Abstract
Femoral head avascular necrosis (AVN) causes the damage of hip joint and related dysfunctions, thus consisting of a clinical challenge. Osteoprotegerin (OPG), receptor activator of nuclear factor κB (RANK) and its ligand (RANKL) all regulate the formation of bones via gene transcriptional regulation for the balance between osteoblasts and osteoclasts. This study thus investigated the expressional profiles of OPG, RANK and RANKL genes in AVN patients, and explored related molecular mediating pathways. Real-time qPCR was used to measure the gene expression of OPG, RANK and RANKL genes in AVN femoral head tissue samples from 42 patients, along with normal tissues. Western blotting analysis was performed to quantify protein levels of OPG and RANKL. There was a trend but not statistically significant elevation of mRNA levels of OPG in femoral head AVN tissues compared to normal tissues (P>0.05). The expression of RNAK and RNAKL, however, was significantly elevated in necrotic tissues (P<0.05). No significant difference in protein levels of OPG or RANKL between groups. The expression of OPG, RANK and RANKL genes exert a crucial role in the progression of AVN, suggesting their roles in mediating bone homeostasis and potential effects on bone destruction.
Keywords: Femoral head avascular necrosis, osteoprotegerin, receptor activator of nuclear factor κB, ligand for receptor activator of nuclear factor κB, gene expression regulation
Introduction
Femoral head avascular necrosis (AVN) is a complicated and common disease in orthopedics. Current intervention methods had limited treatment efficacy, thus frequently causing the damage of femoral head and degradation of hip joints [1]. AVN can be further aggravated by the use of steroid, alcoholic intake, systemic lupus erythematosus, sickle cell anemia, metabolic disorders, tumors or trauma [2,3]. The body’s repaired mechanism may function as the vessel proliferation and temporally active osteoclasts at the necrotic lesion and adjacent tissues at the early stage of AVN. Such self-repair, however, had limited effects as the bone formation by osteoblasts will be inhibited, along with the persistent activation of osteoclasts, which finally lead to the degradation of chondrocytes and breakage of joint surface [4-6]. The dynamic balance between osteoblasts and osteoclasts is mediated by various molecular pathways, which affect the bone formation in a synchronized pattern. The interruption of this balance will break the focal homeostasis of bone metabolism. Osteoprotegerin (OPG), receptor activator of nuclear factor κB (RANK) and its ligand (RANKL) are all known factors for bone formation and degradation. As the first identified molecule in RANK-RANKL-OPG axis, OPG can inhibit the proliferation of osteoclasts via its competitive binding against RANK. The binding between RANKL and homologous RNAK, however, can facilitate the bone reabsorption via a cascade signaling pathway [7,8]. As the pleiotropic roles of those three genes in bone formation and bone reabsorption, they have been widely used in treating osteoporosis, arthritis, vascular diseases, tumor and bone fracture [9-11]. In this study, the expressional profiles of those three genes were evaluated in a total of 42 AVN patients, in parallel with normal controlled individuals, in an attempt to investigate their roles in focal bone formation.
Materials and methods
Patient information
Tissue samples were collected from a total of 44 femoral head AVN patients, all of which received the total hip arthroplasties (THA) in our hospital. Among all patients, two individuals were excluded due to the lower protein contents in their samples. The condition of femoral head AVN was determined to be grade III or grade IV based on association research circulation osseous (ARCO) standards. This study has been approved by the ethical committee of our hospital. Written consents have been obtained from patients and their families.
Sample collection
The femoral head sample collected from the surgery was longitudinally divided into two parts. Total RNA was extracted from both necrotic and normal tissues at comparable sites. The morphology of sectioning surface was recorded during the surgery for the determination of the borderline between necrotic and normal tissues (Figure 1). Necrotic bone tissues were samples from inferior chondrocytes (about 1~3 mm below the cartilage). Normal tissues were collected from the head/neck of the femoral, with more than 1 cm away from the borderline. Collected tissues were rinsed in 0.9% saline for 5 min to clear bone marrow and residual blood. RNA and proteins were then extracted as reported previously [1]. In brief, fresh tissue samples were lysed in Trizol reagents (Invitrogen, UK) for total RNA extraction following manual instructions. RNA was then purified by RNeasy Mini-kit (Qiagen, Germany) and analyzed by 1% agarose gel electrophoresis and UV spectrophotometer. The optical density (OD) ratio at 260/280 nm was between 1.8 and 2.0, suggesting highly purified RNA. We further extracted and cultured cells from seven necrotic tissues along with normal controlled tissues following established protocols [12]. The staining of cultured cells by 85L-2 kit (Sigma-Aldrich, UK) also revealed the existence of living osteoblasts even in necrotic tissues (Figure 2).
