Abstract
Muc-1 is a member of the carbohydrate-binding protein family that contributes to neoplastic transformation, tumor survival, angiogenesis, and metastasis. The aim of this study is to investigate the role of muc-1 in human oral squamous cell carcinoma progression. In this study, we tested our hypothesis that muc-1 regulate oral squamous cell carcinoma cells (SCC-9) malignant biological behaviors, and silencing muc-1 reduced SCC-9 cellular colony forming ability, migration and invasion. Moreover, silenced cells present defects in phosphatidylinositol 3-kinase (PI3K)-serine/threonine kinase (Akt) signaling, and reduced expression/activity of matrix metallopeptidase (MMP)-2/9. Furthermore, in muc-1 siRNA-transfected cells, we detected a decrease in signal transducer and activator of transcription 3 (STAT3) phosphorylation and nuclear translocation. In vivo, muc-1 siRNA cells inoculated subcutaneously in nude mice demonstrated decreased tumor growth and PI3K-Akt signaling inhibition. These results indicate that muc-1 is a key factor in SCC-9 tumor migration, invasion, and suggesting that muc-1 can be a novel therapeutic target in oral squamous cell carcinoma.
Keywords: Oral squamous cell carcinoma, Muc-1, RNA interference, tumor invasion
Introduction
Oral squamous cell carcinoma (OSCC) is the most common type of head and neck cancer. This cancer has a very poor prognosis, and it is often characterized by aggressive local invasion, early metastasis and poor response to chemotherapy. In the last few decades, our understanding of the molecular biology of OSCC metastasis has increased tremendously. Future, continue to identify the key molecules that control tumor deteriorate and development of novel treatments than can block or inhibit invasion and/or metastasis is important for improving the prognosis of OSCC.
Mucin 1, cell surface associated (Muc-1) or polymorphic epithelial mucin (PEM) is a mucin encoded by the muc-1 gene. Muc1 is a glycoprotein with extensive O-linked glycosylation of its extracellular domain. Recent studies provide evidence that muc-1 plays a critical role in human cancer [1,2]. Muc1 has been determined to be one of the most frequently expressed surface markers in metastatic breast cancers [3]. Muc-1 cooperates with receptor tyrosine kinases and promotes an invasive phenotype in breast tumorigenesis [4]. In addition to breast tumorigenesis, muc-1 confers an invasive phenotype and drives progression of primary melanoma cells and enhances their metastatic capability via activation of the PI3K-Akt pathway [5]. Activation of PI3K has been linked to mitogenesis, differentiation, survival, migration, invasion, and actin cytoskeletal reorganization. The PI3K-Akt pathway is a major regulator of STAT3, which can promote oncogenesis by being constitutively active through various pathways, and matrix metallopeptidase-2/9 (MMP-2/9) activity [6]. Collectively, these results suggest that endogenous muc-1 regulates cell metastasis in a variety of cancers. In contrast, many of the functions of muc-1 in OSCC progression, invasion, and metastasis remain unclear.
In the present study, to investigate how muc-1 regulates oral squamous cell carcinoma cells migration and invasion, we examined effects of transient muc-1 silencing by RNA interference on OSCC cell line SCC-9. We found that silencing muc-1 decreased the SCC-9 cells migration and invasion abilities, and reduced the phosphorylated PI3K (p-PI3K), phosphorylated Akt (p-Akt) and STAT3 transcriptional activation. Furthermore, silencing of muc-1 decreased MMP-2/9 levels and activity in SCC-9. These effects are consequence of a down-regulation of PI3K-Akt signaling pathway, and eventually lead to an impairment of in vivo tumorigenesis.
Materials and methods
Cell culture and siRNA transfection
OSCC-derived cell lines (HO-1-u-1, KOSC-2, SCC-22, HO-1-N-1, and Tca8113) and human normal oral keratinocyte, hNOK were purchased from Cell Resource Center, Shanghai Institutes for Biological Sciences. Cells were cultured in DMEM/F12 or DMEM, respectively, and supplemented with 10% (v/v) fetal bovine serum, 100 units/mL penicillin and 100 units/ml streptomycin. All cells were maintained in a humidified incubator with 5% CO2 at 37°C. Muc-1-siRNA (siMuc-1) was produced by GenePharma Co, Ltd (Shanghai, China). Transient transfection was performed using the Lipofectamine RNAi MAX reagent (Invitrogen) and following the manufacturer’s instructions.
