Abstract
Hepatocellular carcinoma (HCC) is a highly malignant disease, and its outcome of routine therapies is poor. Comprehensive treatment including gene therapy is an important way to improve patients’ prognosis and survival. In this study, we successfully constructed a triple-controlled cancer-selective oncolytic adenovirus, QG511-HA-Melittin, carrying melittin gene, in which the hybrid promoter, hypoxia-response element (HRE)-AFP promoter, was used to control viral E1a expression targeting AFP-positive cancer cells in hypoxia microenviroment, and the E1b-55 kDa gene was deleted in cancer cells with p53-deficiency. The cytological experiments found that the viral replication of QG511-HA-Melittin was increased to 12800-folds in Hep3B cells within 48 h, and 130-folds in SMMC-7721, but the virus did not replicate in L-02 cells. QG511-HA-Melittin had a strong inhibition effect on AFP-positive HCC cell proliferation, such as Hep3B and HepG2, whereas, there was low or no inhibition effect of QG511-HA-Melittin on AFP-negative cancer cells SMMC-7721 and normal cells L-02. In the in vivo experiment, compared with the blank control group, QG511-HA-Melittin can significantly inhibit the growth of HCC xenografts (P<0.05). The survival of mice in QG511-HA-Melittin group was much longer than that of the blank control group. Both in vitro and in vivo experiments manifested that QG511-HA-Melittin exerts an inhibitory effect on HCC cells, which may provide a new strategy for HCC biotherapy.
Keywords: Hepatocellular carcinoma, oncolytic adenovirus, melittin gene, gene therapy, targeting mechanism
Introduction
As an important part in comprehensive treatments of hepatocellular carcinoma (HCC), gene therapy has provided therapeutic benefits to HCC patients and has been the focus of intense pre-clinical and clinical research during recent years [1,2].
However, traditional gene therapy showed the limited transfection, little selectivity and poor efficiency when the replication-deficient viral vectors or non-viral vectors were used to transfer anti-tumor transgenes. Safety, repeatability, and efficiency are still the key problems in HCC gene therapy. The appearance and application of cancer-selective replicating oncolytic viral vectors provided a promising strategy for treatment of solid tumors, including HCC [3]. The representative oncolytic viral vectors are ONYX-015 with adenoviral E1b-55 kDa gene deletion to target p53-deficiency cancer cells [4,5] AdSurp and AdeSurp with adenoviral E1a gene under the control of survivin promoter to target broad-spectrum of Survivin-positive cancer cells [6], AdCEAp with E1a gene under the control of CEA promoter to target CEA-positive cancer cells [7], AdE2F or AdEHE2F with E1a gene or E4 gene, respectively, under the control of E2F1 promoter to target E2F1-positive cancer cells [8,9], CNHK500 or CNHK600 with E1a gene under the control of human telomerase reverse transcriptase (hTERT) promoter and E1b gene under the control of hypoxia-response element (HRE) to target hTERT-positive cancer cells in hypoxia microenviroment [10,11], and Ad-AFP-E1A-E1B (Ä55 kDa) with both E1a gene and E1b-55 kDa gene under the control of AFP promoter to target HCC cells [12]. Oncolytic adenoviruses are promising as therapeutic agents in cancer treatment. These viruses are genetically modified to target, infect and replicate in cancer cells causing them to lyse.
Melittin is a water-soluble toxic peptide and derived from the bee venom [13]. It has multiple effects, including anti-bacterium, anti-virus and anti-inflammation [14]. Several studies showed that melittin has a strong effect on tumor cells such as induction of HCC cell apoptosis [15]. Melittin could suppress Rac1-dependent pathway and inhibit tumor cell metastasis by reducing cell motility and migration, suggesting that melittin is a potential therapeutic agent for HCC [14]. A recombinant adenovirus harboring melittin gene changed some biological behaviors of tumor cells and presented anti-tumor effect [16]. The combination of melittin with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) was proposed a promising therapeutic approach in the treatment of TRAIL-resistant human cancer, such as HCC [17]. Melittin could inhibit the growth of BEL-7402 cell xenografts in nude mice [18], and had inhibitory effect on breast cancer cells [19]. It was also demonstrated that meilltin inhibited the development and angiogenesis in human cervical cancer and breast cancer cells [19,20], or induced apoptotic cell death in ovarian cancer cells [21]. When the oncolytic adenovirus is inserted with the melittin gene, the gene copies are increased along with the viral replication specifically in tumor cells, thus enhancing the inhibitory effect on tumor cells. We hope the melittin gene will be easily and specifically introduced to tumor cells by the cancer-selective replicating adenovirus and efficiently expressed transgene along with the viral replication to reach therapeutic concentration in tumor cells.
