Abstract
Previous studies indicate that the Nrf2-ARE signaling pathway plays a neruo-protective role in glia cell, however, the mechanism was also elusive. This study aims to explore the inhibitive function of all-trans-retinoic (ATRA) on Nrf2-ARE pathway in intracerebral hemorrhage (ICH), and investigate the mechanism. In this study, the femoral artery injection method was employed to establish ICH model. The model rats were randomly divided into four groups, including Sham group, ICH group, ATRA group and DMSO group. The neurological scores were evaluated for the four groups at different time points. Hematoxylin-Eosin staining was used to stain the CD11b positive glia cells. Double immunofluorescence staining method was utilized to observe the co-expression of HO-1, NF-κB, Nrf2 and TNF-α and CD11b marker in glia cells. Western blot assay was used to detect the Nrf2 protein (total and binding Nrf2), HO-1, NF-κB and TNF-α proteins in every group. The results indicated that neurologiclal scores were significantly decreased in ATRA group compared to ICH gorup (P < 0.05). The glia cells were significantly activated and accumulated in ICH rats. ATRA significantly decreased co-expression of Nrf2, HO-1 and CD11b, and increased co-expression of NF-κB, TNF-α and CD11b of glia cells. ATRA significantly decreased total Nrf2 expression and increased binding Nrf2 expression in ATRA group compared to ICH group (P < 0.05). ATRA decreased anti-oxygen protein Nrf2 and HO-1, and increases inflammatory factors NF-κB and TNF-α. In conclusion, the application of ATRA could inhibit the neuro-protective function effectively by blocking the Nrf2-ARE pathway in glia cells.
Keywords: Intracerebral hemorrhage, Nrf2-ARE pathway, all-trans-retinoic, glia cells
Introduction
Intracerebral hemorrhage (ICH) is a kind of serious and acute central nervous system disease [1,2]. The occurrence of cerebral hemorrhage may influence the brain function on specific cognitive abilities, and cause the cognitive dysfunction [3,4]. Many studies have discovered that the diseases in intracerebral hemorrhage, cerebral ischemia could activate the Nrf2-ARE pathway [5-8]. The activated Nrf2 could also trigger the expression of ARE regulated heme oxygenase (HO-1), quinine reduction (NQO1), and play the role of anti-oxidative and anti-inflammatory and neuroprotective function [9,10]. Other reports also indicated that the Nrf2-ARE signaling pathway could play the neruoprotective role by regulating the activation of glial cells [6,7]. The glial cells were the important immunological cells in central nerve system. Especially, in the ICH, the stationary status of glial cells was transformed to the active status, and activating and proliferating quickly, and playing the neuroprotective functions [8-10].
In this study, we observed the active status of glial cells in ICH. In order to explore the inhibitive function of all-trans-retinoic (ATRA) on Nrf2-ARE pathway in ICH, we used the ATRA to inhibit the combining of Nrf2 and ARE, and observe the expression of Nrf2, HO-1, nuclear factor kB (NF-κB) and tumor necrosis factor α (TNF-α). This study could provide the novel strategy and clue for the clinical therapy of ICH.
Materials and methods
Experimental animals and reagents
Male SD rats were purchased from Slack Jiangda Laboratory Animal Co., Ltd. (Changsha, Hunan, China). The body weights range from 250 to 300 g.
ATRA and DMSO were purchased was purchased from Sigma, CA, USA. The rabbit anti-rat Nrf2 polyclonal was purchased from Abcam (ab13243, ENG, UK). Rabbit anti-rat NF-κB polyclonal (#8242) and rabbit anti-rat TNFα polyclonal (#11948) were purchased from Cell Signaling (MA, USA). Mouse anti-rat CD11b polyclonal antibody was purchased from Sigma (70-Mab1445, CA, USA). The goat anti-rabbit polyclonal antibody labeled with Cy3 fluorescein and goat anti-mouse polyclonal antibody labeled with fluorescein isothiocyanate (FITC), rabbit anti-rat β-actin polyclonal antibody were purchased from Abcam, UK.
