Abstract
Aim: To investigate the effect of mitogen-activated protein kinase 7 (MAPK7) in ovarian cancer metastasis and to explore its potential mechanism. Methods: pcDNA-MAPK7 and siRNA-MAPK7 vectors were transfected into the human ovarian cell line OVCAR-3 based on gene silencing and overexpression methods. Effects of MAPK7 overexpression and silencing on OVCAR-3 cells proliferation, cell invasion, and migration were analyzed using the MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) assay, Matrigel methods, and Markered methods respectively. In addition, effect of MAPK7 expression on extracellular matrix (ECM) associated protein was detected using Western blot. Results: Compared with the controls, MAPK7 was up-regulated when cells were transfected with pcDNA-MAPK7 plasma, as well as MAPK7 was sliced when cells were transfected with siRNA-MAPK7 plasma (P<0.05). Besides, biological function analysis performed that overexpression of MAPK7 significantly increased OVCAR-3 cell proliferation, invasion, and migration (P<0.05), while these effects were inhibited by MAPK7 silencing (P<0.05). Additionally, MAPK7 overexpression increased type II collagen expression (P<0.05). However, there was no significant difference between MAPK7 expression and type I collagen expression (P>0.05). Conclusion: Our data implied the up-regulated MAPK7 might contribute to ovarian cancer metastasis through up-regulating type II collagen expression and then were involved in cell biological processes such as cell proliferation, invasion, and migration. MAPK7 may be a potential therapeutic target in the clinical treatment for ovarian cancer.
Keywords: Ovarian cancer, MAPK7, cell proliferation, cell invasion, metastasis
Introduction
Ovarian cancer is the third primary malignant tumors of reproductive system in female, which has increasing morbidity worldwide in recent years [1]. Mortality of ovarian cancer is high and the five-year survival rate is poor, which is approximately to 30% [2]. Pathogenesis of ovarian cancer is complicate, indicating its hard diagnose and difficult treatment [3]. Clinical treatment and diagnosis methods for ovarian cancer are mainly surgery and chemotherapy, which have side effects and result in unsatisfactory cure effect on patients due to its easy metastasis and the hard detection resulted from few clinical features in early stage [4,5]. Therefore, it is necessary to investigate the mechanism of ovarian cancer cell proliferation and invasion for the cancer treatment in clinical.
Mitogen-activated protein kinase 7 (MAPK7) is a member of the MAP kinase family that act as an integration point for multiple biochemical signals, and are involved in a wide variety of cellular processes such as proliferation, differentiation, transcription regulation and development [6,7]. Recently, studies have referred that MAPK7 may be a potential tumor biomarker for many cancers in clinical. For instance, Ivana reports that MAPK7 is the target gene for breast cancer during its development [8], and Dartel refers that abnormal amplification of MAPK7 is the genetic character in high-grade osteosarcoma and can be the potential biomarker for tumor prognosis [9,10]. Also, recent evidence mentioned that MAPK7 is associated with poor survival of breast cancer through MEK5-ERK5 pathway after systemic treatments [11,12]. Despite several articles have developed the significant functions of MAPK7 in tumor metastasis and prognosis, the role of MAPK7 in ovarian cancer has not been described in previous papers.
In this present study, we used the human ovarian cancer cell line of OVCAR-3 to specifically up-regulating and down-regulating the expression of MAPK7 through gene silencing method. Comprehensive experimental methods such as MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) assay and Matrigel method were used to detect the effects of MAPK7 expression on ovarian cancer cell proliferation, migration and invasion, and the effect of MAPK7 on extracellular matrix associated proteins expressions. This study aimed to explore the role of MAPK7 on ovarian cancer metastasis and to provide basis for MAPK7 application for ovarian cancer target diagnose in clinical.