Here we constructed an adenovirus, QG511-HA-Melittin, in which the hybrid promoter HRE-AFP was used to regulate the E1a, and the E1b-55 kDa gene was deleted, finally generating a triple-controlled oncolytic adenovirus carrying the melittin gene. We not only observed its specific replication capability in different HCC cell lines, but also explored its targeting inhibition to HCC both in the in vitro and in vivo experiments.
Materials and methods
Cell culture
The following cells were obtained from the Laboratory of Viral and Gene Therapy, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University: Hep3B and HepG2 (AFP-positive human hepatocellular carcinoma cell line), SMMC-7721 (AFP-negative human hepatocellular carcinoma cell line), L-02 (normal human liver cell line), and Human embryonic kidney (HEK) 293 cell line. All the cells were cultured in DMEM media (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich), 4 mmol/L L-glutamine, 100 units/mL penicillin and 100 ìg/mL streptomycin. Cells were grown in a humidified atmosphere at 37°C in 5% CO2 and then sub-cultured after treatment with trypsin mixture (Lonza) for 2 to 5 min at room temperature, followed by washing and re-suspension in complete media.
Construction of the viruses
Plasmids, pMD-Melittin and pHRE-AFP containing the melittin gene and the hybrid promoter HRE-AFP, respectively, were synthesized by TAKARA. The hybrid promoter HRE-AFP was inserted into adenoviral genome to control E1a gene, the E1b-55 kDa gene was deleted, and the melittin gene expressing cassette was inserted into E3 region, finally generating the replicative adenovirus QG511-HA-Melittin. The replicative adenovirus QG511-HA without melittin gene was also constructed as the control virus. The two adenoviruses were amplified in HEK293 cells and purified by ultra-centrifugation on cesium chloride (CsCl) gradients. The titers of QG511-HA-Melittin and QG511-HA were detected with the Tissue Culture Infectious Dose 50 (TCID50) method established by Qbigene (IIIkich, France), and expressed as plague-forming units per milliliter (pfu/ml).
Replicative adenovirus QG511-HA-Melittin mediated Melittin melittin expression in HCC cells
To confirm the expression of melittin gene by the Replicative adenovirus QG511-HA-Melittin targeting HCC cells, the virus-infected HCC cells and normal cells were examined with RT-PCR. The tested cells were cultured in 6-well plates at a concentration of 1×105 cells/well, and infected with adenovirus QG511-HA-Melittin and QG511-HA at a multiplicity of infection (MOI) of 5.0 pfu/cell. All the parental and infected cells were cultured for 48 h, harvested and isolated total RNA for amplify the melittin expression by RT-PCR. The specific primers for RT-PCR were Primer IX (TCC TCC TCG TAT TAG AAA C) and Primer X (CTA CTG TTG TCT CTT CCT).
In vitro viral replication assay
To observe the targeting replication of QG511-HA-Melittin, the HCC cell lines (Hep3B, HepG2 and SMMC-7721) and normal cells (L-02) were seeded in 6-well plates at a density of 1×105 cells/well, and infected with the adenoviruses QG511-HA-Melittin and QG511-HA at an MOI of 5.0 pfu/cell. After infection for 0, 48, or 96 h, the cells were harvested and the cell lysates were prepared by three cycles of freezing and thawing, and then centrifuged. Serial dilutions of the lysates were subsequently tittered on HEK293 cells according toTCID50 method.
