Abstract
As one of the important complications of diabetes, diabetic retinopathy (DR) presented high incidence worldwide. Hyperglycemia is an important promoting factor for DR occurrence and development. It can damage retinal endothelial cell, resulting in retinal structure and function disorder. Studies have shown that miR-200b may involve in regulating DR occurrence and development, but its specific function and mechanism have not been elucidated. This study aimed to investigate miR-200b effect and mechanism on human retinal endothelial cells (hRECs) under high glucose environment. hRECs were cultured under high glucose or normal environment. Real time PCR was applied to detect miR-200b expression. MiR-200b was transfected to hRECs and MTT was used to detect its effect on hRECs proliferation under high glucose environment. Real time PCR and Western blot were performed to determine VEGF and TGFβ1 expression in the retina endothelial cells. MiR-200b expression decreased significantly under high glucose environment, whereas hRECs proliferated obviously. Compared with normal control, VEGF and TGFβ1 mRNA and protein expression increased markedly (P < 0.05). After miR-200b transfection, miR-200b expression increased, while VEGF and TGFβ1 mRNA and protein expression decreased obviously. Compared with high glucose group, hRECs proliferation was inhibited (P < 0.05). MiR-200b can regulate RECs growth and proliferation by changing VEGF and TGFβ1 expression to delay DR.
Keywords: Diabetic retinopathy, retinal endothelial cell, VEGF, TGFβ1
Introduction
As one of the most important diabetes complications, diabetic retinopathy (DR) is the leading cause of blindness in adult [1,2]. According to WHO survey, there were up to 360 million patients suffered from diabetes worldwide. It was expected to reach 1 billion till 2030 [3,4]. The study found that diabetic retinopathy (DR) was closely related to retinal microvascular system damage caused by high glucose environment [5]. DR can change retinal structure, leading to its metabolism and function disorder. Retinal microvascular endothelial cells were responsible to supply nutritional requirements for retina nerve. They played a key role in the protection of the vision by maintaining blood-retinal barrier and removing toxins and inflammatory factors [6,7].
MicroRNAs, also known as miRNAs or small RNAs, widely existed in animals and plants that was a kind of short chain noncoding RNA with the regulating function [8]. MiRNA can degrade mRNA and regulate protein translation through its complete or incomplete pairing with target genes, or by inhibiting the downstream target protein expression [9]. MiRNAs were mainly investigated in cancer, while they were lack of study in other diseases. MiR-200b was a new discovered miRNAs, and was reported to participate in diabetes occurrence and development [10]. It was found that miR-200b expression decreased significantly in diabetic patients, and can promote diabetes development. Thus, miR-200b was thought to play a role in diabetes occurrence and development [11]. Further study showed that miR-200b may be involved in DR occurrence and development [12]. But the specific function and mechanism of miR-200b in DR was still unclear. For hyperglycemia state can cause retinal endothelial cell (REC) injury directly or indirectly, it can lead to visual dysfunction and DR occurrence. At the same time, vascular endothelial growth factor (VEGF) and transforming growth factor β1 (TGFβ1) were confirmed to be involved in the regulation of DR lesions [13,14]. This study tended to study miR-200b impact on RECs under high glucose and effect in regulating VEGF and TGFβ1, to analyze its role in DR.
Materials and methods
Reagents and instruments
Human retinal endothelial cells were bought from Angio-Proteomie (USA). Human microvascular endothelial cell medium was got from Cell Applications. Penicillin-streptomycin and EDTA were purchased from Hyclone (USA). DMSO and MTT were bought from Gibco. Enzyme-EDTA was acquired from Sigma. PVDF membrane was got from Pall Life Science. RNA extraction kit, reverse transcription kit, and lipo2000 were bought from Invitrogen. Western blot related reagents were from Beyotime (Shanghai, China). ECL reagent was got from Amersham Biociences. Rabbit anti human VEGF and TGFβ1 antibodies, and HRP tagged IgG secondary antibody was got from Cell signaling (USA). DNA amplifier was bought from PE Gene Amp PCR System 2400 (USA). Other common reagents were from Sangon (Shanghai, China).
hRECs culture and grouping
hRECs at 3rd-8th generations were seeded in dish at 1×106 cells/cm2 with neural cell medium containing no fetal bovine serum DMEM medium (containing 100 U/ml penicillin and 100 μg/ml streptomycin), 5.5 mmol/L glucose. The cells were randomly divided into three groups, including normal control group: the cells were cultured under normal condition; High glucose group: high glucose environment was applied to induced culture cells, and the hRECs in logarithmic stage was maintained in microvascular endothelia cells medium with 33 mmol/L glucose after 72 h; MiR-200b: RGC-5 cells transfected with miR-200b were cultured under high glucose.