Materials and methods
Cell culture and cell transfection
Human ovarian cancer OVCAR-3 cell line (purchased from ScienCell Research Laboratories, USA) was cultured in RPMI-1640 medium supplemented with 20% fetal bovine serum (FBS) and 0.01 mg/mL bovine insulin at 37°C in an atmosphere of 5% CO2. The liquid nitrogen stored OVCAR-3 cells were dissolved in water at 37°C and then centrifuged at 1000 rpm for 3 min. Cells were resuspended with 1 mL fresh RPMI-1640 medium containing 20% FBS, then 2 mL fresh RPMI-1640 medium was mixed with cells and incubated at 37°C. After being cultured for 24 h, cells (1×107) were injected onto Petri dishes with addition of RPMI-1640 medium supplemented with 20% FBS.
OVCAR-3 cells were randomly separated into four groups: pcDNA3.1-MAPK7 of 1 μg/mL and siRNA-MAPK7 of 1 μg/mL plasma (purchased from Invitrogen, USA) were transfected into OVCAR-3 cells: cells without plasma transfection were in Blank group; and cells transfected with plasma without MAPK7 sequence were in control group. Cell transfections were conducted using Lipofectamine 2000 Transfection Reagent (Invitrogen, CA, USA) according to manufacturer’s protocol [13]. The transfected cells were cultured for 4 h, and then normal medium was added. After incubation for 48 h, cells were harvested for further detection.
RT-PCR analysis
Total RNA extraction from OVCAR-3 cells collected at 48 h was conducted using TRIzol Reagent (Invitrogen, CA, USA) as previously described [14] and was treated with RNse-free Dnase I (Promega Biotech, USA). Consequently, concentration and purity of isolated RNA were measured with SMA 400 UV0VIS (Merinton, Shanghai, China). Purified RNA at density of 0.5 μg/μL with nuclease-free water was used for cDNA synthesis with the PrimerScript 1st Strand cDNA Synthesis Kit (Invitrogen, USA). Expressions of targets in OVCAR-3 cells were detected in an Eppendorf Mastercycler (Brinkman Instruments, Westbury, NY) using the SYBR ExScript RT-qPCR Kit (Takara, China). The total reaction system of 20 μL volume was as follows: 1 μL cDNA from the above PCR, 10 μL SYBR Premix EX Taq, 1 μL each of the primers (10 μM), and 7 μL ddH2O. The PCR program was as follows: denaturation at 50°C for 2 min; 95°C for 10 min; followed by 45 cycles of 95°C for 10 s, and 60°C for 1 min. Melting curve analysis of amplification products was performed at the end of each PCR to confirm that only one product was amplified and detected. Phosphoglyceraldehyde dehydrogenase (GAPDH) was chosen as the internal control. Primers used for targets amplification were as follows: MAPK7 sense, 5’-ATGAACCCTGCCGATATTG-3’ and MAPK7 anti-sense, 5’-CTTTGAGAATGCTCCCATG-3’; and GAPDH sense, 5’-TATGATGATATCAAGAGGGTAGT-3’, and GAPDH anti-sense, 5’-TGTATCCAAACTCATTGTCATAC-3’.
Analysis of cell proliferation
Effect of target gene expression on cell proliferation ability was assessed using MTT assay as previously described [15]. Briefly, OVCAR-3 cells cultured in RPMI1640 medium containing 20% FBS at pogarithmic stage (5×103) were transfected into the 96-well plates. After for 24 h cultivation, supernatant was abandoned and followed with addition of 20 μL MTT every 24 h and incubation for 4 h. After that, 150 μL dimethylsulfoxide (DMSO) was used to mix with the cells for 10 min. Absorbance of cells in each well was observed at 570 nm under an absorption spectrophotometer (Olympus, Japan). All experiments were conducted independently for 3 times.
Cell migration assessment
Shortly, cells in each group were cultured in 6-well plates with the density of 5×105 per well [16]. After being confluented, a wound was made across the well with a 200 μL pipette tip. The wound was photographed immediately and then observed the document cellular migration cells across the gap wound using an inverted microscope (×10 magnification). The width of the gap was detected in 5 different visuals and the average width was calculated. All experiments were conducted independently for 3 times.