Establishment of rat ICH model and MRI verification
The establishing method was performed according to the previous study [11]. The rats were subjected to fasting without water deprivation 8 h before surgery. At 3 mm lateral to the right side of the anterior fontanelle and a penetration depth of 5 mm, 50 μl autologous blood was slowly injected into the basal ganglia to establish a rat ICH model. The Signal Excite HD 3.0 T MR scanner (GE, MI, USA) was used to examine the hematoma 3 h after the ICH had been established. When a notable round, oval or irregularly-shaped hematoma was discovered, the rat was considered as the model rat. When there was blood reflux into the needle tract, blood in the ventricles or death, the rat was excluded from the study, and can’t be considered as the model rat.
Trial grouping
Selecting the healthy SPF SD rats (weighing 250-300 g). There were a total of 90 successfully establised hemorrhage model rats for the experiment. The model rats were randomly divided into four groups, including Sham group, ICH group, ATRA group and DMSO group. For the above 4 groups, each group was divided into 5 subgroups, including 6 h, 1 d, 2 d, 3 d, 7 d (after ICH) subgroups, and each subgroup contains 5 rats. Furthermore, we also selected 5 health SPF rats as the control group.
Neurological score
Garcia 18-points test [12] was performed to assess the neurological function of the rats, and those with a score ≥ 3 were considered usable subjects. The Garcia 18-points scores were obtained 24 h before surgery. Also, the Garcia 18-point scores were evaluated at 6 h, 1 d, 2 d, 3 d and 7 d after surgery, respectively.
Rats treatment and tissues selection
The rats were anesthetized and the heart was rapidly exposed, a perfusion tube was inserted from the left ventricle to the root of the aorta, and a small cut was made on the right atrial appendage with scissors. Then, 200 ml 4°C saline was infused until the effluent fluid was clear, and 4% paraformaldehyde was used for perfusion until the limbs of the rat were rigid. The animal was then decapitated and its brain collected, placed in 4% paraformaldehyde for 24 h, and subsequently subjected to frozen sectioning at a slice thickness of 5 μm for immunofluorescence staining.
Hematoxylin-eosin staining for glia cells
The isolated tissues were embedded in tissue freezing medium (Triangle Biomedical Science, NC, USA) and immediately frozen in isopentane at -50°C. Total 4 μm cryosections were cut on a Microm HM 500 M Cryostat and placed on Superfrost Plus Gold glass slides (Menzel, Germany). Slides were subsequently fixed in 70% ethanol. The sections were stained with hematoxylin staining, and all of the procedures were performed according to the manufacturer’s instruction.
Immunofluorescence analysis for co-expression
The immunofluorescence analysis was employed to the co-expression of Nrf2-ARE downstream factors (HO-1, NF-κB, Nrf2 and TNF-α) and glial cells (CD11b marked). The brief processes of the immunofluorescence analysis were performed as the followings: Nrf2, HO-1, NF-κB and TNF-α positive cells were detected as follows. ① The brain tissues were fixed in pre-cooling paraformaldehyde for 15 min; ② PBS rinse was performed for 5 min × 3 times, followed by membrane rupture using 0.25% triton for 15 min; ③ Blocking using 10% BSA for 60 min; ④ PBS rinse was performed for 5 min × 3 times, followed by incubation with primary antibody at 4°C overnight (about 12 hours); ⑤ PBS rinse was performed for 10 min × 3 times, followed by incubation with second antibody at room temperature for 2 hours in dark; ⑥ PBS rinse was performed for 10 min × 3 times, followed by DAPI staining for 15 min; ⑦ PBS rinse was performed for 5 min × 3 times, followed by mounting with glycerol, and fluorescent microscope was used for observation and taking images (with LI-COR imaging analysis system, Biosciences, Lincoln, NE, U.S.). At 400 magnifications, images from 5 non-overlapping fields were analyzed for cell counts and the mean was calculated.