Figure 1.
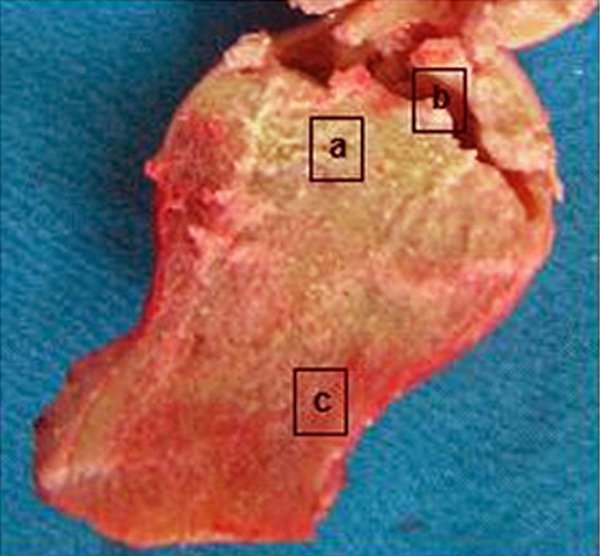
Femoral head tissue sample. a. Necrotic bone tissues; b. Breakage of cartilage; c. Normal tissues.
Figure 2.
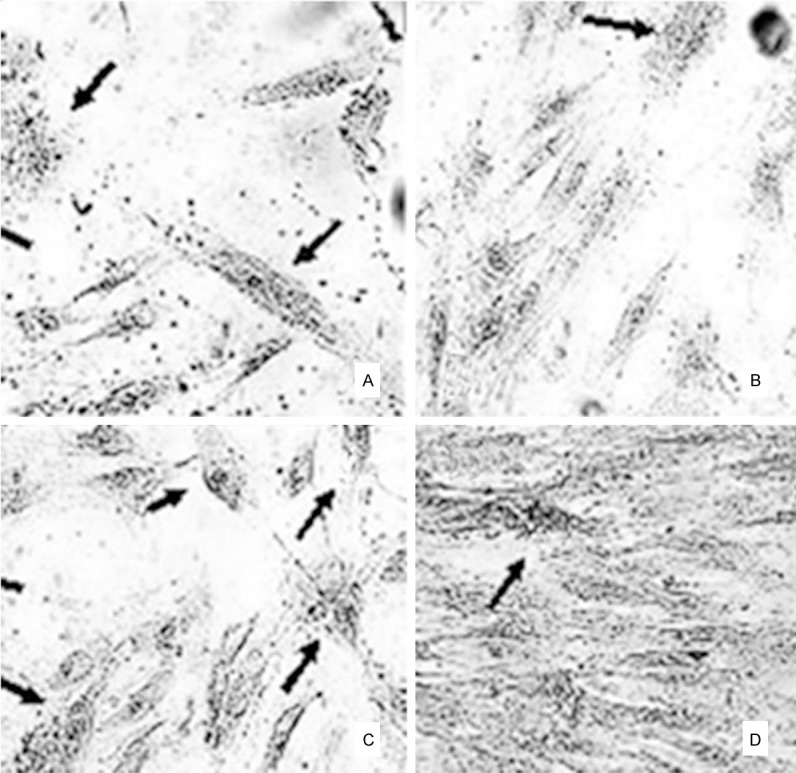
Cultured cells from femoral head samples. A and B. Normal tissues; C and D. Necrotic bone tissues. Arrows, osteoclasts by alkaline phosphate. Magnification, 40×.