Immunohistochemical detections
A total of 40 OSCC radical resection tumor samples were randomly collected at Department of Stomatology, The Third Affiliated Hospital of Beijing University of Chinese Medicine. All the procedures were approved by Ethics Committee of Beijing University of Chinese Medicine. Deparaffinized tumor sections were stained with muc-1 antibody. Detection was done with avidin-biotin-HRP complex (Thermo scientific) and diaminobenzidine as chromogen. Nuclei were counterstained with hematoxylin. Muc-1 positive area was counted in five random high-power fields per section and was reported as a percentage of positive area in each tumor sections.
Cell proliferation assay
Cell proliferation was measured by a methylthiazol tetrazolium (MTT) assay. Cancer cells (2.0×103 cells/well) were seeded in 96-well microtiter plates in a total volume of 100 μl/well. Transfection was performed when the cells were 30%-50% confluent. Following transfection, 10 μl/well of CCK-8 was added for 4 h. The absorbance of each well was determined at 450 nm using a microtiter plate reader (Molecular Devices). The results are expressed as the mean±SD of three independent experiments. MTT assays were made in triplicate.
Anchorage-independent cell growth in soft agar
After transfection, SCC-9 cells (5×104) suspended in 2 ml medium, 0.3% agar, and 10% FBS were plated over the bottom agar pre-solidified with 3 ml medium containing 0.6% agar and 10% FBS. Colony formation was then carried out by photographing through the microscope after 14 days. Five random fields under microscope were taken and colony formation unities were counted [7].
Wound healing assay
SCC-9 cells migration was determined using a scratch assay. The transfected cell lines were seeded on a 24-well plate and allowed to reach confluence. After scratching the bottom of the well with a pipette tip, the monolayer of cells was washed three times with PBS to remove the detached cells. The remaining adherent cells were incubated in medium containing 1% FBS for 24 h; this medium was then replaced with medium containing 10% FBS. Space filling by cell migration was evaluated 48 h later using bright-field microscopy. The experiments were performed in triplicate [8].
Cell invasion assay
Cell invasion was tested using a BD BioCoat Matrigel Invasion Chamber (Becton-Dickinson,). Cells were harvested 48 h after transfection. The cells were re-suspended in serum-free DMEM and then added to the upper chamber at a density of 2×105 cells/well. Cell invasion into the Matrigel was determined after 24 h of culture at 37°C. The membrane containing invading cells was fixed using methanol and stained with HE, and invasion was quantified by light microscopy after removing the non-invading cells on the upper side of the membrane with cotton swabs [9].
Western blotting
Cells were harvested following treatment with siMuc-1 or scrambled siRNA (siCTL) for 48 h. Protein was extracted in lysis buffer. Protein concentrations were measured using a BCA Protein Assay Kit. Fifty micrograms of protein was loaded on a 12% SDS-PAGE gel, followed by protein separation and electroblotting onto a polyvinylidene difluoride plus membrane. The membrane was labeled with the following primary antibodies: anti-muc-1, anti-Akt, anti-phosphorylated Akt, anti-PI3K, anti-phosphorylated PI3K, anti-MMP-2, anti-MMP-9 or anti-GAPDH. Anti-GAPDH was used to monitor equal loading in each lane. Anti-mouse HRP-conjugated secondary antibodies were incubated in 5% BSA in PBST buffer for 1.5 h at room temperature. All antibodies were obtained from Abcam. Immunoreactivity was detected using an enhanced chemiluminescence detection system (Amersham). For densitometric analysis of Western blots, Alpha Image software (Alpha Innotech) was used.
Matrix metalloprotein (MMP) activity assay
The activity of MMP-9 and MMP-2 were determined by QuickZyme MMPs activity assay (QucikZyme BioSciences) according to the manufacturer’s instructions [10]. Briefly, after transfection for 48 h, SCC-9 were washed with fresh medium and replaced with serum-free medium. After additional 24 h, the medium was collected and centrifuged at 10000 g for 10 min. Respective supernatant was added to the 96-well strip coated with MMP-9 antibody or MMP-2 antibody and incubated at 4°C overnight. After washing with wash buffer for 3 times, 50 µl assay buffer was added into the well, followed by adding 50 µl detection reagent. After incubation at 37°C for 1 h, OD405 was measured with Microplate Reader (Bio-Tek).