Cytotoxicity assay in vitro
MTT method was used to assess the cytotoxicity of QG511-HA-Melittin and QG511-HA in Hep3B, HepG2, SMMC-7721 and L-02 cells. The cells were grown to confluent in 96-well plates and infected with QG511-HA-Melittin and QG511-HA with a series of MOIs (MOI=0, 0.1, 0.5, 1, 2, 5, 10, 20, 50, and 100). The cell viability was determined after 96 h post infection. Cells were exposed to 2% crystal violet in 20% methanol for 15 min, then washed with distilled water and documented.
Antitumor efficacy in vivo
Animal experiments were performed according to the SIBS Guideline for the Care and Use of Laboratory Animals. Male BALB/c nude mice (4-6 weeks old) were purchased from the SLAC Laboratory Animal Center (Shanghai, China). To establish HCC xenograft tumors, 2×107 Hep3B cells in 0.2 ml MEM were subcutaneously injected into the right flank of each mouse. When the tumor diameter reached 5-8 mm, mice were randomly divided into 3 groups as follows: 8 mice for Group QG511-HA-Melittin, 8 mice for Group QG511-HA, and 8 mice for Group NS as blank control. All the mice were exposed to 2×108 pfu virus through 5 times of intratumoral injections, one time another day. After the viral injections, tumor sizes were measured on Day 1, 3, 5, 7, and 9 with a caliper in two dimensions and tumor volume was calculated as length (mm) × width (mm) × width (mm)/2.
When the efficacy observation ended, half of mice in every group were sacrificed by anesthetization. Tumors were removed, fixed in 10% neutral formaldehyde for 12 h, embedded with paraffin and sectioned. Hematoxylin/eosin staining was performed to examine the necrosis of tumor cells and surrounding tissue cells. The other half mice in every group were continuously raised for overall survival observation.
Data analysis
All data were expressed as the mean ± S.D. and analyzed through independent sample t-test and one-way ANOVA with SPSS base 13.0. Results were considered statistically significant when P<0.05.
Results
Adenovirus QG511-HA-Melittin mediated Melittin expression targeting HCC cells
We successfully constructed the replicated adenovirus QG511-HA-Melittin, in which the hybrid promoter HRE-AFP was used to regulate the E1a, and the E1b-55 kDa gene was deleted, the melittin expressing cassette was inserted into E3 region. The negative control virus QG511-HA without melittin gene was also constructed (Figure 1A). To confirm the expression of melittin gene by the replicative adenovirus QG511-HA-Melittin targeting HCC cells, the virus-infected HCC cells and normal cells were examined with RT-PCR. Under the control of hybrid promoter HRE-AFP, the virus replicates and mediates melittin expression specifically in HCC cells, the melittin gene was amplified along with the viral replication and expressed efficiently in the AFP-positive HCC cells, but not in the AFP-negative HCC cells and normal cells (Figure 1B).
Figure 1.
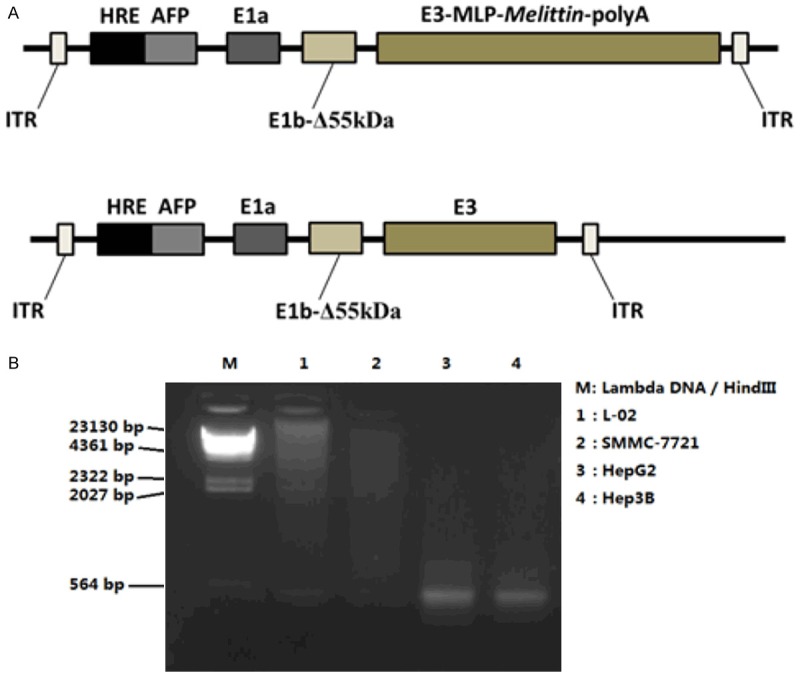
A. Schematic representation of the adenovirus, in which in which the hybrid promoter HRE-AFP was used to regulate the E1a, and the E1b-55 kDa gene was deleted, the Melittin expressing cassette was inserted into E3 region. The negative control virus QG511-HA without Melittin gene was also constructed. B. Indicated cells were infected with QG511-HA-Melittin, and examined with RT-PCR.
