Abstract
Mucinous ovarian cancer accounts for almost 10% of epithelial ovarian cancer, though patients in the early stage have an excellent prognosis, patients with advanced disease have a poor outcome and the molecular mechanism remains unclear. In this study, we retrieved database and found a secretory protein TFF1, which plays a tumor suppressor role in gastric cancer, is highly expressed in mucinous ovarian cancer, not serous or any other kind of ovarian cancer. Furthermore, the highly expressed TFF1 indicates a poor clinical outcome. Further biological function analysis revealed that TFF1 not only promotes cell proliferation but also invasion and chemoresistance, and these oncogenic effects might through regulating the activity of Wnt/β-catenin signaling and the level of Twist. Taken together, we found TFF1 plays an oncogenic role in mucinous ovarian cancer, unlike its suppressive role in gastric cancer.
Keywords: Trefoil Factor 1, mucinous ovarian cancer, cell proliferation, cell invasion, chemoresistance, Wnt/β-catenin signaling
Introduction
Ovarian cancer is responsible for 4% of deaths from cancer in women [1]. Pathologically, ovarian cancer can be divided into three subgroups: epithelial, stromal and germ cell tumor [2] and epithelial ovarian cancer accounting for more than 90% of diagnosed ovarian cancer, and 10% of patients suffering from epithelial ovarian cancer are mucinous ovarian carcinoma [3]. They appear to have a different natural history from the other histological types in terms of presentation, response to therapy and outcome [4]. Mucinous ovarian cancer usually diagnosed at stage I, though infiltrative growth pattern is less frequent, it appears to follow a more aggressive course [5]. For mucinous ovarian cancer diagnosis, CK 7 and CK 20 were usually applied to immunohistochemistry, while it was not enough for diagnosis [6]. Molecular studies have identified that the overexpression of k-ras and mutation of p53 in mucinous ovarian cancer [7], while the detailed molecular mechanisms of ovarian cancer is still unclear.
In current study, we found that TFF1 is specified highly expressed in mucinous ovarian cancer. TFF1 is a 9 KD extracellular matrix protein, which belongs to the trefoil family [8]. Previously study has revealed that loss of TFF1 promotes gastric tumorigenesis through Wnt/β-catenin signaling [9], and both miRNA and DNA methylation resulted in the loss of TFF1 in gastric cancer [10,11]. Except gastric cancer, TFF1 also plays a role in breast cancer. In breast cancer, the level of TFF1 is induced by estrogen and TFF1 stimulates breast cancer cell migration by acting as a chemoattractant [12]. While the mRNA level of TFF1 exhibited an inverse association with tumor size, histological grade [13]. These findings suggested that TFF1 might exert its effects in a context dependent manner. However, the role of TFF1 in ovarian cancer has not been reported to date. In this study, we reported the expressed pattern and the biological functions of TFF1 in ovarian cancer.
Materials and methods
Cell culture
In this study, OMC-3 and MCAS were derived from mucinous adenocarcinomas. Caov-3 was derived from high-grade serous ovarian cancer. OMC-3 was purchased from RIKEN Cell Bank of Japan, MCAS were obtained from the Japanese Collection of Research Bioresources Cell Bank and Caov-3 cell lines were purchased from ATCC. OMC-3 cells were grown in Ham’s F12 medium; MCAS cells were grown in minimum essential medium and Caov-3 were grown in Dulbecco’s modified Eagle medium. All those cells were maintained under standard culture conditions (37°C, 5% CO2).
RNA isolation and quantitative real-time PCR
Total RNA was purified from ovarian cancer cells using TRIzol (Invitrogen) following the manufacturer’s protocol. RNA (1 μg) was reverse transcribed using SuperScript Reverse Transcriptase III (Invitrogen). Quantitative real time PCR was performed using SYBR green Supermix (ABI) in ABI 7500 PCR system. Housekeeping gene GAPDH was used as an internal standard. Primers’ sequences were as following, TFF1, forward: 5’-GGAGAACAAGGTGATCTGCG-3’; reverse: 5’-AATTCTGTCTTTCACGGGGG-3’. GAPDH, forward: 5’-CTGGGCTACACTGAGCACC-3’; reverse: 5’-AAGTGGTCGTTGAGGGCAATG-3’.