RNA isolation and RT-PCR
Total RNA was extracted from cells 48 h after transfection using Trizol. Template cDNA was synthesized from 1 μg of total RNA with a Quantscript RT Kit (TIANGEN, Shanghai, China) using random primers and a ribonuclease inhibitor. One microliter of the cDNA sample was mixed with 19 μL of a master reaction mix. PCR was performed for 40 cycles (denaturation at 95°C for 15 s, annealing at 60°C for 1 min and extension at 60°C for 1 min) using a RealMasterMix Kit (SYBR Green) and a 7500 Real Time PCR System (America). The RT-PCR procedure for muc-1 was based on a protocol described previously. β-actin was used as an internal reference. The following primer sets were used: muc-1, 5’-GGCCACTGATTGTGCCTTAT-3’ (forward); 5’-TGCAACCTTGAAGTGGTCAG-3’ (reverse); β-catenin, 5’-GCCGGCTATTGTAGAAGCTG-3’ (forward); 5’-GAGTCCCAAGGAGACCTTCC-3’ (reverse). The experiments were performed in triplicate.
Fluorescent distribution of STAT3
SCC-9 grown on coverslips were transfected with siMuc-1 and cultured for 48 h. These cells were fixed with 4% paraformaldehyde for 15 min, incubated with Triton-X-100 for 15 min and blocked in 1% BSA. The cells were then incubated with a monoclonal mouse anti-STAT3 antibody (Abcam) for 1 h, followed by incubation with a secondary Alexa Fluor 555-conjugated goat anti-mouse IgG antibody (Invitrogen) for 1 h. The coverslips were washed and soaked in ddH2O for 5 s, mounted onto glass microscope slides with Vectashield mounting medium with DAPI, and examined with an Olympus FluoView FV1000 confocal microscope. Experiments were performed in duplicate and repeated at least three independent times.
Subcutaneous xenograft models
After transfected with either scrambled siRNA or siMuc-1, SCC-9 cells (3×106) were subcutaneously implanted into female, BALB/c nude mice to build non-small cell lung cancer xenograft. Tumor volume and mice body weight were measured every 3 days. Tumor volume was calculated as mm3 =0.5× length (mm) width (mm)2. After sacrificing mice on day 25, tumors tissues will be harvested for western blotting. All animal experiments were carried out in compliance with the Guidelines for the Institute for Experimental Animals, Center of Hunan University Of Chinese Medicine.
Statistical analysis
Statistical analyses were carried out using SPSS software (Ver 16.0, USA). The results are presented as the mean ± SD, and statistical significance was determined using an ANOVA and the SNK-q test. A value of <0.05 was considered to be statistically significant.
Results
Evaluation of muc-1 expression in OSCC-derived cell lines
We performed qRT-PCR and immunoblotting using OSCC-derived cell lines (Sa3, HO-1-u-1, KOSC-2, Ca9-22, HO-1-N-1, HSC-2, and HSC-3) and HNOK. Expression analyses indicated that both transcription and translation products of muc-1 were highly expressed in OSCC-derived cell lines compared with the HNOK (Figure 1A and 1B). We also analyzed the muc-1 protein expression in primary OSCCs and paired normal oral tissues from 40 patients using the IHC. As shown in Figure 1C, strong muc-1 immunoreactions were detected in the tumor tissue in the OSCCs.
Figure 1.
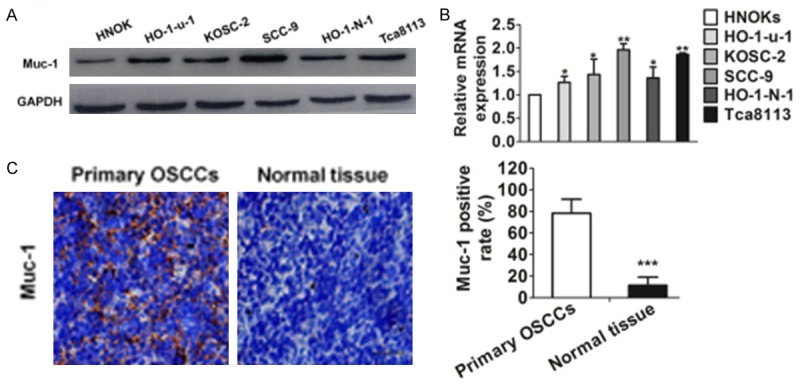
Evaluation of muc-1 expression in OSCC-derived cell lines. A. Immunoblotting analysis of muc-1 protein in the OSCC-derived cell lines and HNOK. Muc-1 protein expressions are up-regulated in all OSCC-derived cell lines examined compared with that in the HNOK. B. Quantification of muc-1 mRNA levels in OSCC-derived cell lines by qRT-PCR analysis. All OSCC-derived cell lines have significant up-regulation of muc-1 mRNA compared with that in the HNOK (*P<0.05 and **P<0.01 versus HNOK). C. Evaluation of muc-1 protein expression in primary OSCCs. Representative IHC results for muc-1 protein in normal tissue and primary OSCCs. Strong muc-1 immunoreactivity is detected in primary OSCCs. Normal oral tissues show almost weak immunostaining. Scale bars, 50 μM. Bars are represented as the mean ± SD, n=3, *P<0.001 versus normal tissue).