MiR-200b mimics tranfection
MiR-200b mimics was synthesized by Genepharma in Shanghai. The sequence of miR-200b mimics was 5’-UAAUACUGCCUGGUAAUGAUGAC-3’. Lipo2000 reagent was used to transfect miR-200b to hRECs under high glucose environment. hRECs in logarithmic growth phase seeded in 6-well plate at 3×106 cells/cm2 and maintained in 5% CO2 incubator at 37°C for 12 h. Then lipo2000 mixed with miR-200b mimics were added to the cells and incubated for 6 h. The cells were further cultured after changing the medium.
Real-time PCR
mRNAs were extracted from hRECs by Trizol. Real-time PCR was applied to detect target gene expression using the primers in Table 1. PCR reaction contained 52°C for 1 min, followed by 35 cycles including 90°C for 30 s, 58°C for 50 s and 72°C for 35 s. Gene expression levels were quantified relative to the expression of GAPDH using an optimized comparative Ct (2-ΔCt) value method.
Table 1.
Primer sequence
| Gene | Forward 5’-3’ | Reverse 5’-3’ |
|---|---|---|
| GADPH | AGTGCCAGCCTCGTCTCATAG | CGTTGAACTTGCCGTGGGTAG |
| Mir-200b | AGCGGCTCATCTAAACAATGG | GGCGCACATTCTCTCCGTA |
| VEGF | AAACTGTCAGCTCGGTCAGA | TCAGGGGCCGATTAAAGCTC |
| TGFβ1 | GCCAGGATATGAGTTTGGGA | GGGTGCATGTCTGCTCCTGT |
MTT
The cells were seeded in 96-well plate at 3000/well with five replication. 20 μl MTT solution at 5 g/L was added to each well and the plate was incubated for 4 h. After removing the supernatant, 150 μl DMSO was added for 10 min and the absorbance value at 570 nm was read to calculate cell proliferation rate.
Western blot
Total protein was extracted from hRECs by lysate with ultrasound. After centrifuged at 10000× g for 15 min, the supernatant was moved to new Ep tube and stored at -20°C. The protein was separated by 10% SDS-PAGE electrophoresis and transferred to PVDF membrane. After blocked by 5% skim milk for 2 h, the membrane was incubated with VEGF antibody (1:1000) or TGFβ1 antibody (1:2000) at 4°C overnight. After washed by PBST, the membrane was further incubated with goat anti rabbit secondary antibody (1:2000) for 30 min and imaged with chemiluminescent agent. The band was calculated by Quantity one software with four replication.
Statistical analysis
All statistical analyses were performed using SPSS16.0 software (Chicago, IL). Numerical data were presented as means and standard deviation (X̅ ± S). Differences between multiple groups were analyzed by one-way ANOVA. P < 0.05 was considered as significant difference.
Results
MiR-200b expression in hRECs
Real-time PCR was applied to detect miR-200b expression in hRECs. As shown in Figure 1, miR-200b expressed highly in normal hRECs, while it decreased obviously under high glucose environment (P < 0.05). MiR-200b mimics transfection to hRECs under high glucose environment can elevate miR-200b level significantly (P < 0.05).
Figure 1.