Western blot analysis for Nrf2, HO-1, NF-κB and TNF-α expression
Brain tissues were isolated, sliced and snap-frozen in liquid nitrogen and immediately homogenized in ice-cold RIPA lysis buffer (Sigma, USA) containing a cocktail of protease inhibitors (Roch, Switzerland). Denatured total cell lysates were run in SDS-PAGE, transferred to nitrocellulose membranes and blocked overnight at 4°C in 5% milk. The blocked membranes were incubated overnight at 4°C with the anti-Nrf2 (1:1000, ab31163, Abcam, U.K.), anti-HO-1 (1:2000, ab13243, Abcam), anti-NF-κB (1:1000, CST#8242, Cell Signaling Technology, U.S.), and anti-β-actin (1:1000, 70-Mab1445, Sigma) followed by 1 hour incubation at room temperature with an HRP-conjugated secondary antibody. The Tow-color Laser Odyssey Infrared Imaging System (Gene Company Limited, Hong Kong, China) was used to scan the membranes, and the images were analyzed using Odyessy Version 3.0 software. Signals were normalized against β-actin and the results were expressed as a percentage of the negative control signal.
Statistical analysis
Significant differences between groups were determined by Student’s t test. The mean and standard deviations of the result between groups was used and P value < 0.05 was taken as statistically significant. Analyses were performed with the SPSS 17.0 statistical software package (Microsoft, CA, USA).
Results
Neurological score
There was no obvious neurological function deficit in Sham group, and the neurological score achieved the higher levles steadily (Figure 1). The neurological deficits and neurologiclal scores were significantly decreased in ICH group and ATRA group, and achieved the lowest levle 48 hours after intracerebral hemorrhage (Figure 1, P < 0.05). The death rat in ATRA group was significantly higher compared to the ICH group (Figure 1, P < 0.05). The changes of neurological function in ATRA group was initiated from 1 day after intracerebral hemorrhage, and ended at the 7th day (Figure 1). Moreover, no effects of DMSO treatement on neurological function and score were discovered.
Figure 1.
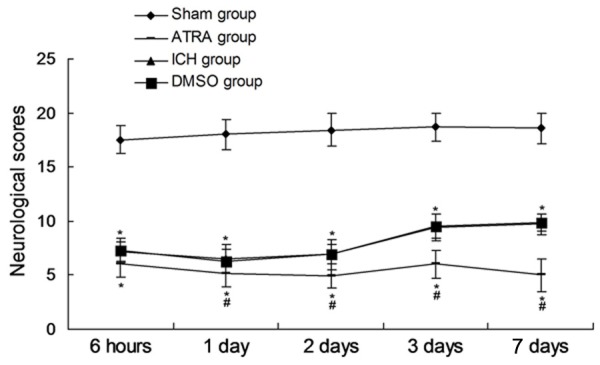
Neurological socres of every group at different time points after intracerebral hemorrhage. *P < 0.05 represents the neurological score in ICH or ATRA group compared to Sham group. #P < 0.05 represents the neurological score in ATRA group compared to ICH group.
Expression of glia cells
From the Figure 2, the CD11b positive glia cells were obviously observed arrounding the intracerebral localization 6 hours (Figure 2A, 2B). The results indicated that the glia cells were significantly activated and accumulated. Furthermore, the CD11b positive glia cells achieved the peak at 3 days after intracerebral hemorrhage (Figure 2C, 2D). There were only a few CD11b positive glia cells in the Sham group.
Figure 2.