Silencing muc-1 reduced mobility of human oral squamous cell carcinoma cells
A number of previous studies have indicated that muc-1 is associated with proliferation, migration, and invasion in several cancers. To elucidate the role of muc-1 in these processes of OSCC-derived cell lines, we used siRNA to silence muc-1 in SCC-9 cells (RT-PCR and western blotting showed SCC-9 cells expressed high levels of muc-1) and subsequently examined its behaviors. Significant inhibition of muc-1 at protein expression level was detected 48 h following transient gene silencing of muc-1, whereas there was no significant difference between control cells and siCTL transfected cells (Figure 2A).
Figure 2.
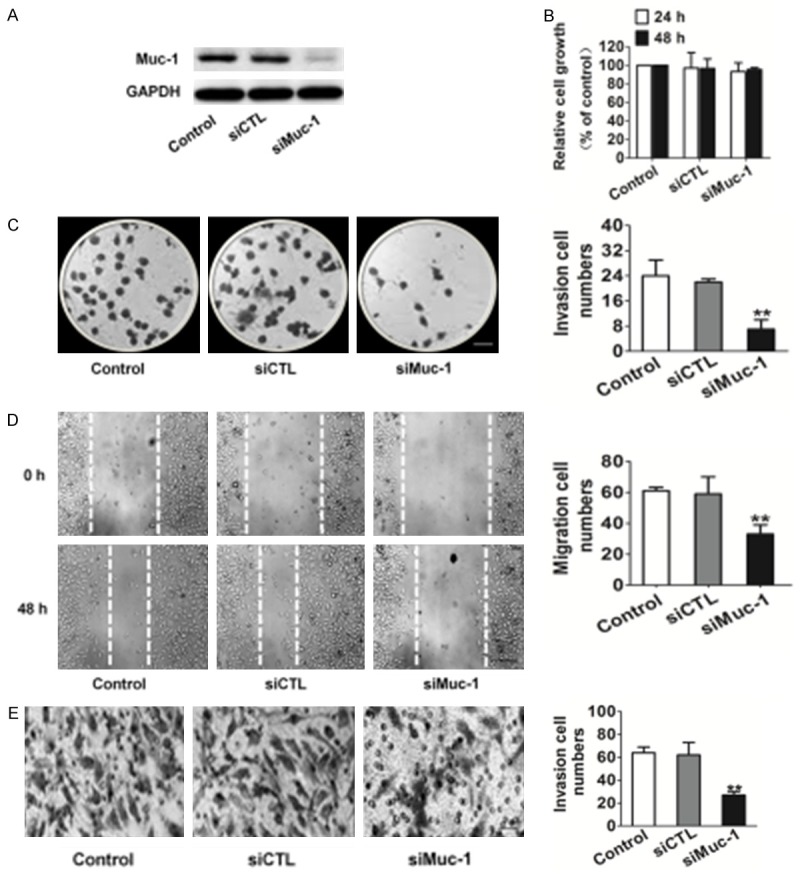
Effects of muc-1 silencing on SCC-9 cell clone formation motility. A. Immunoblotting analysis shows that the muc-1 protein levels in siMuc-1 cells have decreased markedly compared with that in mock cells. B. Proliferation assays indicated that muc-1-siRNA exerted no inhibition on SCC-9 cells proliferation in vitro. C. Clone formation assay indicated that muc-1 siRNA inhibited SCC-9 cell colony-forming effectively. Scale bars: 1 mm. D. Representative examples of wound healing experiment results from control and siRNA-muc-1 SCC-9 cells. Representative pictures of the wound distance were taken at each time point as indicated. Scale bars: 50 µm. E. SCC-9 motility was also estimated by Transwell assay. Representative pictures were taken after staining with crystal violet. Scale bars: 50 µm. Invaded cells transfected with scrambled siRNA or muc-1-siRNA were evaluated on the basis of the mean values from five fields for each group (Bars are represented as the mean ± SD, n=3, **P<0.01 versus control cells).