Western blot
Cells were lysed in WB/IP lysis buffer (P0013, Beyotime), all the procedures were following the manufacturer’s protocol. Subsequently the cell lysates were boiled in 5× SDS-PAGE loading buffer for 10 min and then resolved by 12% SDS-PAGE and transferred to nitrocellulose membrane. The following antibodies were used in this study: TFF1 (Proteintech), GAPDH (Proteintech), β-catenin (CST). Bound antibodies were visualized with the ECL kit (Thermo Scientific).
Small interfering RNA treatment
Cells were tranfected with small interfering RNA (siRNA, GenePharma) targeting TFF1 or with a scrambled siRNA as a negative control. The sequences of si RNAs were as following. Si-#1, 5’-AGACACTTCTGCAGGGATCT-3’, si-#2, 5’-GCTGTTTCGACGACACCGT-3’. Transfections were performed using Lipofectamine RNAi MAX reagent (Invitrogen), all the procedures were following the manufacturer’s procotol.
Overexpression of TFF1
To overexpress TFF1, vectors containing the ORF of TFF1 were obtained from Genecopoeia. We then transfected 293T cells using lipofectamine 2000 (Invitrogen) with those vectors and all the procedures were according to the manufactures protocols. The supernatant media containing virus was collected by centrifugation to remove cellular contaminant. The resulting viruses were used to infect indicated cells, and then integrated cells were selected by 2 μg/ml puromycin for 2 weeks. The alterations of TFF1 in those cells were confirmed by western blot before further analysis.
CCK-8 cell viability assays
Cells were seeded into a 96-well plate at 3×103 cells per well with 100ul cultured medium or medium which containing indicated concentration of cisplatin (Sigma-Alorich) and cultured at 37°C, 5% CO2. The cell viability was quantified by addition 10 μl of cell counting kit (CCK-8, Dojindo). After 1.5 hours incubation, the plates were monitored by Power Wave XS microplate reader (BIO-TEK) at an absorbance 450 nm. Cisplatin was resolved by DMSO.
Transwell cell invasion assays
We performed transwell cell invasion assays using transwell chamber (BD Biosciences, USA). In brief, 100 μl matrigel was injected into the upper chamber and then incubated in 37°C for 2 hours. Then cells were seeded into the upper chamber at a density of 4×104 cells per 100 µl per chamber and maintained in serum-free medium, and lower chambers were filled with 700 µl complete medium. Cells were incubated for 48 hours at 37°C, 5% CO2. Non-invaded cells retaining on the upper surface were scrubbed with a cotton swab. The invaded cells were fixed and then stained with 0.1% crystal violet.
Statistical analysis
Data present here were expressed as the mean ± standard deviation. The Student’s t-test was used for comparisons between groups and P<0.05 was considered statistically significant difference.
Results
The level of TFF1 is up-regulated in mucinous ovarian cancer
We retrieved Oncomine database and found that the mRNA level of TFF1 is significantly up-regulated in mucinous ovarian cancer in two independent GSE data profiles (Figure 1A and 1B). We further fond that TFF1 is not only elevated in mucinous ovarian cancer, the highly expressed TFF1 also predicts a poor clinical outcome (Figure 1C). TFF1 is a poorly studied gene in ovarian cancer to data, so the specified expression pattern of TFF1 in mucinous ovarian cancer, not serous or clear cell adenocarcinoma, inspired us to explore its biological functions in ovarian cancer, especially in mucinous ovarian cancer. We then examined the mRNA and protein level of TFF1 in four ovarian cancer cell lines, and OMC-3 and MCAS were isolated from mucinous ovarian tissues. Caov-3 and OVCAR3 were derived from serous ovarian carcinoma. We found that both the mRNA and protein level of TFF1 were highly expressed in OMC-3 and MCAS cells (Figure 1D). We then silenced TFF1 in these two cells and overexpressed TFF1 in Caov-3 cell lines. As presented in Figure 2A, both knockdown and overexpression of TFF1 were remarkably altered the level of TFF1 in these cell lines.