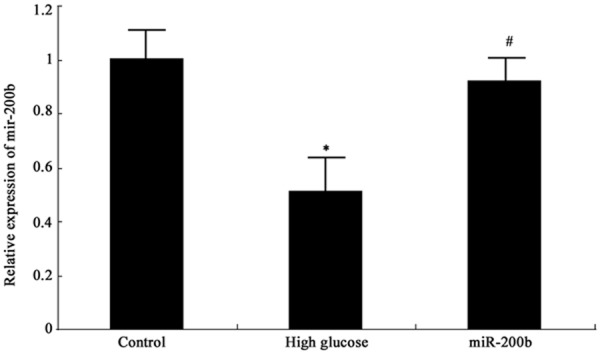
MiR-200b expression in hRECs *P < 0.05, compared with normal control; #P < 0.05, compared with high glucose group.
MiR-200b effect on hRECs proliferation
MTT was performed to test miR-200b effect on hRECs proliferation under high glucose. It was found that high glucose environment promote hRECs proliferation significantly (P < 0.05). After transfecting miR-200b mimics, hRECs proliferation under high glucose was suppressed markedly (P < 0.05) (Figure 2). It revealed that miR-200b reduction can promote retinal endothelial cell growth significantly, and targeting miR-200b can inhibit retinal endothelial cell proliferation.
Figure 2.
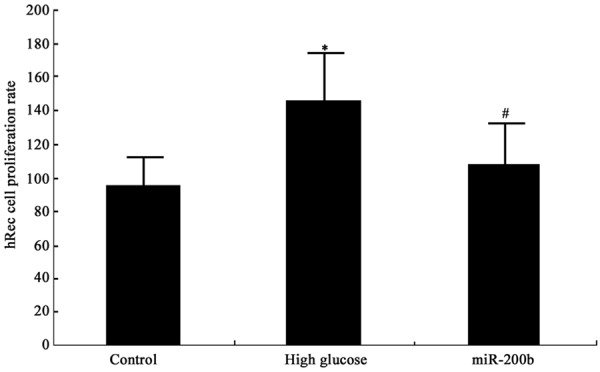
MiR-200b impact on hRECs proliferation *P < 0.05, compared with normal control; #P < 0.05, compared with high glucose group.
MiR-200b impact on VEGF and TGFβ1 mRNA expression in hRECs
MiR-200b impact on VEGF and TGFβ1 mRNA expression in hRECs under high glucose was detected by real-time PCR. VEGF and TGFβ1 mRNA expression in hRECs elevated significantly under high glucose (P < 0.05). MiR-200b mimics transfection can suppress VEGF and TGFβ1 mRNA expression in hRECs under high glucose obviously (P < 0.05) (Figure 3).
Figure 3.
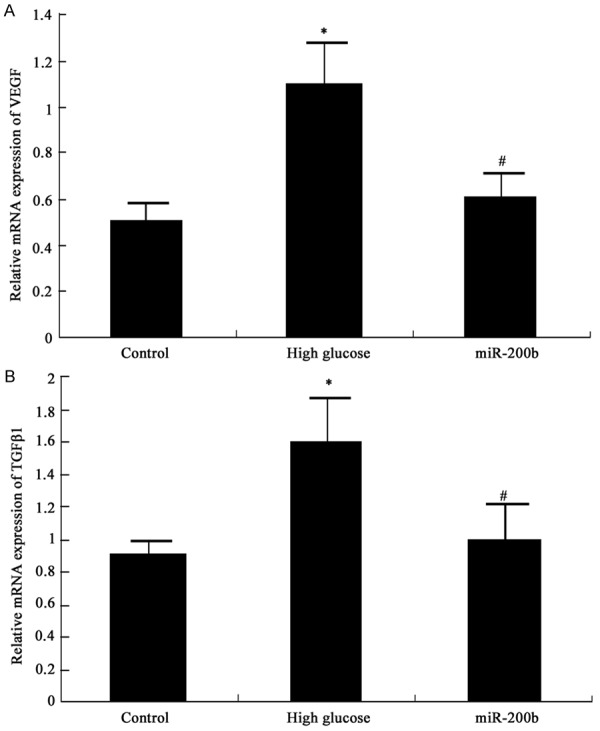
MiR-200b impact on VEGF and TGFβ1 mRNA expression in hRECs A MiR-200b impact on VEGF mRNA in hRECs B MiR-200b impact on TGFβ1 mRNA in hRECs *P < 0.05, compared with normal control; #P < 0.05, compared with high glucose group.