Targeting replication of virus QG511-HA-Melittinin in HCC cells
In order to analyze the replication capability of the adenovirus, the viral replication experiments were performed to evaluate the selective replication of QG511-HA-Melittin in different cells. The results showed that QG511-HA-Melittin was replicated efficiently in AFP-positive cell lines, Hep3B and HepG2, but replicated weakly in AFP-negative cell lines, SMMC-7721 and normal cells L-02. At 48 h after viral infection, the replication of QG511-HA-Melittinin was increased to 12800 folds in Hep3B and 5000 folds in HepG2, only 130 folds and 196 folds increased in L-02 and SMMC-7721 cells, respectively. At 96 h after viral infection, the replication specificity of QG511-HA-Melittin was slowed down (Figure 2).
Figure 2.
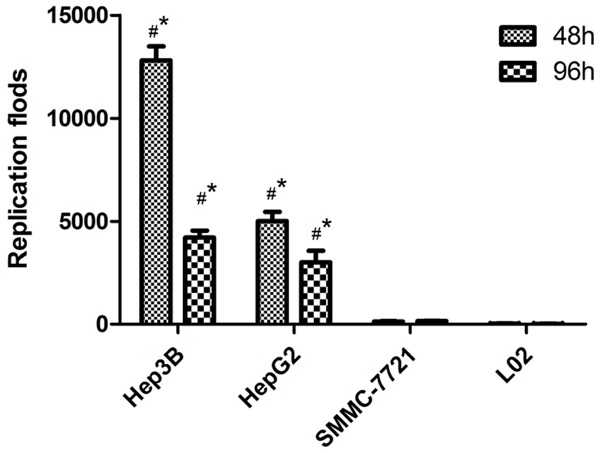
Replication folds of QG511-HA-Melittin in indicated cells at 48 h and 96 h after viral infection. Compared with 0 h in different cells, *P<0.01, #P<0.01.
Cytotoxic activity of QG511-HA-Melittinon in vitro
The cytotoxic activity of QG511-HA-Melittin in different cell lines was investigated. Compared with that of the control virus QG511-HA without Melittin gene, QG511-HA-Melittin has an obvious killing effect on Hep3B and HepG2 cells, the effective dosages for killing 50% cells (ED50) of QG511-HA-Melittin in Hep3B and HepG2 were MOI=5 and 10 pfu/cell, respectively. The survival of SMMC-7721 or L-02 was hardly affected by QG511-HA-Melittin and QG511-HA, indicating that QG511-HA-Melittin can specifically inhibit the proliferation of AFP-positive tumor cells and has the limited effect on normal cells and AFP-negative tumor cells (Figure 3).
Figure 3.