We observed cell proliferation of the cell lines for 72 h, and detected no differences between siMuc-1 and siCTL transfected cells for SCC-9 cell lines (Figure 2B). In anchorage-independent cell growth assay, only control and siCTL transfected SCC-9 cells could form more colonies in soft agar, an in vitro hallmark of cell transformation. Strikingly, however, the ability of SCC-9 to form colonies was completely abrogated by muc-1 silencing (Figure 2C). In addition, the migration capacity was reduced in siMuc-1 transfected cells compared to the control groups (Figure 2D). Moreover, Transwell assay confirmed the inhibitory effect of muc-1 silencing on invasion. Furthermore, we determined that muc-1 silencing affected cell invasion similarly to migration of the SCC-9. Specifically, we found that the number of invasion cells on the Matrigel membrane was markedly decreased in siMuc-1 treated cells compared with control cells (Figure 2E). Collectively, these results indicate that muc-1 modulates OSCC-derived cell lines SCC-9 migration and invasion but not cell proliferation in vitro.
Silencing muc-1 reduced the level of STAT3 and inhibited PI3K-Akt signaling
It has been reported that muc-1 plays a role in PI3K-Akt signaling in breast, colon, and gastric cancer cells by interacting with STAT3. Accordingly, we analyzed the PI3K-Akt signaling pathway that was mediated by muc-1 and that was associated with cell motility. We found that silencing the muc-1 gene significantly reduced the protein levels of PI3K and Akt in SCC-9 cells (Figure 3A).
Figure 3.
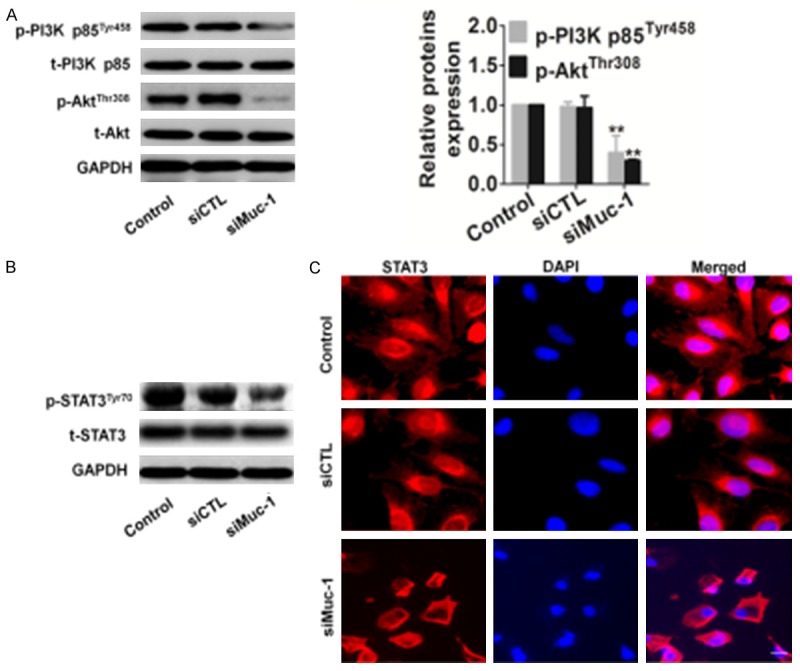
Effects of muc-1 silencing on the PI3K/Akt pathway. A. Expression of PI3K and Akt in muc-1-silenced cells. After transfection of cells with either scrambled siRNA or muc-1-siRNA, the total cells were isolated and PI3K/Akt expression levels were detected by western blotting. GAPDH was used as a loading control. B. SCC-9 cells transfected using the same conditions as described above and total protein was extracted, the total and phosphorylated (p) STAT3 were detected. C. SCC-9 cells were transfected, and then cells were fixed and incubated with primary antibody against STAT3. SCC-9 cells were immunostained with anti-rabbit FITC-conjugated secondary antibody and then stained with DAPI. The specimens were visualized and photographed using a fluorescence microscope. Scale bars: 20 µm. Blue depicts the nucleus and green depicts localization of STAT3 (Bars are represented as the mean ± SD, n=3, **P<0.01 versus control cells).
To further investigate the PI3K-Akt signaling pathway that mediated STAT3 expression, we assessed the expression of total and phosphorylated STAT3 in SCC-9 cells transfected with either siCTL or siMuc-1 by western blotting. Phosphorylated form of STAT3 is important requisite for cancer cell migration and invasion. We found that the expression level of STAT3 remained unchanged after silencing muc-1, whereas the phosphorylated form of the STAT3 was strongly reduced by this transfection (Figure 3B). We also examined the subcellular distribution of STAT3 in both control- and siMuc-1 transfected SCC-9 cells by immunofluorescence staining and confocal microscopy. These experiments showed that STAT3 was distributed in the cytoplasm and nuclei of siCTL transfected cell, and in the cytoplasm and membranes of siMuc-1 transfected cells (Figure 3C). These results indicate that phosphorylation of PI3K and Akt occurs before the downregulation of STAT3 during muc-1 silencing in SCC-9 cells.