Figure 1.
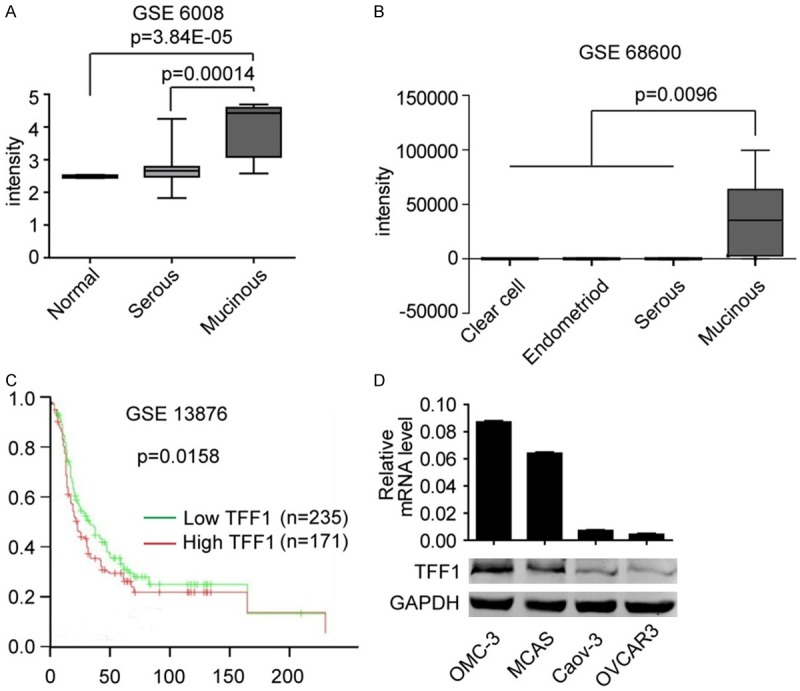
TFF1 is highly expressed in Mucinous ovarian cancer. Data analyzed from GEO dataset GSE6008 (A) and GSE68600 (B) suggests that the level of TFF1 is significantly higher in mucinous ovarian cancer than in normal ovary and other kind of ovarian cancer. (C) Data analyzed from GSE13878 suggests that highly expressed TFF1 indicates a poor prognosis in patients. (D) Both mRNA and protein level of TFF1 is higher in mucinous ovarian cancer cell line OMC-3 and MCAS than in other kind of ovarian cancer cell line Caov-3 and OVCAR3.
Figure 2.
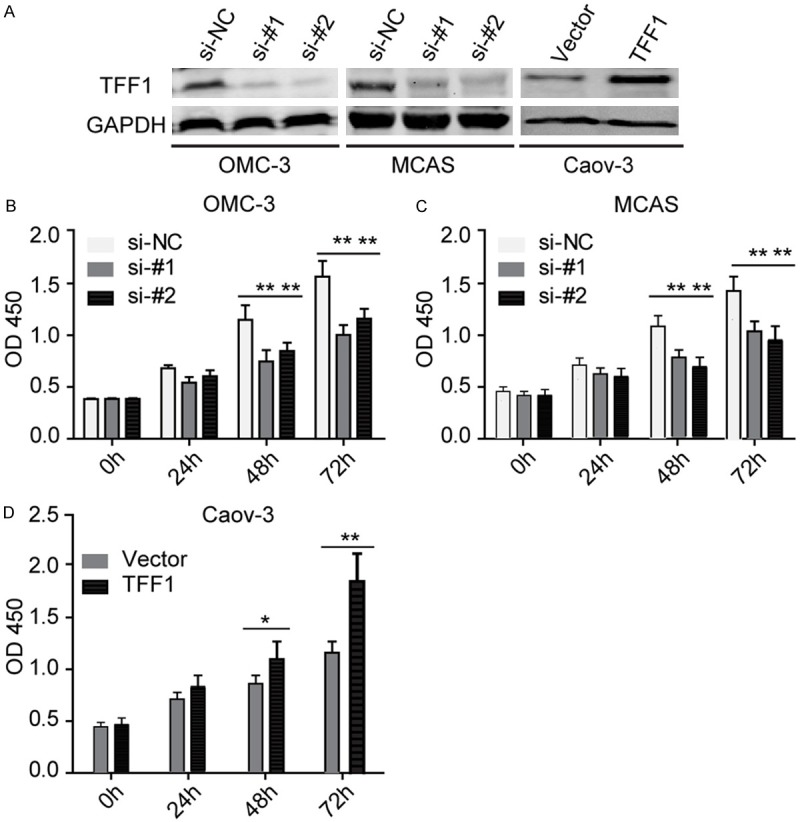
TFF1 elevates ovarian cancer cell viability. A: The level of TFF1 was significantly reduced and increased when TFF1 was knockdown and overexpressed respectively in indicated cell lines, GAPDH was used as loading control. B, C: Silencing TFF1 inhibits OMC-3 and MCAS cell viability significantly in indicated time points, **P<0.01. D: Overexpression of TFF1 remarkably elevates Caov-3 cell viability, *P<0.05, **P<0.01. All the experiments were triplicate.
TFF1 elevates the abilities of cell viability and invasion in ovarian cancer