Real-time qPCR
To quantify the gene expression of OPG, RANK and RANKL genes, we employed fluorescent qPCR technique. The in vitro reverse transcription was performed using RNA as the template. PCR system contained 2 μL cDNA template, 0.5 μM specific primers, 1 mM MgCl2 and 2 μL polymerase. The parameters were: initial denature (95°C, 10 min); 45 cycles containing denature (95°C, 15 sec), annealing (54°C, 10 sec) and elongation (72°C, 16 sec). House-keeping gene with stable transcription levels such as h-PBGD gene was used as the reference to quantify the expression level of target genes. A cycle threshold (Ct) value for cDNA copy number was determined from the standard curve of each gene and the reference. The relative expression level was determined by 2-ΔΔCt method.
DNA oligonucleotide primers and hybridization probes were synthesized by TIB Molbiol (Germany). Fluorescent labeling was conjugated on the adjacent sites of hybridization probe. In brief, recipient fluorescent molecule LC Red 640 labeled the 5’-end of the first probe, while fluorescein (FITC, 3FL) was used to label the 3’-end in the second probe. The probe wit 5’-label prevents the polymerase elongation during 3’-phosphrylation in PCR. Sequences of primers and hybridization probes were listed in Table 1. The lengths of PCR products for OPG, RANKL and RANK genes were 147 bp, 164 bp and 132 bp, respectively.
Table 1.
Primer and probe sequences used in qPCR
| Target gene | Primer/probe name | Oligonucleotide sequence (5’->3’) |
|---|---|---|
| OPG | Sense primer | 5’-gaagc tggaa cccca gag-3’ |
| Antisense primer | 5’-gtgtt gcatt tcctt tctga gtta-3’ | |
| 1st probe | 5’-caatt tgtgt gtttt ctaca gggtg tt-FL | |
| 2nd probe | 5’-LC640-agatg acgtc tcatt tgaga agaac ccat-PH | |
| RANKL | Sense primer | 5’-gcaaa aggaa ttaca acata tcgtt-3’ |
| Antisense primer | 5’-acttt atggg aacca gatgg g-3’ | |
| 1st probe | 5’-ccaga tctaa ccatg agcca tccac c-FL | |
| 2nd probe | 5’-LC640-tcgct ttctc tgctc tgatg tgctg tg-PH | |
| RANK | Sense primer | 5’-aggga aagca ctcac agcta at-3’ |
| Antisense primer | 5’-acatg ctccc tgctg acc-3’ | |
| 1st probe | 5’-agtgg agata aggag tcctc aggtg aca-FL | |
| 2nd probe | 5’-LC640-ttgtg tcagt acaca cacgg caaac tt-PH |
Western blotting
Both necrotic and normal tissues were firstly rinsed by PBS and were then lysed using NET-Triton lysis buffer (containing 0.01 M Trsi-Cl, 0.1 M NaCl, 1 mM EDTA pH 7.4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% proteinase inhibitor). Proteins were separate by electrophoresis on Nu-PAGE-Tris aceate gel (Invitrogen, CA) and were transferred to PVDF membrane (BioRad, UK). Non-specific binding sites were blocked by 5% defatted milk powder, mouse anti-RANKL monoclonal antibody (1:100, Santa-Cruz, US) or human anti-OPG monoclonal antibody (1:250, Abcam, UK) was added to detect the protein expression. Human anti-actin monoclonal antibody (1:500, Serotec, UK) was used as an internal reference. After overnight incubation, anti-mouse/human IgG conjugated with horseradish peroxidase (HRP) (1:500, Abcam, UK) was added to amplify the signal. ELC detection reagent was then used to visualize the positive protein bands. Radioactive autography was performed under the exposure of XAR-5 films (Kodak, Japan). Protein bands were quantified by a laser scanner (Epson, Japan). The relative expression of proteins was calculated as the ratio of OD values of target protein bands against actin.
Statistical analysis
SPSS 16.0 software package was used to analyze all collected data, which were presented as mean ± standard deviation (SD) or medians with minimal/maximal values. Wilcoxon rank-sum test was used in between-comparison, a statistical significance was defined when P<0.05.