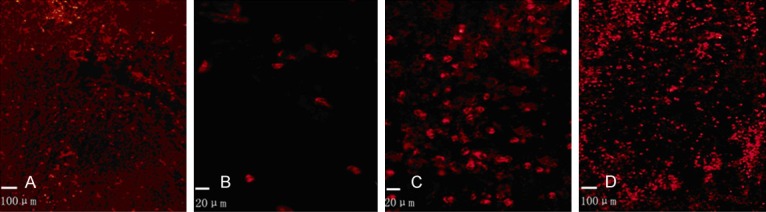
CD11b positive glia cells observation with Hematoxylin-Eosin staining. CD11b positive glia cells expression was examined in ICH group 6 hours after intracerebral hemorrhage at the magnification of 200 × (A) and 400 × (B). CD11b positive glia cell expression was examined in ICH group 3 days after intracerebral hemorrhage at the magnification of 200 × (C) and 400 × (D).
Co-expression of Nrf2, HO-1, NF-κB, TNF-α and glial cells
In order to explore the effects of Nrf2, HO-1, NF-κB, TNF-α on ICH perifocal tissues, co-expression of Nrf2, HO-1, NF-κB, TNF-α were examined by double immunofluorescence staining. The resutls indicated that all of the factors (Nrf2, HO-1, NF-κB, TNF-α) and CD11b co-expression positive glia cells reached a peak on day 3 (Figure 3). From Figure 1A and 1B, we could found that ATRA significantly decreased the co-expression of Nrf2 (Figure 1A) and HO-1 (Figure 2B) and CD11b of glia cells compared to the ICH group. However, the ATRA could significantly increase the co-expression of NF-κB (Figure 1C) and TNF-α (Figure 1D) and CD11b of glia cells compared to the ICH group. The above data suggest that the ATRA could inhibit the neuroprotection of Nrf2-ARE pathway, and enhance the inflammatory response of glia cells.
Figure 3.
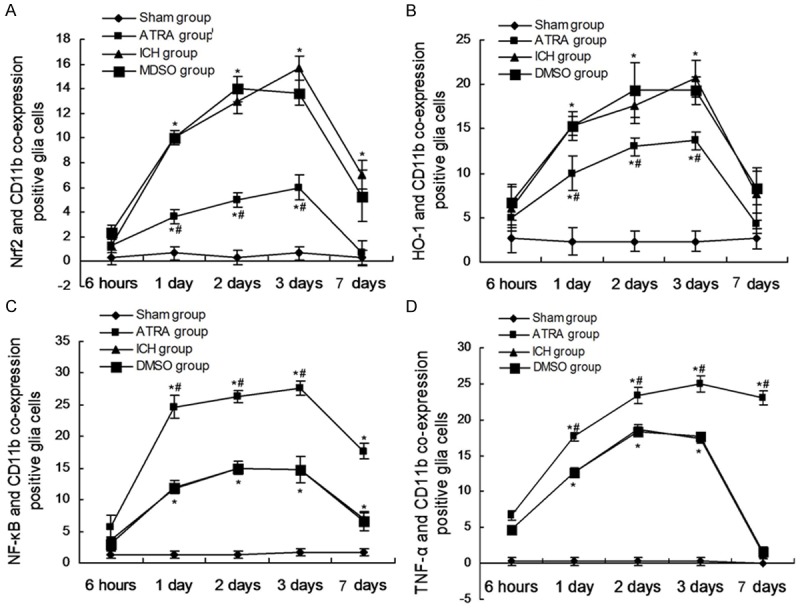
Nrf2, HO-1, NF-κB, TNF-α and CD11b co-expression positive glial cells. A. Statistical analysis of Nrf2 and CD11b co-expresion positive glial cells. B. Statistical analysis of HO-1 and CD11b co-expresion positive glial cells. C. Statistical analysis of NF-κB and CD11b co-expresion positive glial cells. D. Statistical analysis of TNF-α and CD11b co-expresion positive glial cells. *P < 0.05 represents the co-expression positive glial cells in ICH or ATRA group compared to Sham group. #P < 0.05 represents the co-expression positive glial cells in ATRA group compared to ICH group.