MiR-200b impact on VEGF and TGFβ1 protein expression in hRECs
Western blot was further applied to determine miR-200b mimics transfection effect on VEGF and TGFβ1 protein expression in hRECs and similar results with mRNA expression was observed. VEGF and TGFβ1 protein expression level elevated in hRECs under high glucose (P < 0.05), and their levels were restrained after miR-200b mimics transfection (P < 0.05) (Figures 4 and 5). It suggested that high glucose environment can inhibit miR-200b expression in hRECs, and increase VEGF and TGFβ1 mRNA and protein expression, further cause retinal endothelial cell structure and function disorder. Targeting miR-200b to promote its expression can downregulate VEGF and TGFβ1 mRNA and protein expression, thus improve DR.
Figure 4.
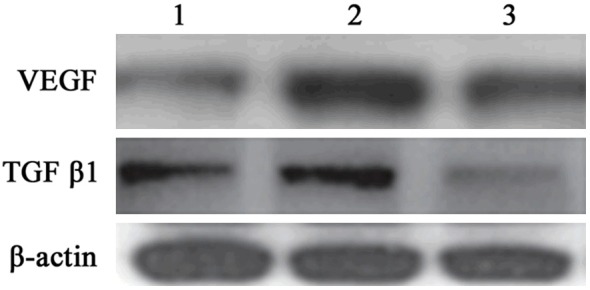
MiR-200b impact on VEGF and TGFβ1 protein expression in hRECs. 1 control; 2, high glucose group; 3, miR-200b group.
Figure 5.
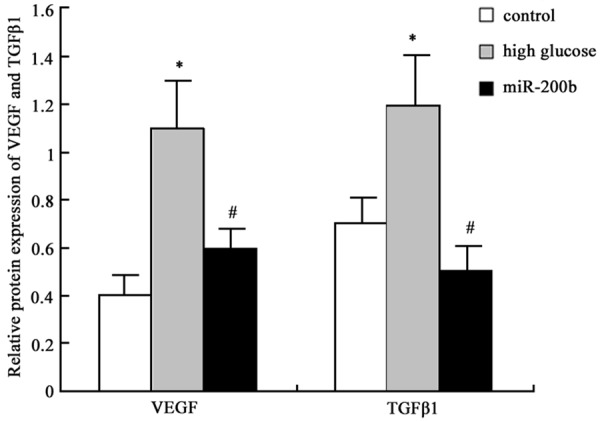
MiR-200b impact on VEGF and TGFβ1 protein expression in hRECs analysis *P < 0.05, compared with normal control; #P < 0.05, compared with high glucose group.
Discussion
DR was a common diabetic microvascular complication that can lead to retinal microvascular progressive damage. It seriously affected the patients’ physical and mental health, and brought heavy mental and economic burden to the society [15]. Although medical kept on progress, DR treatment effect was still not satisfied. High glucose environment in diabetic patients can lead to retinal endothelial cells suffered a series of endocrine metabolism changes. High blood glucose is an important factor for diabetes complications occurrence and development that can cause organ structure and function abnormity [16,17]. Retinal endothelial cells structure and function changes were the main pathological mechanism in the chronic process of diabetes. High blood glucose can result in retinal endothelial cell dysfunction including promoting cell proliferation and neovascularization through regulating endothelin and VEGF [18,19].
As voted one of the top ten important discoveries, miRNAs participated in various processes including cell proliferation, apoptosis, signal transduction, differentiation, hormone secretion, lipometabolism and maintaining the potential of embryonic stem cells. It can regulate the body’s growth and development to make the body adapt to the environment. Recently, multiple studies focused on miRNAs effect in the regulation of tumor occurrence, development, invasion, metastasis, and other biological features [9]. Few researches investigated miRNAs in DR. MiR-200b was thought to be involved in regulating diabetes occurrence and development [10], but its role in DR had not been elucidated. Through hRECs culture and high glucose environment treatment, our study confirmed that miR-200b level decreased in hRECs under high glucose state. It was further revealed that miR-200b can inhibit hRECs abnormal proliferation by transfecting miR-200b mimics.