Cell invasion assay
For cell invasion ability assay, Matrigel method was used as previously described [17]. Briefly, cells in each group cultured at 48 h were incubated in serum-free RPMI-1640 medium containing 0.01% serum albumin (BSA, Sigma, USA) for 24 h. The upper layer of Transwell was enveloped with serum-free RPMI-1640 medium supplemented with 50 mg/L Matrigel and then air-dried at 4°C. After being sucked out the medium, 50 μL fresh serum-free medium containing 10 g/L BSA was added and then cultured for 30 min at 37°C. After that, Transwell was put into the 24-well plates and cultured with DMEM (Dulbecco’s Modified Eagle Medium) medium (Sigma, USA) mixed with 10% FBS. Then cells in Transwell were suspended with serum-free DMEM medium. After 48 h, Transwell in each group was washed with PBS buffer to remove the upper cells on microporous membrane, followed with fixed in ice-cold alcohol. Finally, Transwell from each group was stained with 0.1% crystal violet for 30 min, and then decolorated with 33% acetic acid. The absorbance of eluents was observed at OD 570 nm using a microplate reader (Biotech, USA). Transwell in control group was treated without Matrigel. Total experiments were conducted independently for 3 times.
Western blot
OVCAR-3 cells cultured at 48 h in each group were lapped with RIPA assay (radioimmunoprecipitation, Sangon Biotech, China) lysate containing PMSF (phenylmethanesufonyl fluoride, Sigma, USA), and then were centrifuged at 12,000 rpm for 10 min at 4°C. Supertanant was collected for the measurement of protein concentrations using BCA protein assay kit (Pierce, Rochford, IL). For Western blotting [18], 25 μg protein per cell lysate was subjected to a 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transferred onto a Polyvinylidencefluoride (PVDF) membrane (Mippore). Then the PVDF membrane was blocked in Tris Buffered Saline Tween (TBST) containing 5% non-fat milk for 1 h. Consequently, the membrane was incubated with rabbit anti-human antibodies (type I and type II collagen, 1:100 dilution, Invitrogen, USA) and overnight at 4°C. Then membrane was incubated with hoseradish peroxidase labeled goat anti-rat secondary antibody (1:1000 dilution) at room temperature for 1 h. Finally, PVDF membrane was washed with 1×TBST buffer for 10 min with 3 times. Detection was conducted using the development of X-ray after chromogenic substrate with an enhanced chemiluminescence (CEL) method. Additionally, ß-actin (Sigma, USA) served as the internal control.
Statistical analysis
All data were expressed as mean ± standard error of mean (SEM). Independent sample t-test was used to calculate the difference between two groups using the graph prism 5.0 software (GraphPad Prism, San Diego, CA). Post-hoc Tukey-test was used to calculate the difference among groups. P<0.05 was considered as statistically significant.
Results
MAPK7 expression in OVCAR-3 cells
After being transfected with siRNA-MAPK7, expression of MAPK7 in ovarian OVCAR-3 cells was significantly declined compared with that in control or in Blank group (P<0.05). Otherwise, when OVCAR-3 cells were transfected with pcDNA-MAPK7, expression of MAPK7 was significantly increased compared with that in control or in blank group (P<0.05) (Figure 1).
Figure 1.