ATRA triggers the expression changes of binding and total Nrf2 protein
In order to discuss the effects of ATRA on the binding and total Nrf2 protein expression, the two forms of Nrf2 were detected by western blot assay. Figure 4 showed taht the total Nrf2 was significantly decreased in ATRA group compared to the ICH group (Figure 4A, 4B, P < 0.05) at 6 hours and 2 days. However, the binding Nrf2 protein was significantly increased in ATRA group compared to the ICH group (Figure 4A, 4C, P < 0.05).
Figure 4.

Examination of the total Nrf2 and binding Nrf2 expression. A. Western blot assay for the total and binding Nrf2 protein expression. B. Statistical analysis for the total Nrf2 expression. C. Statistical analysis for the binding Nrf2 expression. *P < 0.05 represents the total or binding Nfr2 protein expression in ATRA grup compared to ICH group.
ATRA decreases anti-oxygen protein and increases inflammatory factors
To explore the mecahnism of the effects of ATRA on the glia cells activation, the anti-oxygen protein and inflammatory factors were examined. The results indicated that the ATRA significantly decreased the HO-1 expression level in ATRA group compared to ICH group (Figure 5A, P < 0.05). However, the inflammatory factor, NF-κB (Figure 5B, P < 0.05) and TNF-α (Figure 5C, P < 0.05) increased significantly in ATRA group compared to ICH group.
Figure 5.
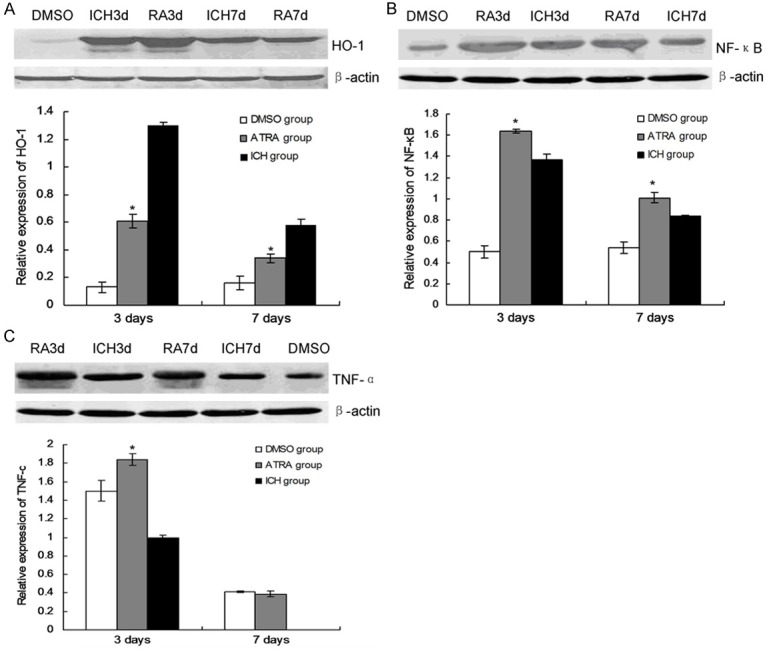
HO-1, NF-κB and TNF-α expression in ATRA treated rat brain tissues. A HO-1 expression and statistical analysis. B. NF-κB expression and statistical analyis. C. TNF-α expression and statistical analysis. *P < 0.05 represents the HO-1, NF-κb or TNF-α protein expression in ATRA grup compared to ICH group.