Firstly, we performed CCK-8 cell viability assays. Surprisingly, both loss and gain function assays revealed that TFF1 significantly elevates the cell viability (Figure 2B-D). We then used these transfected cell lines performed transwell cell invasion assays, and we found that TFF1 also remarkably promotes ovarian cancer cell invasion (Figure 3). These findings remind us that TFF1 plays an aggressive role in mucinous ovarian cancer progression.
Figure 3.
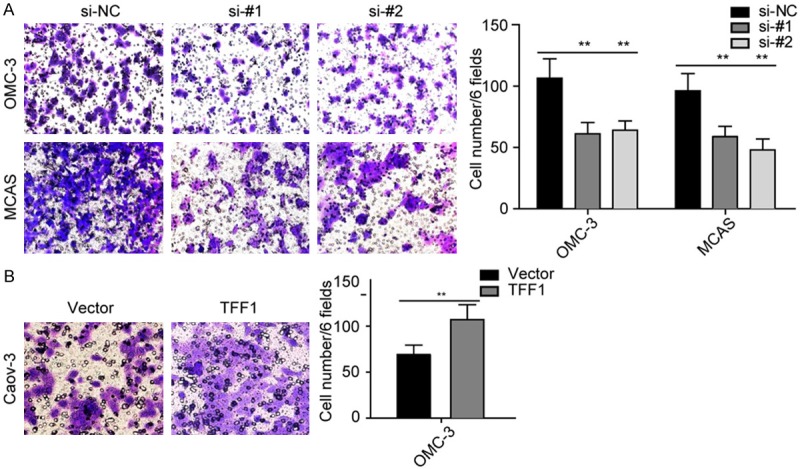
TFF1 promotes cell invasion. A: Silencing TFF1 inhibits OMC-3 and MCAS cell invasion in vitro. B: Overexpression of TFF1 promotes Caov-3 cell invasion significantly. **P<0.01. All the experiments were triplicate.
TFF1 promotes ovarian cancer cell chemoresistance to cisplatin
Considering that chemoresistance is a malignant characteristic in ovarian cancer, we then used these transfected cell lines performed chemosensitivity assays. As showed in Figure 4, we treated these cells with different concentration of cisplatin for 72 hours and then examined the cell viability and we found that TFF1 enhances ovarian cancer cells the ability of chemoresistance to cisplatin.
Figure 4.
