Shi GX, Andres DA, Meyerson M. Oncogenic RIT1 mutations in lung adenocarcinoma. Oncogene. 2014;33:4418–23. doi: 10.1038/onc.2013.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez-Seguí I, Makishima H, Jerez A, Yoshida K, Przychodzen B, Miyano S, Shiraishi Y, Husseinzadeh HD, Guinta K, Clemente M, Hosono N, McDevitt MA, Moliterno AR, Sekeres MA, Ogawa S, Maciejewski JP. Novel recurrent mutations in the RAS-like GTP-binding gene RIT1 in myeloid malignancies. Leukemia. 2013;27:1943–6. doi: 10.1038/leu.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JT, Liu W, Kuang ZH, Chen HK, Li DJ, Feng QS, Liu QC, Hu B. Amplification of RIT1 in hepatocellular carcinoma and its clinical significance. Ai Zheng. 2003;22:695–9. [PubMed] [Google Scholar]
- 15.Li JT, Liu W, Kuang ZH, Zhang RH, Chen HK, Feng QS. Mutation and amplification of RIT1 gene in hepatocellular carcinoma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:43–6. [PubMed] [Google Scholar]
- 16.Shi GX, Cai W, Andres DA. Rit-mediated stress resistance involves a p38-mitogen-and stress-activated protein kinase 1 (MSK1) dependent cAMP response element-binding protein (CREB) activation cascade. J Biol Chem. 2012;287:39859–68. doi: 10.1074/jbc.M112.384248. [DOI] [PMC free article] [PubMed] [Google Scholar]