Results
Patient information
Of all 42 AVN patients, there were 23 women (10 with menopause) and 19 males. The average age was 43±2.3 years old (20~70 years old). Based on ARCO standard, 4 patients were at stage III while the other 38 patients were at stage IV. A total of 12 patients were smokers. In an epidemiological survey, 15 patients had steroid medicines, one has received kidney transplantation, three of them had sickle cell anemia, three patients were complicated with systemic lupus erythematosus while four patients are alcoholics. The other four patients had no identified risk factor for AVN.
mRNA levels of OPG, RANKL and RANK
The ratio of OPG/h-PBGD mRNA in necrotic bone tissues was higher than normal tissues but with no statistical significance (Figure 3, P>0.05). The expression of RANK and RANKL was significantly higher in necrotic and normal tissues (P<0.05 in both cases).
Figure 3.
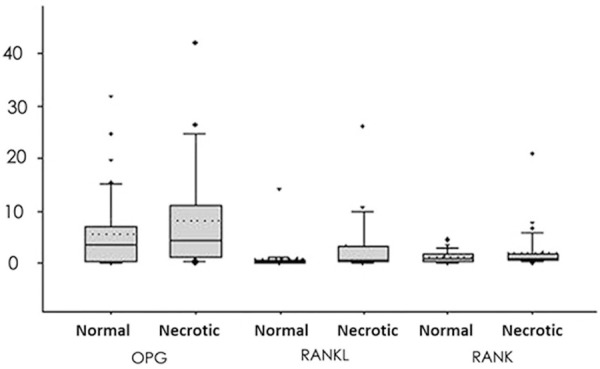
mRNA levels of OPG, RANKL and RANK genes. The box-plot displayed 25% quartile, mean and 75% quartile, in addition to 10% and 90% error bar. Medians were given by dashed lines while outraged data points showed minimal/maximal values. The expression of RANK/RANKL in necrotic tissues was significantly increased compared to controlled ones.
Western blotting
Total proteins from both necrotic and normal tissues were analyzed for expressions of OPG and RANKL. As shown in Figures 4 and 5, OPG protein was expressed in both normal and necrotic tissues with similar levels (P>0.05). The RANKL protein also had similar levels in both tissues (P>0.05). RANK protein, however, was not identified in either tissue.
Figure 4.
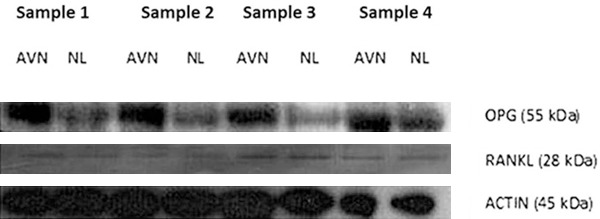
Western blotting for OPG and RANKL proteins. Actin was used as the internal control protein. AVN, necrotic tissues; NL, normal tissues.
Figure 5.
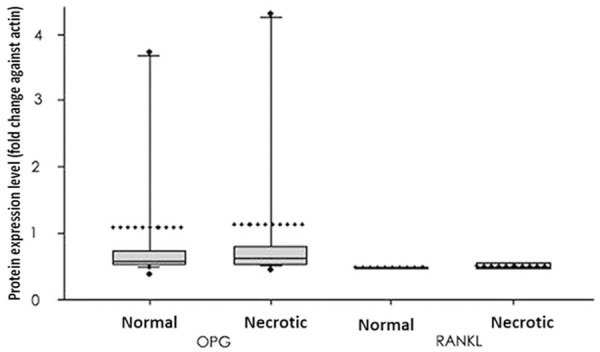
Quantified protein levels of OPG and RANKL. Protein levels were expressed as fold changes against actin in the same tissue and were plotted as the box plot showing 25% quartile, mean and 75% quartile, in addition to 10% and 90% error bar. Medians were given by dashed lines while outraged data points showed minimal/maximal values. No significant has been identified between normal and necrotic tissues in either protein.
Correlation analysis between gene expression and risk factors for AVN
We firstly divided all patients into three age groups: (1) between 20 and 35 years old; (2) between 36 and 50 years old; (5) older than 50 years. The expressional profiles in each group were studied and found no significant correlations between any gene expression level and age (P>0.05). We also compared the gene expression level between women before menopause and those after menopause. The RANK level in necrotic tissues was significantly elevated in menopaused women (3.36 vs. 0.95, P<0.05). Moreover, RANK gene expression was also significantly potentiated in smokers compared to non-smoking patients (2.23 vs. 1.23, P<0.05).