VEGF had a variety of subtypes that can play its role by binding with the corresponding receptor (VEGFR). Most VEGFR located on endothelial cell surface. Their specific binding can change vascular permeability and promote blood vessel formation. Thus, VEGF regulated early stage DR by changing vascular permeability, whereas it participated in the late stage DR through promoting neovascularization [20,21]. TGFβ1 was a kind of isomer of transforming growth factor that belonged to the polypeptide growth factor. It widely distributed in the numerous cells and had many kinds of biological functions. It also played an important role in DR formation by regulating cell growth, differentiation and migration process, adjusting extracellular matrix synthesis and secretion, and participating in immunity. TGFβ1 is a type of strong regulatory factor to promote cell proliferation. It also can upregulate integrin expression, promote extracellular matrix secretion, regulate the interactions between cells and matrix, and chemotaxis macrophages to facilitate neovascularization and fibroblast growth. It also can play a role of immunosuppression by inhibiting antibody formation and lymphocyte proliferation to suppress the cytotoxic effect of CTL, NK, and LAK cells [22,23]. However, there is still lack of investigation about TGFβ1 role in DR. Thus, we intended to study miR-200b effect in retinal endothelial cells. It was confirmed that suppressing miR-200b expression under high glucose environment may promote VEGF and TGFβ1 mRNA and protein expression to facilitate endothelial cell proliferation and neovascularization, leading to retinopathy. Upregulating miR-200b expression by mimics can reduce VEGF and TGFβ1 mRNA and protein expression, and improve endothelial cell structure and function to postpone DR progress.
To sum up, miR-200b can delay DR progress by changing VEGF and TGFβ1 expression to mediate retinal endothelial cell growth and proliferation.
Acknowledgements
Hunan Science and Technology Office of China Scientific Research Project (2014TT2024) and Hunan Science and Technology Office of China Scientific Research Project (2012TT2029).
Disclosure of conflict of interest
None.
References
- 1.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 2.Mysona BA, Matragoon S, Stephens M, Mohamed IN, Farooq A, Bartasis ML, Fouda AY, Shanab AY, Espinosa-Heidmann DG, El-Remessy AB. Imbalance of the nerve growth factor and its precursor as a potential biomarker for diabetic retinopathy. Biomed Res Int. 2015;2015:571456. doi: 10.1155/2015/571456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park YH, Shin JA, Han JH, Park YM, Yim HW. The association between chronic kidney disease and diabetic retinopathy: the Korea National Health and Nutrition Examination Survey 2008-2010. PLoS One. 2015;10:e0125338. doi: 10.1371/journal.pone.0125338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boynton GE, Stem MS, Kwark L, Jackson GR, Farsiu S, Gardner TW. Multimodal characterization of proliferative diabetic retinopathy reveals alterations in outer retinal function and structure. Ophthalmology. 2015;122:957–967. doi: 10.1016/j.ophtha.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia de la Torre N, Fernandez-Durango R, Gomez R, Fuentes M, Roldan-Pallares M, Donate J, Barabash A, Alonso B, Runkle I, Duran A, Rubio MA, Calle-Pascual AL. Expression of Angiogenic MicroRNAs in Endothelial Progenitor Cells From Type 1 Diabetic Patients With and Without Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2015;56:4090–4098. doi: 10.1167/iovs.15-16498. [DOI] [PubMed] [Google Scholar]
- 6.Savage SR, McCollum GW, Yang R, Penn JS. RNA-seq identifies a role for the PPARbeta/delta inverse agonist GSK0660 in the regulation of TNFalpha-induced cytokine signaling in retinal endothelial cells. Mol Vis. 2015;21:568–576. [PMC free article] [PubMed] [Google Scholar]
- 7.Loukovaara S, Gucciardo E, Repo P, Vihinen H, Lohi J, Jokitalo E, Salven P, Lehti K. Indications of lymphatic endothelial differentiation and endothelial progenitor cell activation in the pathology of proliferative diabetic retinopathy. Acta Ophthalmol. 2015;93:512–23. doi: 10.1111/aos.12741. [DOI] [PubMed] [Google Scholar]