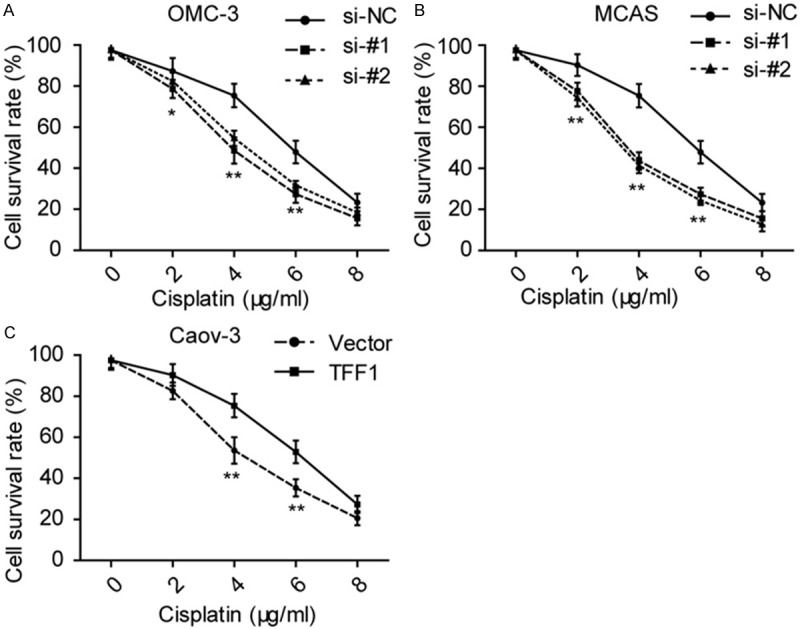
TFF1 elevates ovarian cancer cell the ability of chemoresistance. (A, B) Knockdown of OMC-3 inhibits cell viability when treated OMC-3 (A) and MCAS (B) with indicated concentration of csiplatin. (C) Elevates the level of TFF1 promotes Caov-3 cell viability in indicated concentration of cisplatin. *P<0.05, **P<0.01. All the experiments were triplicate.
TFF1 is related to the Wnt/β-catenin signaling
Our findings remind us that TFF1 plays an aggressive role in mucinous ovarian cancer. We then detected the level of CCND1, c-myc and Twist, which were reported plays an essential role in the development of ovarian cancer and chemoresistance respectively. Surprisingly, as shown in Figure 5A and 5B, the mRNA level of CCND1 and c-myc were positively correlated with the level of TFF1. Furthermore, we found that the level of Twist is related to TFF1 too. As CCND1 and c-myc are the down-stream effecter of Wnt/β-catenin signaling, these findings remind us that TFF1 might in some way regulate the activity of Wnt/β-catenin signaling. Twist has been reported that plays a role in EMT and chemoresistance, our results given a clue that there was some association between TFF1 and Twist.
Figure 5.
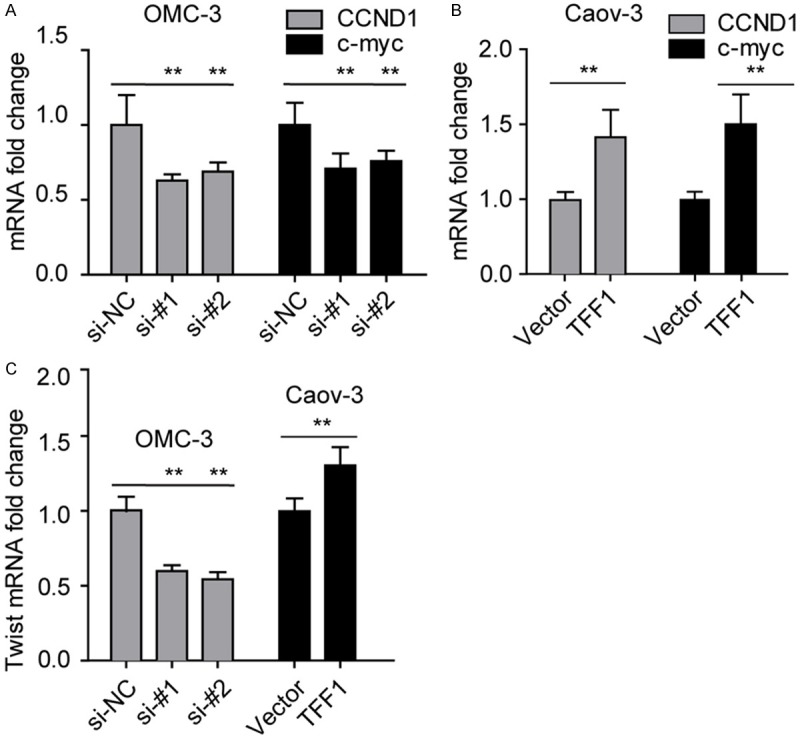
TFF1 elevates the level of CCND1, c-myc and Twist. (A, B) Knockdown of TFF1 in OMC-3 (A) and MCAS (B) inhibits the expression of CCND1 and c-myc. (C) The level of TFF1 was positive correlated with the level of Twist. **P<0.01, all the experiments were triplicate.