Discussion
The pathogenesis of AVN in femoral heads involves multiple factors, of which the imbalance between osteoblasts and osteoclasts are crucial due to their mediation on bone formation [13]. The focal ischemia in AVN leads to the necrosis of bones and marrows. Although body’s auto-impairment may fix minor lesion (less than 15% of total femoral head), mostly it does not accomplish the fixation [14,15]. Surgical measures include depressurization, bone dissection and bone transplantation with/without blood vessels. In severe cases, total hip joint replacement is necessary. However, repeated surgeries and postoperative complications often affects the patient’s life quality [16].
The growth, development and maintenance of bones are closely regulated by the interaction between osteoclasts, which facilitate bone formation, and osteoclasts for bone reabsorption. OPG, RANKL and RANK have been reported to be important factors in maintaining balances between those two cells [17] as the dysregulation of those factors may lead to the occurrence of various orthopedics diseases [12,18,19]. In this study, we investigated the gene expressions of OPG, RANKL and RANK in femoral head samples of AVN patients. Our results showed elevated but not statistically significant difference of OPG mRAN in necrotic tissues. The mRNA of RANKL and RANK, however, were highly expressed in necrotic tissues when compared to control tissues. Protein levels of either OPG or RANKL, were not significantly changed between necrotic and normal tissues. In summary, inconsistent patterns between mRNA and protein expressions existed for those genes, suggesting further post-translational regulation in necrotic tissues.
This study, for the first time, suggested relatively elevated OPG mRNA in necrotic bone tissues, although with no statistical significance. This may be caused by decreased activity of osteoclast, which can disrupt the balance between osteoblasts and osteoclast, further compromising bone reformation. There have been studies reported the elevated bone density and osteopetrosis in mice with OPG-overexpression. In contrast, the gene knockout of OPG may lead to early onset osteoporosis. A recent clinical survey showed significant correlation between gene expressions of OPG, RANKL and RANK with the risks of bone fracture, suggesting the crucial role of OPG in postnatal bone development. Other studies focused on mutation of RANK or OPG and diseases such as osteolysis, Paget’s disease and progressive bone deformity, further supporting the interaction among those three factors in maintain normal bone volumes [20-23]. In our study, the elevation of OPG mRNA in necrotic tissues was consistent with imaging results and mouse model with higher bone density [24]. Whether the elevate OPG is the result of AVN or just one etiological factor for ostesopetrosis, however, needs to be elucidated.
In a comparison between RANKL/OPG ratios between necrotic and normal tissues, no significant differences have been identified. However, both gene expression levels were elevated in necrotic tissues, reflecting the requirement of osteoblasts in bone reformation. Genes responsible for osteoblasts maturation and functions, such as bone morphological proteins (BMPs), related transcriptional factors (RUNX2) and other signaling molecules have been demonstrated to be related with bone volume abnormality and bone diseases [25,26]. For example, recent studies showed the decreasing bone volume by active osteoclasts, which are induced by BMPs via RANKL-OPG pathway, making BMPs as key regulators for osteoclasts [27,28].
In summary, the expression of OPG, RANK and RANKL genes are closely related with progression of femoral head AVN. Those genes may affect bone homeostasis and may exert certain roles in bone reabsorption and long-term hip joint breakage.
Disclosure of conflict of interest
None.