- 8.Orang AV, Barzegari A. MicroRNAs in colorectal cancer: from diagnosis to targeted therapy. Asian Pac J Cancer Prev. 2014;15:6989–6999. doi: 10.7314/apjcp.2014.15.17.6989. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release. 2014;194:238–256. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz MA, Feng B, Chakrabarti S. Polycomb repressive complex 2 regulates MiR-200b in retinal endothelial cells: potential relevance in diabetic retinopathy. PLoS One. 2015;10:e0123987. doi: 10.1371/journal.pone.0123987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y, Feng B, Chen S, Chu Y, Chakrabarti S. Mechanisms of endothelial to mesenchymal transition in the retina in diabetes. Invest Ophthalmol Vis Sci. 2014;55:7321–7331. doi: 10.1167/iovs.14-15167. [DOI] [PubMed] [Google Scholar]
- 12.Murray AR, Chen Q, Takahashi Y, Zhou KK, Park K, Ma JX. MicroRNA-200b downregulates oxidation resistance 1 (Oxr1) expression in the retina of type 1 diabetes model. Invest Ophthalmol Vis Sci. 2013;54:1689–1697. doi: 10.1167/iovs.12-10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin ES, Sorenson CM, Sheibani N. Diabetes and retinal vascular dysfunction. J Ophthalmic Vis Res. 2014;9:362–373. doi: 10.4103/2008-322X.143378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajamani U, Jialal I. Hyperglycemia induces Toll-like receptor-2 and -4 expression and activity in human microvascular retinal endothelial cells: implications for diabetic retinopathy. J Diabetes Res. 2014;2014:790902. doi: 10.1155/2014/790902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Liu L, Wu J, Yue S, Geng J, Lian J, Teng W, Huang D, Chen L. Incidence Density and Risk Factors of Diabetic Retinopathy Within Type 2 Diabetes: A Five-Year Cohort Study in China (Report 1) Int J Environ Res Public Health. 2015;12:7899–7909. doi: 10.3390/ijerph120707899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castilho A, Madsen E, Ambrosio AF, Veruki ML, Hartveit E. Diabetic hyperglycemia reduces Ca2+ permeability of extrasynaptic AMPA receptors in AII amacrine cells. J Neurophysiol. 2015;114:1545–1553. doi: 10.1152/jn.00295.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baptista FI, Castilho AF, Gaspar JM, Liberal JT, Aveleira CA, Ambrosio AF. Long-term exposure to high glucose increases the content of several exocytotic proteins and of vesicular GABA transporter in cultured retinal neural cells. Neurosci Lett. 2015;602:56–61. doi: 10.1016/j.neulet.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Monaghan K, McNaughten J, McGahon MK, Kelly C, Kyle D, Yong PH, McGeown JG, Curtis TM. Hyperglycemia and Diabetes Downregulate the Functional Expression of TRPV4 Channels in Retinal Microvascular Endothelium. PLoS One. 2015;10:e0128359. doi: 10.1371/journal.pone.0128359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2015 doi: 10.1016/j.survophthal.2015.06.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Behl T, Kaur I, Goel H, Kotwani A. Significance of the antiangiogenic mechanisms of thalidomide in the therapy of diabetic retinopathy. Vascul Pharmacol. 2015 doi: 10.1016/j.vph.2015.07.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Zhang Q, Steinle JJ. Beta-adrenergic receptor agonist decreases VEGF levels through altered eNOS and PKC signaling in diabetic retina. Growth Factors. 2015;33:192–9. doi: 10.3109/08977194.2015.1054990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko H. Geraniin inhibits TGF-beta1-induced epithelial-mesenchymal transition and suppresses A549 lung cancer migration, invasion and anoikis resistance. Bioorg Med Chem Lett. 2015;25:3529–34. doi: 10.1016/j.bmcl.2015.06.093. [DOI] [PubMed] [Google Scholar]
- 23.Kamath VV, Krishnamurthy S, Satelur KP, Rajkumar K. Transforming growth factor-beta1 and TGF-beta2 act synergistically in the fibrotic pathway in oral submucous fibrosis: An immunohistochemical observation. Indian J Med Paediatr Oncol. 2015;36:111–116. doi: 10.4103/0971-5851.158842. [DOI] [PMC free article] [PubMed] [Google Scholar]