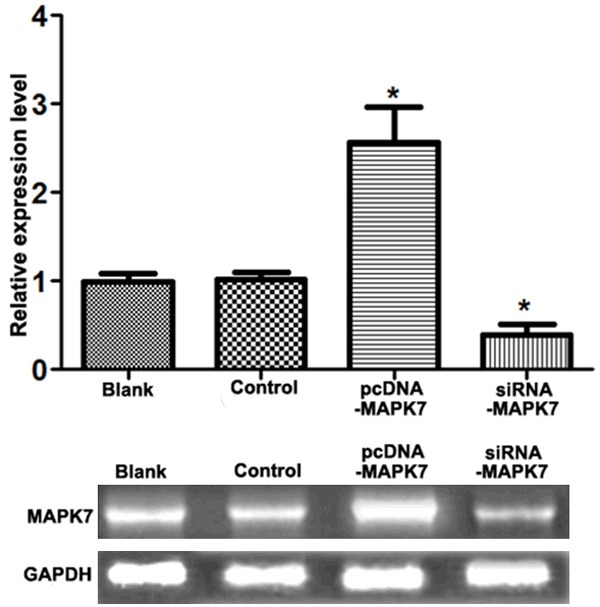
Expression of MAPK7 in OVCAR-3 cells. When cells were transfected with pcDNA-MAPK7 plasma, expression of MAPK7 increased about 2.5 fold change compared with the control groups, while MAPK7 expression was decreased approximately to 31% by transfecting with siRNA-MAPK7. *P<0.05, compared with the control group.
MTT assay
Effect of MAPK7 expression on OVCAR-3 cell proliferation was detected using MTT assay (Figure 2). After being transfected with pcDNA-MAPK7 plasma for 24 h, relative cell viability of OVCAR-3 cell was significantly increased compared with that in Blank or control group (P<0.05). However, OVCAR-3 cell viability was significantly inhibited by transfecting with siRNA-MAPK7 plasma (P<0.05), indicating that overexpression of MAPK7 would accelerate OVCAR-3 cell proliferation.
Figure 2.
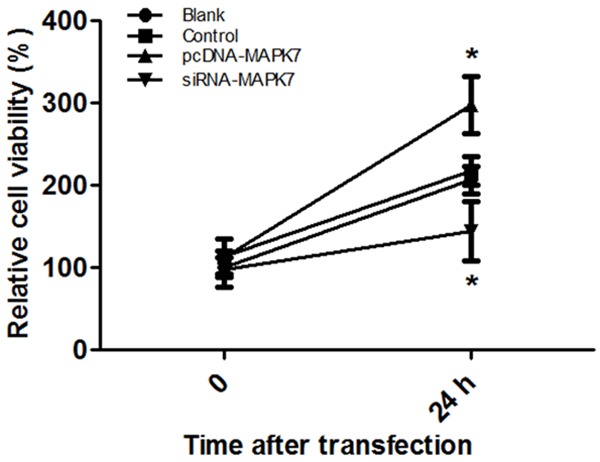
Effect of MAPK7 expressions on OVCAR-3 expression. When cells were transfected with blank pcDNA, the relative OVCAR-3 cell viability was about 2.1 fold. After being transfected with pcDNA-MAPK7, cell viability was raised to 2.7 fold; however, cell viability was dropped to 1.3 fold when cells were transfected with siRNA-MAPK7 plasma. *P<0.05, compared with the control group.
Cell migration assay
Influence of MAPK7 expression on OVCAR-3 cell migration ability was detected (Figure 3). Under the observation of microscope, when OVCAR-3 cells were transfected with pcNDA-MAPK7 plasma, there were more migrate cells than that in Blank or in control group, but there was few migrate cells transfected with siRNA-MAPK7, suggesting that overexpression of MAPK7 would promote OVCAR-3 cell migration (Figure 3A).
Figure 3.
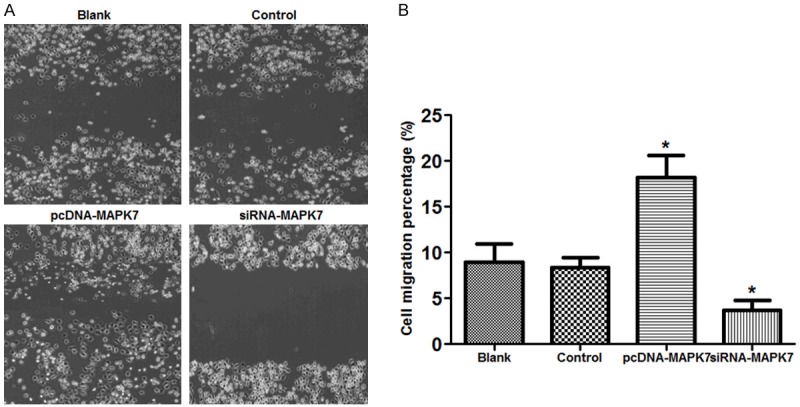
Influence of MAPK7 expression on OVCAR-3 cells migration. A: Migrate cells in each group, there were many migrated cells in pcDNA-MAPK7 group compared with the other three groups; B: There were about 19.2% migrate cells in OVCAR-3 pcDNA-MAPK7 group, while that was significantly dropped to 3.7% in siRNA-MAPK7 group. *P<0.05, compared with the control group. Eight views were randomly selected in this study.