Silencing muc-1 decreased MMP-2 and MMP-9 expression
MMP-2/9 is involved in the breakdown of the extracellular matrix, which is crucial for cell migration and invasion. MMP-2 and MMP-9 have been suggested to be an important downstream target of the PI3K-Akt signaling pathway in tumor. To test if MMP-2/9 might be a mediator of PI3K-Akt signaling induced migration and invasion in SCC-9 cells, we analyzed MMP-2/9 levels by Western blotting. MMP-2 and MMP-9 protein levels were significantly lower in siMuc-1 treated cells than in the corresponding controls (Figure 4A). Quantification of MMP-2 and MMP-9 activities using a fluorogenic assay showed a significantly decrease in extracellular MMP-2 and MMP-9 activity in SCC-9 cells after silencing muc-1 (Figure 4B).
Figure 4.
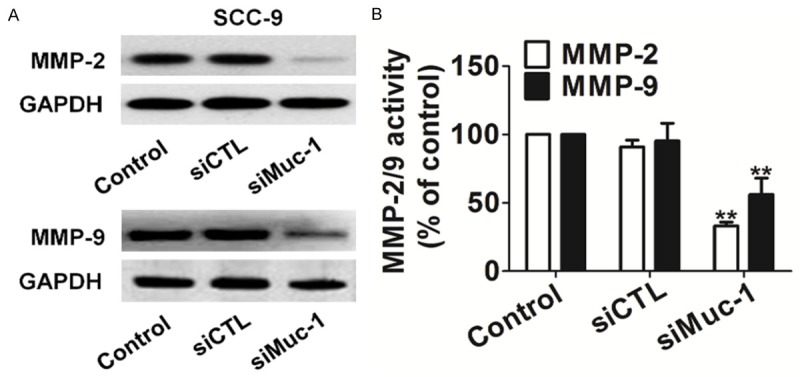
Effects of muc-1 silencing on MMPs activity. A. Total cell lysates were isolated after control- or siMuc-1 transfection and MMP-2/9 expression levels were detected by western blotting. B. Quantification of MMP-2/9 activity. Bars are represented as the mean ± SD, n=3, **P<0.01 versus control cells.
Muc-1 siRNA impaired the growth of OSCC-derived cell in vivo
To further investigate the role of muc-1 in tumoral growth in vivo, siMuc-1 and control SCC-9 cells were injected subcutaneously into the backs of female nude mice, respectively (6 mice in each group), and tumor growth was monitored for 25 days (Figure 5A). According to our in vitro findings, the mean tumoral volume (Figure 5B) and tumor weight (Figure 5C) of the siMuc-1 group was significantly smaller compared to the control. Furthermore, there was no significant difference in weight between control group and siMuc-1 treated groups (Figure 5D). Phosphorylation of PI3K, Akt, and STAT3 expression was checked on tumor tissue after SCC-9 cells injection into nude mice to confirm those results in vitro (Figure 5E). In addition, siMuc-1 also resulted in down-regulation of PI3K-Akt downstream molecules including MMP-2 and MMP-9 (Figure 5E). At the same time, we observed a significant decrease in the phosphorylation of STAT3 in the mice injected siMuc-1 cells (Figure 5E), which was consistent with the results of SCC-9 cell lines in vitro.
Figure 5.