References
- 1.Baelde HJ, Cleton-Jansen AM, van Beerendonk H, Namba M, Bovée JV, Hogendoorn PC. High quality RNA isolation from tumours with low cellularity and high extracellular matrix component for cDNA microarrays: application to chondrosarcoma. J Clin Pathol. 2001;54:778–82. doi: 10.1136/jcp.54.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorkman A, Svensson PJ, Hillarp A, Burtscher IM, Rünow A, Benoni G. Factor V leiden and prothrombin gene mutation: risk factors for osteonecrosis of the femoral head in adults. Clin Orthop Relat Res. 2004:168–72. [PubMed] [Google Scholar]
- 3.Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86:631–7. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 5.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–35. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 6.Celik A, Tekis D, Saglam F, Tunali S, Kabakci N, Ozaksoy D, Manisali M, Ozcan MA, Meral M, Gülay H, Camsari T. Association of corticosteroids and factor V, prothrombin, and MTHFR gene mutations with avascular osteonecrosis in renal allograft recipients. Transplant Proc. 2006;38:512–6. doi: 10.1016/j.transproceed.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 7.Chong B, Hegde M, Fawkner M, Simonet S, Cassinelli H, Coker M, Kanis J, Seidel J, Tau C, Tüysüz B, Yüksel B, Love D International Hyperphosphatasia Collaborative Group. Idiopathic hyperphosphatasia and TNFRSF11B mutations: relationships between phenotype and genotype. J Bone Miner Res. 2003;18:2095–104. doi: 10.1359/jbmr.2003.18.12.2095. [DOI] [PubMed] [Google Scholar]
- 8.Cundy T, Hegde M, Naot D, Chong B, King A, Wallace R, Mulley J, Love DR, Seidel J, Fawkner M, Banovic T, Callon KE, Grey AB, Reid IR, Middleton-Hardie CA, Cornish J. A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum Mol Genet. 2002;11:2119–27. doi: 10.1093/hmg/11.18.2119. [DOI] [PubMed] [Google Scholar]
- 9.Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, Koller DL, Li G, Liu CT, Minster RL, Moayyeri A, Vandenput L, Willner D, Xiao SM, Yerges-Armstrong LM, Zheng HF, Alonso N, Eriksson J, Kammerer CM, Kaptoge SK, Leo PJ, Thorleifsson G, Wilson SG, Wilson JF, Aalto V, Alen M, Aragaki AK, Aspelund T, Center JR, Dailiana Z, Duggan DJ, Garcia M, Garcia-Giralt N, Giroux S, Hallmans G, Hocking LJ, Husted LB, Jameson KA, Khusainova R, Kim GS, Kooperberg C, Koromila T, Kruk M, Laaksonen M, Lacroix AZ, Lee SH, Leung PC, Lewis JR, Masi L, Mencej-Bedrac S, Nguyen TV, Nogues X, Patel MS, Prezelj J, Rose LM, Scollen S, Siggeirsdottir K, Smith AV, Svensson O, Trompet S, Trummer O, van Schoor NM, Woo J, Zhu K, Balcells S, Brandi ML, Buckley BM, Cheng S, Christiansen C, Cooper C, Dedoussis G, Ford I, Frost M, Goltzman D, González-Macías J, Kähönen M, Karlsson M, Khusnutdinova E, Koh JM, Kollia P, Langdahl BL, Leslie WD, Lips P, Ljunggren Ö, Lorenc RS, Marc J, Mellström D, Obermayer-Pietsch B, Olmos JM, Pettersson-Kymmer U, Reid DM, Riancho JA, Ridker PM, Rousseau F, Slagboom PE, Tang NL, Urreizti R, Van Hul W, Viikari J, Zarrabeitia MT, Aulchenko YS, Castano-Betancourt M, Grundberg E, Herrera L, Ingvarsson T, Johannsdottir H, Kwan T, Li R, Luben R, Medina-Gómez C, Palsson ST, Reppe S, Rotter JI, Sigurdsson G, van Meurs JB, Verlaan D, Williams FM, Wood AR, Zhou Y, Gautvik KM, Pastinen T, Raychaudhuri S, Cauley JA, Chasman DI, Clark GR, Cummings SR, Danoy P, Dennison EM, Eastell R, Eisman JA, Gudnason V, Hofman A, Jackson RD, Jones G, Jukema JW, Khaw KT, Lehtimäki T, Liu Y, Lorentzon M, McCloskey E, Mitchell BD, Nandakumar K, Nicholson GC, Oostra BA, Peacock M, Pols HA, Prince RL, Raitakari O, Reid IR, Robbins J, Sambrook PN, Sham PC, Shuldiner AR, Tylavsky FA, van Duijn CM, Wareham NJ, Cupples LA, Econs MJ, Evans DM, Harris TB, Kung AW, Psaty BM, Reeve J, Spector TD, Streeten EA, Zillikens MC, Thorsteinsdottir U, Ohlsson C, Karasik D, Richards JB, Brown MA, Stefansson K, Uitterlinden AG, Ralston SH, Ioannidis JP, Kiel DP, Rivadeneira F. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001:19–33. doi: 10.1097/00003086-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hadjigeorgiou G, Dardiotis E, Dardioti M, Karantanas A, Dimitroulias A, Malizos K. Genetic association studies in osteonecrosis of the femoral head: mini review of the literature. Skeletal Radiol. 2008;37:1–7. doi: 10.1007/s00256-007-0395-2. [DOI] [PubMed] [Google Scholar]