Cell invasion assay
Effect of MAPK7 expression on OVCAR-3 cell invasion ability was assessed using Matrigel method (Figure 4). The results showed that there were more invasion cells (10.6 cells/visual field) in pcDNA-MAPK7 group compared with the controls, while invasion cells were significantly declined by siRNA-MAPK7 transfection (2.3 cells/visual field) (P<0.05).
Figure 4.
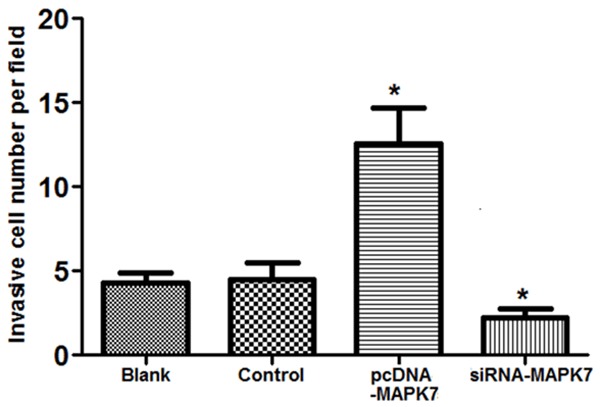
Effect of MAPK7 expressions on OVCAR-3 cell invasion. When MAPK7 was overexpressed in OVCAR-3 cells, invasion cells was significantly increased (about 10.6 cells/visual) compared with the control group. However, invasion cells were significantly decreased when MAPK7 was silencing in OVCAR-3 cells (about 2.3 cells/field). *P<0.05, compared with the control group.
Collagen expression in OVCAR-3 cells
In order to assess the influence of MAPK7 expression on intracellular collagen expression, Western blotting analyze was used to detect the expressions of type I and II collagen in OVCAR-3 cells (Figure 5). The results showed that there was no significant difference of type I collagen expression in OVCAR-3 cells among the four groups (P>0.05, Figure 5A). However, type II collagen expression was significantly increased in pcDNA-MAPK7 group while that was significantly decreased in siRNA-MAPK7 group compared with the Blank and control groups, indicating that overexpression of MAPK7 would enhance type II collagen expression in OVCAR-3 cells (P<0.05, Figure 5B).
Figure 5.
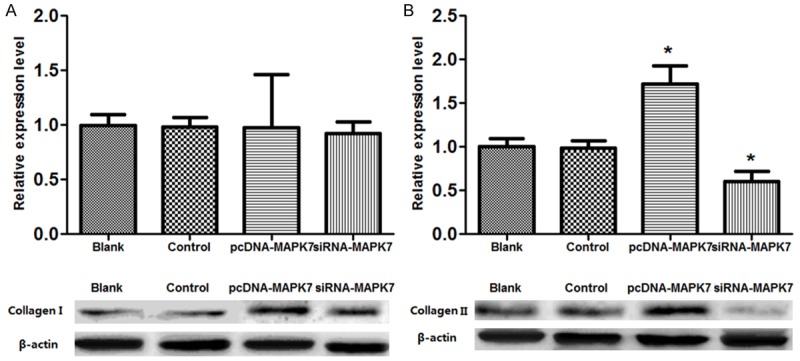
Western blotting analyze of collagen expression in OVCAR-3 cells. A: Expression of type I collagen in pcDNA-MAPK7 group was 1.2 fold change of control or Blank group, while that in siRNA-MAPK7 group was 1.1 fold change of the control group; B: Expression of type I collagen in pcDNA-MAPK7 group was 1.8 fold change of control or Blank group, while that in siRNA-MAPK7 group was 0.7 fold change of the control group. *P<0.05, compared with the control group.