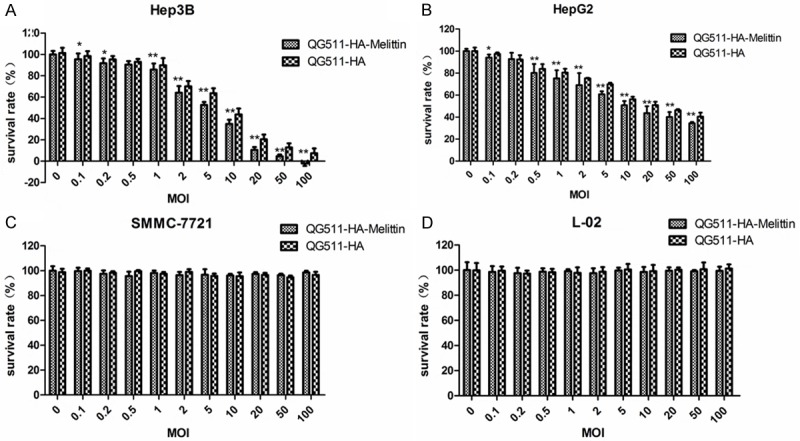
The survival rate of QG511-HA-Melittin in indicated cell lines. The cells were grown to confluent in 96-well plates and infected with QG511-HA-Melittin with a series of MOIs (MOI=0, 0.1, 0.5, 1, 2, 5, 10, 20, 50, and 100). The cell viability was determined after 96 h post infection. MTT method was used to assess the cytotoxicity. QG511-HA was used as the control. Compared with MOI=0 group, *P<0.05, **P<0.01.
Antitumor efficacy of QG511-HA-Melittinon in vivo
Inhibitory effect of QG511-HA-Melittin and QG511-HA on tumor growth was investigated in Hep3B tumor xenograft models in nude mice. The results showed that the inhibition rate of QG511-HA-Melittin and QG511-HA had significant differences compared with that of the NS group (P<0.05), demonstrating that QG511-HA-Melittin and QG511-HA can much more effectively inhibit the growth of the HCC xenografts (Figure 4A). There was significant difference between QG511-HA-Melittin group and QG511-HA group (P<0.05). The overall survival (OS) of mice with HCC xenografts was observed, and the results showed that mice in the QG511-HA-Melittin group and QG511-HA group have longer OS than mice in the NS group (P<0.05; Figure 4B). The median survival was different significantly between QG511-HA-Melittin group and QG511-HA group (P<0.05; Figure 4B).
Figure 4.
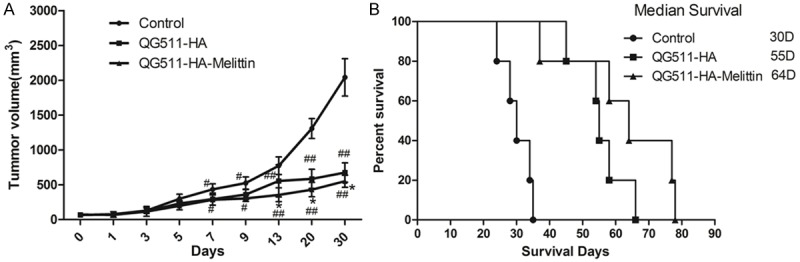
Antitumor effect of QG511-HA-Melittin in nude mice. A. QG511-HA-Melittin and QG511-HA both showed apparent therapeutic effects in mice bearing Hep3B tumor xenograft models, and QG511-HA-Melittin exhibited an even better antitumor efficacy than QG511-HA. Compared with control group, #p<0.01, ##p<0.05; Compared with QG511-HA *P<0.05. B. Mice treated with QG511-HA-Melittin and QG511-HA survived noticeably longer than the control groups, and mice treated with QG511-HA-Melittin presented with a longer median survival time.
Pathological examination of the xenografts tumor
Pathological examination of all the tumor xenografts was implemented by H&E staining in different groups. H&E staining showed that there were more and wider necrotic areas in the QG511-HA-Melittin group and QG511-HA group than in the NS group. More efficient antiangiogenic effect was presented in the QG511-HA-Melittin group with cell shrinkage and progression towards apoptosis (Figure 5). The results indicated the potential tumor inhibition activity of QG511-HA-Melittin and QG511-HA in HCC xenografts.
Figure 5.