Discussion
The key finding from this study is that highly expressed TFF1 plays an oncogenic role in mucinous ovarian cancer. Silencing TFF1 results in antitumor activity like inhibiting cell proliferation and invasion, in mucinous ovarian cancer. High mRNA level of TFF1 expression was associated with worse survival in ovarian cancer patients. Furthermore, we found that the level of TFF1 was positively correlated with the level of CCND1, c-myc and Twist.
TFF1 is a membrane of trefoil family peptides, which are characterized by having at least one copy of the trefoil motif, a 40-amino acid domain that contains three conserved disulfides [8]. There are a large body of paper has suggested that TFF1 is a tumor suppressor in gastric cancer. While in current study, our results indicated that TFF1 is a tumor promoter in mucinous ovarian cancer. Previously study has revealed that estrogen regulated the expression of TFF1 through estrogen receptor alpha (ERα). However, Peter R. et al. reported that ER β but ER α was positive in mucinous ovarian tumors. The oncogenic effects of TFF1 in mucinous ovarian cancer might through any other pathway; it was still need to be fully elucidated in further study.
Previously study has revealed that loss of TFF1 could activate β-catenin signaling in gastric cancer. In current study, considering the inverse effects of TFF1 in mucinous ovarian cancer compared with gastric cancer, we then given the idea that highly expressed TFF1 activates the β-catenin signaling. We then detected the level of CCND1 and c-myc, which are the targets of β-catenin [14,15], and we found the level of CCND1 and c-myc are positively correlated with the level of TFF1. The interesting findings were agreement with the effects of TFF1 in mucinous ovarian cancer. Not only CCND1 and c-myc, but also the level of Twist was also positively associated with TFF1. As we known, Twist plays pivotal role in EMT and chemoresistance by activating AKT2 and Erk 1/2 axis [16,17]. In this study, we found that TFF1 also plays a role in chemoresistance to cisplatin; the level of Twist changed could explain this phenomenon. However, the detailed mechanisms of TFF1 in mucinous ovarian cancer need to be further explored.
In conclusion, in current study, we found a canonical tumor suppressor TFF1 plays an oncogenic role in mucinous cancer development, and the highly expressed TFF1 predicts a poor clinical outcome.
Disclosure of conflict of interest
None.