Discussion
In this paper, we analyzed the effect of MAPK7 on ovarian cancer metastasis via silencing and up-regulating MAPK7 expression in OVCAR-3 cells. Our data performed that overexpression of MAPK7 would significantly promote PVCAR-3 cell proliferation, invasion, and migration, while the effects were reverse when MAPK7 was sliced (P<0.05). Moreover, MAPK7 overexpression would significantly increase the protein level of type II collagen in OVCAR-3 cells (P<0.05), suggesting that MAPK7 expression may be correlated to ovarian cancer metastasis.
Roles of MAPK7 expression in cancer cell proliferation has been mentioned in several kinds of malignancies such as breast cancer and osteosarcoma. Previous evidence presented that several key genes were involved in the prognosis abnormal amplification of chromosome 17p11.2 area in osteosarcoma cells [19]. MAPK7 expression was involved in tumor progression and development in acute myelocytic leukemia [20,21]. In this study, our results showed that MAPK7 overexpression significantly increased OVCAR-3 cell proliferation; we speculated that MAPK7 may be involved in OVCAR-3 cell proliferation and ovarian cancer progression. On the other hand, MAPK7 overexpression significantly increased OVCAR-3 cell migration and cell invasion. Role of MAPK7 in ovarian cancer has not been fully described, however, ERK5 (human MAPK7) has been implicated in tumor cell progression and invasion through affecting cell cycle and cell transformation [22]. It has been proved that MAPK7 was the target gene for miR-143, which was associated with osteosarcoma progression, including invasion and migration [23]. Also, Lee said that intracellular signaling related genes including MAPK7 was correlated to hepatocellular carcinoma metastasis including invasion and migration [24]. ERK5 expression was correlated to invasive phenotype of prostate cancer through involving in cell migration [25]. Thus, we speculated that MAPK7 expression may be involved in ovarian cancer metastasis through affecting cell migration and invasion.
Meanwhile, MAPK7 overexpression significantly increased type II collagen expression in OVCAR-3 cells during ovarian cancer progression. Collagen is the main component for extracellular matrix (ECM), which plays pivotal roles in maintaining skin and vessel elastic, and increasing cartilage lubricity [26,27]. Effect of collagen II in ovarian cancer has not been fully reported. Nevertheless, collagen I and VI have been issued in breast cancer. It has been proved that type I and type VI collagen distributed on connective tissue and the base-membrane respectively [28]. Previous papers have referred that type IV collagen was overexpressed in breast cancer tissue through angiogenesis and tumor [29,30], but type I collagen expression was not correlated with benign or malignant breast cancer [31]. Biological mechanism for tumor migration and invasion was the across the basement membrane through degrading many components of ECM including collagen IV and fibronectin [32,33]. Our data showed that collagen II expression was significantly increased by overexpression of MAPK7 in OVCAR-3 cells, indicating that MAPK7 expression could accelerate collagen II degradation. However, the results performed there was no significant difference between collagen I expression and MAPK7, which suggested that MAPK7 may performed no influence on collagen I expression in affecting ovarian cancer metastasis. Our results were coincidence with previous report, which pointed out the potential role of ERK5 (MAPK7) in normal human nucleus pulposus cells and tissues [34]. Therefore, we speculated that overexpression of MAPK7 may contribute ovarian cancer metastasis through increasing type II collagen expression. Moreover, results of MAPK7 silencing on ovarian cell proliferation, migration, and invasion analysis presented the potential possibility of MAPK7 gene therapy for ovarian patients in clinical.