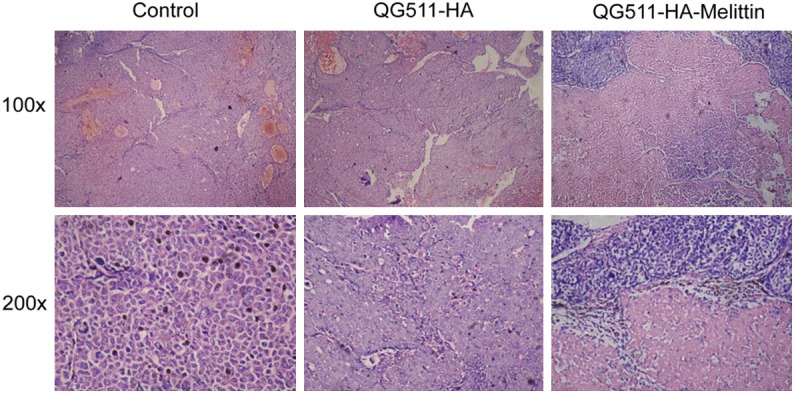
H&E staining, indicating pathomorphological changes. There were more and wider necrotic areas in the QG511-HA-Melittin group and QG511-HA group than in the NS group. More efficient antiangiogenic effect was presented in the QG511-HA-Melittin group with cell shrinkage and progression towards apoptosis.
Discussion
Hepatocellular carcinoma (HCC) accounts for the majority of primary liver cancers. More than 80% of HCC patients occur in less developed countries, particularly in East Asia and sub-Saharan Africa [22]. HCC develops quickly, and the treatments of later-stage HCC are difficult. The possible therapeutic methods include the small molecule tyrosine-kinase inhibitor sorafenib [23], percutaneous ethanol injection [24], trans-arterial chemoembolization (TACE) [25], radiofrequency ablation [26] and TCM [27], All these treatments are rarely curative. Therefore, it is necessary to identify novel therapeutic strategy targeting later-stage HCC. Recently, gene therapy has attracted more attention in the treatment of HCC patients, in which the tumor-selective replicating oncolytic adenoviruses are promising as therapeutic agents [28]. To generate oncolytic adenovirus as transgene vector, it is necessary to limit the replication of virus within cancer cells; the potential mechanism is that manipulating the viral early replicative genes under the control of tumor-specific promoters.
HRE can be used to restrict virus replication in cancer cells in hypoxia microenviroment by controlling viral replicative gene. Hypoxia is an important microenvironment in carcinogenesis, tumor invasion and proliferation. Hypoxia not only alters macrophages to tumor associated macrophage (TAM)-like phenotype, leading to immunosuppression and angiogenesis and inducing angiogenic cytokines by hepatocytes cells [13], but also plays an important role in tumor metastasis. Additionally, it can induce epithelial-mesenchymal transition and chemoresistance in hepatocellular carcinoma [20]. AFP promoter is one of typical tumor specific cis-element which can restrict the expression of therapeutic genes in HCC cells. A strong and HCC-selective promoter by linking the AFP enhancer with the 5’-UTR sequence of the non-tissue-specific, human housekeeping phosphoglycerate kinase (pgk) gene was generated and used to drive expression of DN-PP2Acá, which provided a useful cancer gene therapy strategy to selectively target HCC [29]. Cany et al. also demonstrated that AFP-specific immunotherapy impaired the growth of autochthonous hepatocellular carcinoma in mice [30]. All these investigations demonstrated that both HRE and AFP promoter can regulate viral vector to replicate specifically in various cancer cells, and the inserted hybrid promoter HRE-AFP may provide dual-regulation and allow the oncolytic adenoviral vectors more precisely targeting HCC cells.
Under the control of hybrid promoter HRE-AFP, we successfully constructed the tumor-selective replicating adenovirus, QG511-HA-Melittin, carrying melittin gene which was controlled by the major late promoter (MLP). The virus was also deleted the E1b-55 kDa gene. Therefore, the virus QG511-HA-Melittin is exactly a ripple-regulated oncolytic adenovirus with three targeting mechanism to HCC cells, mainly targeting cancer cells with positive AFP, cells in hypoxia microenviroment and cells with P53-deficiency. The oncolytic adenovirus QG511-HA-Melittin showed tumor-selective replication capability in AFP-positive tumor cell lines. It was demonstrated that the specific inhibition effect of QG511-HA-Melittin on proliferation of AFP-positive HCC was strong, but with limited effect to normal cells or AFP-negative cancer cells. The results proposed a novel treatment in HCC.