- 12.Tuli R, Seghatoleslami MR, Tuli S, Wang ML, Hozack WJ, Manner PA, Danielson KG, Tuan RS. A simple, high-yield method for obtaining multipotential mesenchymal progenitor cells from trabecular bone. Mol Biotechnol. 2003;23:37–49. doi: 10.1385/MB:23:1:37. [DOI] [PubMed] [Google Scholar]
- 13.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97:105–14. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, van Hul W, Whyte MP, Nakatsuka K, Hovy L, Anderson DM. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. 2000;24:45–8. doi: 10.1038/71667. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen HL, Kusk P, Madsen B, Fenger M, Lauritzen JB. Serum osteoprotegerin (OPG) and the A163G polymorphism in the OPG promoter region are related to peripheral measures of bone mass and fracture odds ratios. J Bone Miner Metab. 2004;22:132–8. doi: 10.1007/s00774-003-0461-3. [DOI] [PubMed] [Google Scholar]
- 16.Samara S, Dailiana Z, Varitimidis S, Chassanidis C, Koromila T, Malizos KN, Kollia P. Bone morphogenetic proteins (BMPs) expression in the femoral heads of patients with avascular necrosis. Mol Biol Rep. 2013;40:4465–72. doi: 10.1007/s11033-013-2538-y. [DOI] [PubMed] [Google Scholar]
- 17.Soucacos PN, Beris AE, Malizos K, Koropilias A, Zalavras H, Dailiana Z. Treatment of avascular necrosis of the femoral head with vascularized fibular transplant. Clin Orthop Relat Res. 2001:120–30. doi: 10.1097/00003086-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Whyte MP, Mills BG, Reinus WR, Podgornik MN, Roodman GD, Gannon FH, Eddy MC, McAlister WH. Expansile skeletal hyperphosphatasia: a new familial metabolic bone disease. J Bone Miner Res. 2000;15:2330–44. doi: 10.1359/jbmr.2000.15.12.2330. [DOI] [PubMed] [Google Scholar]
- 19.Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci. 2008;1143:123–50. doi: 10.1196/annals.1443.016. [DOI] [PubMed] [Google Scholar]
- 20.Kamiya N. The role of BMPs in bone anabolism and their potential targets SOST and DKK1. Curr Mol Pharmacol. 2012;5:153–63. doi: 10.2174/1874467211205020153. [DOI] [PubMed] [Google Scholar]
- 21.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–92. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5:618–25. doi: 10.1016/j.coph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Zalavras CG, Malizos KN, Dokou E, Vartholomatos G. The 677C-->T mutation of the methylene-tetrahydrofolate reductase gene in the pathogenesis of osteonecrosis of the femoral head. Haematologica. 2002;87:111–2. [PubMed] [Google Scholar]
- 24.Zupan J, Komadina R, Marc J. The relationship between osteoclastogenic and anti-osteoclastogenic pro-inflammatory cytokines differs in human osteoporotic and osteoarthritic bone tissues. J Biomed Sci. 2012;19:28. doi: 10.1186/1423-0127-19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Grewal R, Perey B, Wilmink M, Stothers K. A randomized prospective study on the treatment of intra-articular distal radius fractures: open reduction and internal fixation with dorsal plating versus mini open reduction, percutaneous fixation, and external fixation. J Hand Surg Am. 2005;30:764–72. doi: 10.1016/j.jhsa.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Trouvin AP, Goeb V. Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging. 2010;5:345–54. doi: 10.2147/CIA.S10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aubin JE, Bonnelye E. Osteoprotegerin and its ligand: a new paradigm for regulation of osteoclastogenesis and bone resorption. Osteoporos Int. 2000;11:905–13. doi: 10.1007/s001980070028. [DOI] [PubMed] [Google Scholar]