Discussion
Nrf2-ARE pathway is the hot fied of anti-oxygen in the recent years, which can induce the expression of endogenous protective genes in many tissues and cells, such as antioxidant enzymes, anti-inflammatory factors and some detoxifying proteins. The activated Nrf2-ARE could enhance the HO-1 expression expression in brain tissues [6,13,14]. HO-1 could mediate many kinds of neuroprotective function, and could also inhibit NF-κB signaling transduction pathway. HO-1 could inhibit the dissociation of I-κB and NF-κB, and disturb the nuclear localization signal (NLS) of p65 subuit in NF-κB [15-17]. The present study indicated that the expression of Nrf-2, HO-1, NF-κB and TNF-α were increased in ICH group compared to DMSO control group and Sham group. Meanwhile, when we treated the rats model with ATRA, the Nrf-2-ARE pathway was inhibited obviously inhibited, and neurological functions were obviously damaged. Our study showed that the expression of Nrf2 protein and HO-1 protein decreased, which is consistent with Wang et al.’s report [18]. This report indicated that sulforaphane (SUL) could activate the Nrf2-ARE pathway, and up-regulate the expression of Nrf2 and HO-1 protein, however, the expression of NF-κB and TNF-α was down-regulated. The above results suggest that ICH could activate the Nrf2-ARE pathway, which play a role in neuro-protection. HO-1 is one critical targeting protein in this pathway to play the neuroprotectiv functions.
In this study, we used the Nrf2 inhibitor [18,19], ATRA, to treat the ICH rat model. There are also some divergences about the function of the ATRA. Wang et al. [18] thought that ATRA could not inhibit the expression of Nrf2, and could inhibit the bingding of Nrf2 to ARE. In order to the effects of ATRA on the Nrf2 activation and potential mechanism, we observed the Nrf2-ARE pathway related factors change after treatment with ATRA. The results indicated that the expression of Nrf2 and HO-1 protein decreased obviously, which suggest that the anti-oxidant adn anti-inflammatory response were significantly decreased. The western blot assay results also showed that the binding Nrf2 protein was increased in ATRA treated rat compared to ICH group, the Nrf2 co-expresison positive glia cells decreased, all of which suggest that the ATRA could inhibit the dissociation of Nfr2 and Keap1. The inhibition of dissociation could decrease the Nrf2 getting into nucleus.
The previous studies [20,21] have been concluded that the activated glia cells volume become bigger, the cell protrusions become more, and the migrating speed become quicker to hematoma arroundings. Some reports [22-24] also indicated that the Nrf2 protiein was activated in many kinds of disease models, and activated the glia cells, which could perform the neuro-protective functions.
In conclusion, The neuro-protective functions of Nrf2-ARE pathway could be activated after ICH of rats. The application of ATRA could inhibit the neuro-protective function effectively by blocking the Nrf2-ARE pathway in glia cells.
Acknowledgements
This study was granted by National Natural Science Foundation of China (No. 81260183).
Disclosure of conflict of interest
None.
References
- 1.Zheng M, Zhu H, Gong Y, Wang D, Xie Q, Tang H, Yang Z, Lu B, Chen X, Wang X. Involvement of GMRP1, a novel mediator of Akt pathway, in brain damage after intracerebral hemorrhage. Int J Clin Exp Pathol. 2013;6:224–229. [PMC free article] [PubMed] [Google Scholar]
- 2.del Zoppo GJ, Frankowski H, Gu YH, Osada T, Kanazawa M, Milner R, Wang X, Hosomi N, Mabuchi T, Koziol JA. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab. 2012;32:919–932. doi: 10.1038/jcbfm.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia PY, Roussel M, Bugnicourt JM, Lamy C, Canaple S, Peltier J, Loas G, Deramond H, Godefroy O. Cognitive impairment and dementia after intracerebral hemorrhage: a cross-sectional study of a hospital-based series. J Stroke Cerebrovasc Dis. 2013;22:80–86. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2012;299:131–135. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Saw CL, Yang AY, Guo Y, Kong AN. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem Toxicol. 2013;62:869–875. doi: 10.1016/j.fct.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Zhao F, Li K, Zhao L, Liu J, Suo Q, Zhao J, Wang H, Zhao S. Effect of Nrf2 on rat ovarian tissues against atrazine-induced anti-oxidative response. Int J Clin Exp Pathol. 2014;7:2780–2789. [PMC free article] [PubMed] [Google Scholar]