Acknowledgements
This work is supported in part by National Natural Science Foundation of China 30772877 (to BL) and Grant from Natural Science Foundation of Shanghai 07ZR14138 (to BL) and 12ZR1437400 (to LW).
Disclosure of conflict of interest
None.
References
- 1.Ling CQ, Wang LN, Wang Y, Zhang YH, Yin ZF, Wang M, Ling C. The roles of traditional Chinese medicine in gene therapy. J Integr Med. 2014;12:67–75. doi: 10.1016/S2095-4964(14)60019-4. [DOI] [PubMed] [Google Scholar]
- 2.Verma IM, Somia N. Gene therapy--promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 3.Gentschev I, Muller M, Adelfinger M, Weibel S, Grummt F, Zimmermann M, Bitzer M, Heisig M, Zhang Q, Yu YA, Chen NG, Stritzker J, Lauer UM, Szalay AA. Efficient colonization and therapy of human hepatocellular carcinoma (HCC) using the oncolytic vaccinia virus strain GLV-1h68. PLoS One. 2011;6:e22069. doi: 10.1371/journal.pone.0022069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DD, Berk AJ. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infec-siRNA tion and DNA transfection. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 5.O’Shea CC, Soria C, Bagus B, McCormick F. Heat shock phenocopies E1B-55K late functions and selectively sensitizes refractory tumor cells to ONYX-015 oncolytic viral therapy. Cancer Cell. 2005;8:61–74. doi: 10.1016/j.ccr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Sun B, An N, Tan W, Cao L, Luo X, Yu Y, Feng F, Li B, Wu M, Su C, Jiang X. Inhibitory effect of Survivin promoter-regulated oncolytic adenovirus carrying P53 gene against gallbladder cancer. Mol Oncol. 2011;5:545–554. doi: 10.1016/j.molonc.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagawa T, Takahashi M, Sato T, Sato Y, Lu Y, Sumiyoshi T, Yamada Y, Iyama S, Fukaura J, Sasaki K, Hamada H, Miyanishi K, Takayama T, Kato J, Niitsu Y. Prolonged survival of mice with multiple liver metastases of human colon cancer by intravenous administration of replicable E1B-55K-deleted adenovirus with E1A expressed by CEA promoter. Mol Ther. 2004;10:1043–1050. doi: 10.1016/j.ymthe.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Alonso MM, Cascallo M, Gomez-Manzano C, Jiang H, Bekele BN, Perez-Gimenez A, Lang FF, Piao Y, Alemany R, Fueyo J. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res. 2007;67:8255–8263. doi: 10.1158/0008-5472.CAN-06-4675. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann D, Wildner O. Restriction of adenoviral replication to the transcriptional intersection of two different promoters for colorectal and pancreatic cancer treatment. Mol Cancer Ther. 2006;5:374–381. doi: 10.1158/1535-7163.MCT-05-0374. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Chen G, Peng L, Wang X, Yang Y, Liu C, Shi W, Su C, Wu H, Liu X, Wu M, Qian Q. Increased safety with preserved antitumoral efficacy on hepatocellular carcinoma with dual-regulated oncolytic adenovirus. Clin Cancer Res. 2006;12:6523–6531. doi: 10.1158/1078-0432.CCR-06-1491. [DOI] [PubMed] [Google Scholar]
- 11.Zhu W, Wei L, Zhang H, Chen J, Qin X. Oncolytic adenovirus armed with IL-24 inhibits the growth of breast cancer in vitro and in vivo. J Exp Clin Cancer Res. 2012;31:51. doi: 10.1186/1756-9966-31-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang F, Ma B, Wang Y, Xiao R, Kong Y, Zhou X, Xia D. Targeting gene-virus-mediated manganese superoxide dismutase effectively suppresses tumor growth in hepatocellular carcinoma in vitro and in vivo. Cancer Biother Radiopharm. 2014;29:403–411. doi: 10.1089/cbr.2014.1642. [DOI] [PubMed] [Google Scholar]