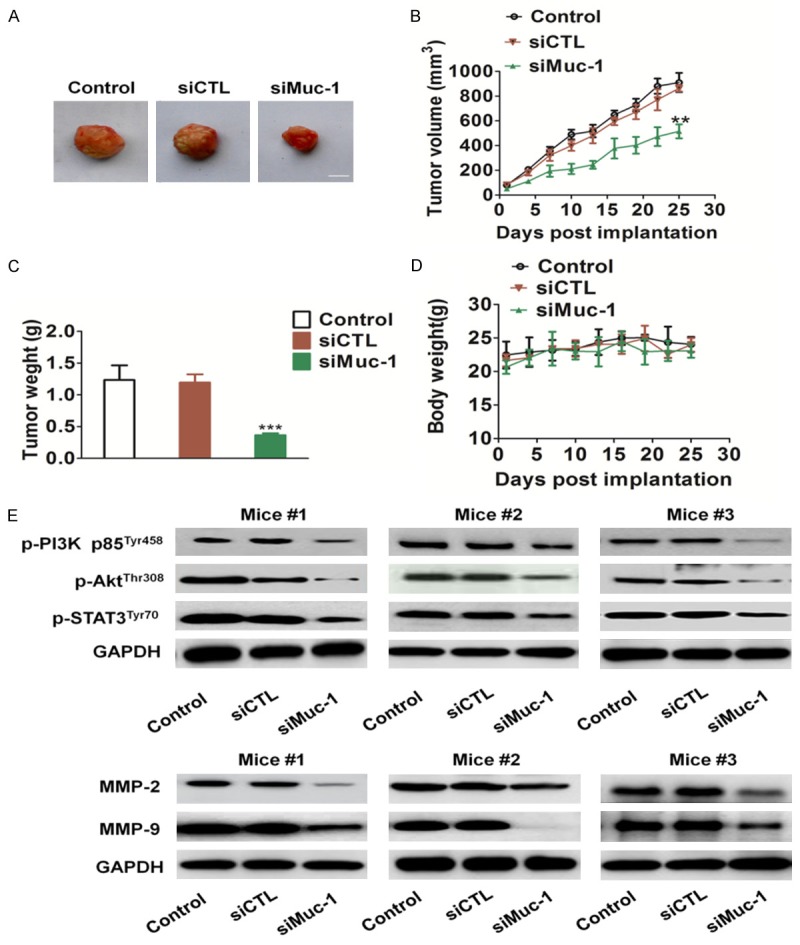
In vivo tumorigenicity of muc-1-siRNA SCC-9 cells. A. Representative pictures of tumor masses from mice were taken after 25 days of implanted with SCC-9 cells or with siMuc-1 CC-9 cells. Scale bars: 1 cm. B and C. Muc-1 silencing resulted in significantly SCC-9 tumor growth inhibition versus mice injected untreated CSS-9 cells. D. There was no significant difference in body weight in the three groups. E. Total tumor tissue lysates were isolated and muc-1 expression levels were detected by western blotting. #, representatives mice number. Scale bars: 50 mm. Bars are represented as the mean ± SD, n=6, ** P<0.01 versus mice injected control SCC-9 cells.
Discussion
Muc-1, a member of the family of mucins, is frequently over-expression in human primary tumors and transmits, amplifies the signals from receptor tyrosine kinases. Great deals of research demonstrate that muc-1 is over-expressed in ovarian, non-small cell lung cancer, gastric cancer and acute myeloid leukemia, and Muc-1 is an oncogenic protein [11,12]. The muc-1 is increasingly implicated in PI3K/Akt signaling and muc-1 can stimulate Akt signaling through its interactions with p85 subunit of PI3K. Through stimulate PI3K signaling, muc-1 contribute to the initiation of several signaling pathways promoting tumor cellular growth, migration, invasion, and metastasis [13]. However, the role of muc-1 in oral squamous cell carcinoma has not been reported.
In this work, we firstly reported a novel role for muc-1 in the migration and invasion of oral squamous cell carcinoma cells. We found that the tumor colony forming units of SCC-9 was significantly inhibited, when the muc-1 gene was disrupted by siRNA. Further investigation with gene silencing of muc-1 showed that the migration and invasion of SCC-9 was significantly decreased as revealed by the wound healing assay and Transell assay. Here, we also examined the key factors and signaling pathways that are mediated by muc-1 and that are associated with SCC-9 cancer cell motility. In this study we examined whether muc-1 silencing affected MMP-2/9 expression in SCC-9. We found that muc-1-siRNA-transfected cells from SCC-9 had drastically reduced MMP-2/9 levels and activity compared with corresponding control cells.
Muc-1 undergoes tyrosine phosphorylation, creating a number of docking sites to mediate interactions with the p85 subunit of PI3K. The interaction of muc-1 with p85 subunit of PI3K is crucial in mediating the PI3K-Akt signaling [14,15]. In this study, using western blotting experiment, we determined that the levels of p-Akt and p-PI3K decreased in siMuc-1 SCC-9 cells. It has been demonstrated that phosphorylated Akt activates its transcription factor STAT3, which acts as transcription activator, is reported plays a role in cell, proliferation, invasion, angiogenesis, and survival. Therefore, we performed immunofluorescence and western blot from control- and siMuc-1 transfected SCC-9 cells, and found that STAT3 phosphorylation was significantly decreased in muc-1-silenced cells.
More convincingly, muc-1 silencing markedly impaired the growth of SSC-9 cells in vivo. Meanwhile, western blot results show that muc-1 silencing could obviously attenuate expressions of PI3K, Akt and MMP-2/9 expression in tumor tissue. All these data presented that muc-1 is involved in the growth of oral squamous cell carcinoma cells SCC-9. Due to the important roles of muc-1 in the growth and migration of oral squamous cell carcinoma cells, it may serve as an attractive target for molecular targeting cancer therapy.