In conclusion, the data presented in this study suggests that up-regulation of MAPK7 might contribute to ovarian cancer metastasis through up-regulating type II collagen expression and then was involved in cell biological processes such as cell proliferation, invasion, and migration. MAPK7 may be a potential therapeutic target for ovarian cancer treatment in clinical. Our study may provide basis for the mechanism exploration of MAPK7 in ovarian cancer metastasis. However, further investigate experimental studies are still needed to provide clinical supports for MAPK7 application on ovarian cancer treatment.
Disclosure of conflict of interest
None.
References
- 1.Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Instit. 2015;107 doi: 10.1093/jnci/dju410. dju410. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, Tenthorey J, Leiser A, Flores-Saaib R, Yu H. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008;14:1065–1072. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z, Bast RC, Yu Y, Li J, Sokoll LJ, Rai AJ, Rosenzweig JM, Cameron B, Wang YY, Meng XY. Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer. Cancer Res. 2004;64:5882–5890. doi: 10.1158/0008-5472.CAN-04-0746. [DOI] [PubMed] [Google Scholar]
- 5.Elit L, Bondy S, Paszat L, Przybysz R, Levine M. Outcomes in surgery for ovarian cancer. Gynecol Oncol. 2002;87:260–267. doi: 10.1006/gyno.2002.6834. [DOI] [PubMed] [Google Scholar]
- 6.Krens SG, Spaink HP, Snaar-Jagalska BE. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006;580:4984–4990. doi: 10.1016/j.febslet.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. 2007;6:3039–3048. doi: 10.1158/1535-7163.MCT-07-0281. [DOI] [PubMed] [Google Scholar]
- 8.Ratkaj I, Stajduhar E, Vucinic S, Spaventi S, Bosnjak H, Pavelic K, Pavelic SK. Integrated gene networks in breast cancer development. Funct Integr Genomics. 2010;10:11–19. doi: 10.1007/s10142-010-0159-2. [DOI] [PubMed] [Google Scholar]
- 9.van Dartel M, Cornelissen PW, Redeker S, Tarkkanen M, Knuutila S, Hogendoorn PC, Westerveld A, Gomes I, Bras J, Hulsebos TJ. Amplification of 17p11. 2~p12, including PMP22, TOP3A, and MAPK7, in high-grade osteosarcoma. Cancer Genet Cytogenet. 2002;139:91–96. doi: 10.1016/s0165-4608(02)00627-1. [DOI] [PubMed] [Google Scholar]
- 10.Tesser-Gamba F, Petrilli AS, de Seixas Alves MT, Garcia Filho RJ, Juliano Y, Toledo SRC. MAPK7 and MAP2K4 as prognostic markers in osteosarcoma. Hum Pathol. 2012;43:994–1002. doi: 10.1016/j.humpath.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Miranda M, Rozali E, Khanna KK, Al-Ejeh F. MEK5-ERK5 pathway associates with poor survival of breast cancer patients after systemic treatments. Oncoscience. 2014;2:99–101. doi: 10.18632/oncoscience.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montero JC, Ocana A, Abad M, Ortiz-Ruiz MJ, Pandiella A, Esparís-Ogando A. Expression of Erk5 in early stage breast cancer and association with disease free survival identifies this kinase as a potential therapeutic target. PLoS One. 2009;4:e5565. doi: 10.1371/journal.pone.0005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalby B, Cates S, Harris A, Ohki EC, Tilkins ML, Price PJ, Ciccarone VC. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Hummon AB, Lim SR, Difilippantonio MJ, Ried T. Isolation and solubilization of proteins after TRIzol® extraction of RNA and DNA from patient material following prolonged storage. Biotechniques. 2007;42:467. doi: 10.2144/000112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Meerloo J, Kaspers GJ, Cloos J. Cancer Cell Culture. Springer; 2011. Cell sensitivity assays: the MTT assay; pp. 237–245. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt GH, Wilkinson MM, Ponder BA. Cell migration pathway in the intestinal epithelium: an in situ marker system using mouse aggregation chimeras. Cell. 1985;40:425–429. doi: 10.1016/0092-8674(85)90156-4. [DOI] [PubMed] [Google Scholar]