- 7.Mani M, Golmohammadi T, Khaghani S, Zamani Z, Azadmanesh K, Meshkini R, Pasalar P. Homocysteine induces heme oxygenase-1 expression via transcription factor Nrf2 activation in HepG2 cell. Iran Biomed J. 2013;17:93–100. doi: 10.6091/ibj.1158.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Son TG, Kawamoto EM, Yu QS, Greig NH, Mattson MP, Camandola S. Naphthazarin protects against glutamate-induced neuronal death via activation of the Nrf2/ARE pathway. Biochem Biophys Res Commun. 2013;433:602–606. doi: 10.1016/j.bbrc.2013.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Nel AE. Role of the nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8:88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 10.Aliaghaei A, Khodagholi F, Ahmadiani A. Conditioned media of choroid plexus epithelial cells induces Nrf2-activated phase II antioxidant response proteins and suppresses oxidative stress-induced apoptosis in PC12 cells. J Mol Neurosci. 2014;53:617–625. doi: 10.1007/s12031-014-0228-4. [DOI] [PubMed] [Google Scholar]
- 11.Deinsberger W, Lang C, Hornig C, Boeker DK. Stereotactic aspiration and fibrinolysis of spontaneous supratentorial intracerebral hematomas versus conservative treatment: a matched-pair study. Zentralbl Neurochir. 2003;64:145–150. doi: 10.1055/s-2003-44617. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. statistical validation. Stroke. 1995;26:627–634. 635. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma inmicroglia/macrophages. Ann Neurol. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 14.Ji L, Wei Y, Jiang T, Wang S. Correlation of Nrf2, NQO1, MRP1, cmyc and p53 in colorectal cancer and their relationships to clinicopathologic features and survival. Int J Clin Exp Pathol. 2014;7:1124–1131. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen-Roetling J, Sinanan J, Regan RF. Effect of iron chelators on methemoglobin and thrombin preconditioning. Transl Stroke Res. 2012;3:452–459. doi: 10.1007/s12975-012-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces cavity size in the brain after intracerebral hemorrhage in aged rats. Acta Neurochir Suppl. 2011;111:185–190. doi: 10.1007/978-3-7091-0693-8_31. [DOI] [PubMed] [Google Scholar]
- 17.Yao C, Wei G, Lu XC, Yang W, Tortella FC, Dave JR. Selective brain cooling in ratsameliorates intracerebral hemorrhage and edema caused by penetrating brain injury: possible involvement of heme oxygenase-1 expression. J Neurotrauma. 2011;28:1237–1245. doi: 10.1089/neu.2010.1678. [DOI] [PubMed] [Google Scholar]
- 18.Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcrip tion factor Nrf2 through activation of retinoic acid recep tor alpha. Proc Natl Acad Sci U S A. 2007;104:19589–19594. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Yang S, Xi G, Song S, Fu G, Keep RF, Hua Y. Microglial activation and brain injury after intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:59–65. doi: 10.1007/978-3-211-09469-3_13. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Yang S, Xi G, Fu G, Keep RF, Hua Y. Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol Res. 2009;31:183–188. doi: 10.1179/174313209X385680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SS, Lim J, Bang Y, Gal J, Lee SU, Cho YC, Yoon G, Kang BY, Cheon SH, Choi HJ. Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: therapeutic relevance to neurodegenerative disease. J Nutr Biochem. 2012;23:1314–1323. doi: 10.1016/j.jnutbio.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Bruck W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the nrf2 antioxidant pathway. Brain. 2011;134:678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 24.Bao LJ, Jaramillo MC, Zhang ZB, Zheng YX, Yao M, Zhang DD, Yi XF. Nrf2 induces cisplatin resistance through activation of autophagy in ovarian carcinoma. Int J Clin Exp Pathol. 2014;7:1502–1513. [PMC free article] [PubMed] [Google Scholar]