- 13.Tu T, Budzinska MA, Maczurek AE, Cheng R, Di Bartolomeo A, Warner FJ, McCaughan GW, McLennan SV, Shackel NA. Novel aspects of the liver microenvironment in hepatocellular carcinoma pathogenesis and development. Int J Mol Sci. 2014;15:9422–9458. doi: 10.3390/ijms15069422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Yu M, He Y, Xiao L, Wang F, Song C, Sun S, Ling C, Xu Z. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology. 2008;47:1964–1973. doi: 10.1002/hep.22240. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Gu W, Zhang C, Huang XQ, Han KQ, Ling CQ. Growth arrest and apoptosis of the human hepatocellular carcinoma cell line BEL-7402 induced by melittin. Onkologie. 2006;29:367–371. doi: 10.1159/000094711. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Ling CQ, Zhang C, Gu W, Li SX, Huang XQ, Zhang YN, Yu CQ. The induced apoptosis of recombinant adenovirus carrying melittin gene for hepatocellularcarcinoma cell. Zhonghua Gan Zang Bing Za Zhi. 2004;12:453–455. [PubMed] [Google Scholar]
- 17.Song CC, Lu X, Cheng BB, DU J, Li B, Ling CQ. Effects of melittin on growth and angiogenesis of human hepatocellular carcinoma BEL-7402 cell xenografts in nude mice. Ai Zheng. 2007;26:1315–1322. [PubMed] [Google Scholar]
- 18.Wang C, Chen T, Zhang N, Yang M, Li B, Lu X, Cao X, Ling C. Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha kinase-NFkappaB. J Biol Chem. 2009;284:3804–3813. doi: 10.1074/jbc.M807191200. [DOI] [PubMed] [Google Scholar]
- 19.Jeong YJ, Choi Y, Shin JM, Cho HJ, Kang JH, Park KK, Choe JY, Bae YS, Han SM, Kim CH, Chang HW, Chang YC. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem Toxicol. 2014;68:218–225. doi: 10.1016/j.fct.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Shin JM, Jeong YJ, Cho HJ, Park KK, Chung IK, Lee IK, Kwak JY, Chang HW, Kim CH, Moon SK, Kim WJ, Choi YH, Chang YC. Melittin suppresses HIF-1alpha/VEGF expression through inhibition of ERK and mTOR/p70S6K pathway in human cervical carcinoma cells. PLoS One. 2013;8:e69380. doi: 10.1371/journal.pone.0069380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258:72–81. doi: 10.1016/j.taap.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, Group SIS. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 24.Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458–464. doi: 10.1016/j.jhep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Hucke F, Sieghart W, Pinter M, Graziadei I, Vogel W, Muller C, Heinzl H, Waneck F, Trauner M, Peck-Radosavljevic M. The ART-strategy: sequential assessment of the ART score predicts outcome of patients with hepatocellular carcinoma re-treated with TACE. J Hepatol. 2014;60:118–126. doi: 10.1016/j.jhep.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Huertas A, Baumann AS, Saunier-Kubs F, Salleron J, Oldrini G, Croisé-Laurent V, Barraud H, Ayav A, Bronowicki JP, Peiffert D. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol. 2015;115:211–216. doi: 10.1016/j.radonc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med. 2014;12:331–335. doi: 10.1016/S2095-4964(14)60038-8. [DOI] [PubMed] [Google Scholar]
- 28.Guo ZS, Thorne SH, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Li DM, Chen K, Chen Z, Zong Y, Yin H, Xu ZK, Zhu Y, Gong FR, Tao M. Development of a gene therapy strategy to target hepatocellular carcinoma based inhibition of protein phosphatase 2A using the alpha-fetoprotein promoter enhancer and pgk promoter: an in vitro and in vivo study. BMC Cancer. 2012;12:547. doi: 10.1186/1471-2407-12-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cany J, Barteau B, Tran L, Gauttier V, Archambeaud I, Couty JP, Turlin B, Pitard B, Vassaux G, Ferry N, Conchon S. AFP-specific immunotherapy impairs growth of autochthonous hepatocellular carcinoma in mice. J Hepatol. 2011;54:115–121. doi: 10.1016/j.jhep.2010.06.027. [DOI] [PubMed] [Google Scholar]