Disclosure of conflict of interest
None.
References
- 1.Budiu RA, Elishaev E, Brozick J, Lee M, Edwards RP, Kalinski P, Vlad AM. Immunobiology of human mucin 1 in a preclinical ovarian tumor model. Oncogene. 2013;32:3664–3675. doi: 10.1038/onc.2012.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng M, Jing DD, Meng XJ. Effect of MUC1 siRNA on Drug Resistance of Gastric Cancer Cells to Trastuzumab. Asian Pac J Cancer Prev. 2014;14:127–131. doi: 10.7314/apjcp.2013.14.1.127. [DOI] [PubMed] [Google Scholar]
- 3.Huyn ST, Burton JB, Sato M, Carey M, Gambhir SS, Wu L. A Potent, Imaging Adenoviral Vector Driven by the Cancer-selective Mucin-1 Promoter That Targets Breast Cancer Metastasis. Clin Cancer Res. 2009;15:3126–3134. doi: 10.1158/1078-0432.CCR-08-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh SK, Uchida M, Yoo B, Ross AW, Gendler SJ, Gong J, Moore A, Medarova Z. Targeted imaging of breast tumor progression and therapeutic response in a human uMUC-1 expressing transgenic mouse model. Int J Cancer. 2013;132:1860–1867. doi: 10.1002/ijc.27872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad R, Rajabi H, Kosugi M, Joshi MD, Alam M, Vasir B, Kawano T, Kharbanda S, Kufe D. MUC1-C Oncoprotein Promotes STAT3 Activation in an Autoinductive Regulatory Loop. Sci Signal. 2011;4:ra9. doi: 10.1126/scisignal.2001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073–1081. doi: 10.1038/onc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam M, Ahmad R, Rajabi H, Kharbanda A, Kufe D. MUC1-C oncoprotein activates ERK→C/EBPβ signaling and induction of aldehyde dehydrogenase 1A1 in breast cancer cells. J Biol Chem. 2013;288:30892–903. doi: 10.1074/jbc.M113.477158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajabi H, Ahmad R, Jin C, Kosugi M, Alam M, Joshi MD, Kufe D. MUC1-C oncoprotein induces TCF7L2 transcription factor activation and promotes cyclin D1 expression in human breast cancer cells. J Biol Chem. 2012;287:10703–10713. doi: 10.1074/jbc.M111.323311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JY, Hiroshima Y, Lee JY, Maawy AA, Hoffman RM, Bouvet M. MUC1 Selectively Targets Human Pancreatic Cancer in Orthotopic Nude Mouse Models. PLoS One. 2015;10:e0122100. doi: 10.1371/journal.pone.0122100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Hu YM, Du YJ, Zhu LR, Qian H, Wu Y, Shi WL. Expressions of MUC1 and vascular endothelial growth factor mRNA in blood are biomarkers for predicting efficacy of gefitinib treatment in non-small cell lung cancer. BMC Cancer. 2014;14:848. doi: 10.1186/1471-2407-14-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JK, Kim JY, Kim HJ, Park KG, Harris RA, Cho WJ, Lee JT, Lee IK. Scoparone Exerts Anti-Tumor Activity against DU145 Prostate Cancer Cells via Inhibition of STAT3 Activity. PLoS One. 2013;8:e80391. doi: 10.1371/journal.pone.0080391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei X, Xu H, Kufe D. Human mucin 1 oncoprotein represses transcription of the p53 tumor suppressor gene. Cancer Res. 2007;67:1853–8. doi: 10.1158/0008-5472.CAN-06-3063. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Shimura T, Yajima T, Kubo N, Araki K, Tsutsumi S, Suzuki H, Kuwano H, Raz A. Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of β-catenin. Int J Cancer. 2011;129:2775–2786. doi: 10.1002/ijc.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D, Chen ZG, Liu SH, Dong ZQ, Dalin M, Bao SS, Hu YW, Wei FC. Galectin-3 gene silencing inhibits migration and invasion of human tongue cancer cells in vitro via downregulating β-catenin. Acta Pharmacol Sin. 2013;34:176–184. doi: 10.1038/aps.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santiago-Gómez A, Barrasa JI, Olmo N, Lecona E, Burghardt H, Palacín M, Lizarbe MA, Turnay J. 4F2hc-silencing impairs tumorigenicity of HeLa cells via modulation of galectin-3 and β-catenin signaling, and MMP-2 expression. Biochim Biophys Acta. 2013;1833:2045–2056. doi: 10.1016/j.bbamcr.2013.04.017. [DOI] [PubMed] [Google Scholar]