- 17.Shaw LM. Cell Migration. Springer; 2005. Tumor cell invasion assays; pp. 97–105. [DOI] [PubMed] [Google Scholar]
- 18.Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, Schilder RJ, Ozols RF, Testa JR. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13:4261–4270. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 19.Both J, Wu T, Bras J, Schaap GR, Baas F, Hulsebos TJ. Identification of novel candidate oncogenes in chromosome region 17p11. 2-p12 in human osteosarcoma. PLoS One. 2012;7:e30907. doi: 10.1371/journal.pone.0030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casas S, Ollila J, Aventín A, Vihinen M, Sierra J, Knuutila S. Changes in apoptosis-related pathways in acute myelocytic leukemia. Cancer Genet Cytogenet. 2003;146:89–101. doi: 10.1016/s0165-4608(03)00102-x. [DOI] [PubMed] [Google Scholar]
- 21.Goellner S, Steinbach D, Schenk T, Gruhn B, Zintl F, Ramsay E, Saluz HP. Childhood acute myelogenous leukaemia: association between PRAME, apoptosis-and MDR-related gene expression. Eur J Cancer. 2006;42:2807–2814. doi: 10.1016/j.ejca.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Reddy KB, Nabha SM, Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003;22:395–403. doi: 10.1023/a:1023781114568. [DOI] [PubMed] [Google Scholar]
- 23.Osaki M, Takeshita F, Sugimoto Y, Kosaka N, Yamamoto Y, Yoshioka Y, Kobayashi E, Yamada T, Kawai A, Inoue T. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther. 2011;19:1123–1130. doi: 10.1038/mt.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CF, Ling ZQ, Zhao T, Fang SH, Chang WC, Lee SC, Lee KR. Genomic-wide analysis of lymphatic metastasis-associated genes in human hepatocellular carcinoma. World J Gastroenterol. 2009;15:356. doi: 10.3748/wjg.15.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsay A, McCracken S, Soofi M, Fleming J, Yu A, Ahmad I, Morland R, Machesky L, Nixon C, Edwards D. ERK5 signalling in prostate cancer promotes an invasive phenotype. Br J Cancer. 2011;104:664–672. doi: 10.1038/sj.bjc.6606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 27.Deyl Z, Mikšık I, Eckhardt A. Preparative procedures and purity assessment of collagen proteins. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;790:245–275. doi: 10.1016/s1570-0232(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 28.Maynes R. Structure and function of collagen types. Elsevier; 2012. [Google Scholar]
- 29.Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev. 2005;15:97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortes-Reynosa P, Robledo T, Macias-Silva M, Wu SV, Salazar EP. Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol. 2008;27:220–231. doi: 10.1016/j.matbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Borrirukwanit K, Lafleur MA, Mercuri FA, Blick T, Price JT, Fridman R, Pereira JJ, Leardkamonkarn V, Thompson EW. The type I collagen induction of MT1-MMP-mediated MMP-2 activation is repressed by αVβ3 integrin in human breast cancer cells. Matrix Biol. 2007;26:291–305. doi: 10.1016/j.matbio.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Yuen SM, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1080. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altankov G, Bl GB. Studies Of Fibronectin-Cancer Cells Interactions Under Static And Dynamic Conditions. Different Adhesive Behavior Of High Metastatic And Low Metastatic Subclone Of Nci-H460 [Google Scholar]
- 34.Liang W, Fang D, Ye D, Zou L, Shen Y, Dai L, Xu J. Differential expression of extracellular-signal-regulated kinase 5 (ERK5) in normal and degenerated human nucleus pulposus tissues and cells. Biochem Biophys Res Commun. 2014;449:466–470. doi: 10.1016/j.bbrc.2014.05.042. [DOI] [PubMed] [Google Scholar]