References
- 1.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 2.Karst AM, Drapkin R. Ovarian cancer pathogenesis: a model in evolution. J Oncol. 2010;2010:932371. doi: 10.1155/2010/932371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan RJ Jr, Alvarez RD, Armstrong DK, Boston B, Burger RA, Chen LM, Copeland L, Crispens MA, Gershenson D, Gray HJ, Grigsby PW, Hakam A, Havrilesky LJ, Johnston C, Lele S, Matulonis UA, O’Malley DM, Penson RT, Remmenga SW, Sabbatini P, Schilder RJ, Schink JC, Teng N, Werner TL National Comprehensive Cancer Network. Epithelial ovarian cancer. J Natl Compr Canc Netw. 2011;9:82–113. doi: 10.6004/jnccn.2011.0008. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie R, Talhouk A, Eshragh S, Lau S, Cheung D, Chow C, Le N, Cook LS, Wilkinson N, McDermott J, Singh N, Kommoss F, Pfisterer J, Huntsman DG, Kobel M, Kommoss S, Gilks CB, Anglesio MS. Morphologic and Molecular Characteristics of Mixed Epithelial Ovarian Cancers. Am J Surg Pathol. 2015;39:1548–57. doi: 10.1097/PAS.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez IM, Prat J. Mucinous tumors of the ovary: a clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol. 2002;26:139–152. doi: 10.1097/00000478-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002;21:391–400. doi: 10.1097/00004347-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Fujita M, Enomoto T, Murata Y. Genetic alterations in ovarian carcinoma: with specific reference to histological subtypes. Mol Cell Endocrinol. 2003;202:97–99. doi: 10.1016/s0303-7207(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Fujii R, Kida N, Takahashi H, Ohkubo S, Fujino M, Ohta M, Hayashi K. Complete primary structure of the human estrogen-responsive gene (pS2) product. J Biochem. 1990;107:73–76. doi: 10.1093/oxfordjournals.jbchem.a123014. [DOI] [PubMed] [Google Scholar]
- 9.Soutto M, Romero-Gallo J, Krishna U, Piazuelo MB, Washington MK, Belkhiri A, Peek RM Jr, El-Rifai W. Loss of TFF1 promotes Helicobacter pylori-induced beta-catenin activation and gastric tumorigenesis. Oncotarget. 2015;6:17911–22. doi: 10.18632/oncotarget.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, Chen GB, Huang QW, Chen X, Liu JJ, Xu W, Huang XX, Liu YP, Xiao CX, Wu DC, Guleng B, Ren JL. miR218-5p regulates the proliferation of gastric cancer cells by targeting TFF1 in an Erk1/2-dependent manner. Biochim Biophys Acta. 2015;1852:970–979. doi: 10.1016/j.bbadis.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho R, Kayademir T, Soares P, Canedo P, Sousa S, Oliveira C, Leistenschneider P, Seruca R, Gött P, Blin N, Carneiro F, Machado JC. Loss of heterozygosity and promoter methylation, but not mutation, may underlie loss of TFF1 in gastric carcinoma. Lab Invest. 2002;82:1319–26. doi: 10.1097/01.lab.0000029205.76632.a8. [DOI] [PubMed] [Google Scholar]
- 12.Prest SJ, May FE, Westley BR. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J. 2002;16:592–594. doi: 10.1096/fj.01-0498fje. [DOI] [PubMed] [Google Scholar]
- 13.Gillesby BE, Zacharewski TR. pS2 (TFF1) levels in human breast cancer tumor samples: correlation with clinical and histological prognostic markers. Breast Cancer Res Treat. 1999;56:253–65. doi: 10.1023/a:1006215310169. [DOI] [PubMed] [Google Scholar]
- 14.Röhrs S, Kutzner N, Vlad A, Grunwald T, Ziegler S, Müller O. Chronological expression of Wnt target genes Ccnd1, Myc, Cdkn1a, Tfrc, Plf1 and Ramp3. Cell Biol Int. 2009;33:501–508. doi: 10.1016/j.cellbi.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Muncan V, Sansom OJ, Tertoolen L, Phesse TJ, Begthel H, Sancho E, Cole AM, Gregorieff A, de Alboran IM, Clevers H. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol Cell Biol. 2006;26:8418–8426. doi: 10.1128/MCB.00821-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Li L, Zeng J, Wu K, Zhou J, Guo P, Zhang D, Xue Y, Liang L, Wang X. Twist confers chemoresistance to anthracyclines in bladder cancer through upregulating P-glycoprotein. Chemotherapy. 2012;58:264–72. doi: 10.1159/000341860. [DOI] [PubMed] [Google Scholar]


