Abstract
Objectives: Tankyrase 1 (TNKS1), a poly (ADP-ribose) polymerase, regulates telomere length and apoptosis in cells, overexpression of which occurred in non-small cell lung cancer (NSCLC). This study investigated TNKS1 single-nucleotide polymorphisms (SNPs) for association with a risk in NSCLC development in a Chinese population. Methods: NSCLC cases and healthy controls of 500 each were recruited for genotyping of 24 TNKS1 SNPs. The association between genotype and NSCLC risk was evaluated by computing the odds ratio (OR) and 95% confidence interval (CI) with multivariate unconditional logistic regression analyses. Haploview software was to analyze association between haplotypes and NSCLC risk. Results: TNKS1 rs6601328 A allele was associated with a lower risk in developing NSCLC and adenocarcinoma (ADC) (OR=0.71; 95% CI, 0.51-0.99 and OR=0.70; 95% CI, 0.50-0.99), whereas TNKS1 rs11991621 C allele (OR=1.44; 95% CI, 1.03-2.03), rs11991621 C/C (OR=1.44, 95% CI, 1.03-2.35; P=0.03), and rs10503380 G/G (OR= 1.56, 95% CI, 1.09-2.50, P=0.02) were associated with a higher risk in developing NSCLC or ADC in females and rs6601328 A/A major allele (OR=1.39; 95% CI, 1.00-1.92; P=0.047) and rs7015700 G/G (OR= 1.51, 95% CI, 1.04-2.21) was associated with an increased NSCLC or ADC risk in males but a reduced NSCLC risk (OR=0.63; 95% CI, 0.42-0.96) and ADC risk (OR=0.64; 95% CI, 0.42-0.97) in females. Haploview showed that there were three Haplotype Blocks associated with NSCLC risk. However, TNKS1 rs12541709 C/C was associated with protective effect against ADC (OR=0.75; 95% CI, 0.56-0.99; P=0.04) in this Chinese population. Conclusion: TNKS1 SNPs (rs11991621 rs10503380, and rs7015700) were associated with NSCLC risk, whereas rs6601328 and rs12541709 inversely associated with NSCLC or ADC risk in this Chinese population.
Keywords: Non-small cell lung cancer, genetic susceptibility, TNKS1, single nucleotide polymorphisms
Introduction
Lung cancer is a significant health problem and one of the leading causes of cancer death in the world [1]. Lung cancer is significantly increased in China [2], although lung cancer incidence has been declining in the US [3]. Histologically, non-small cell lung cancer (NSCLC) is the most common histological type of lung cancer, accounting for approximately 85% of all lung cancer, most of which are diagnosed at the advanced stages of disease and lead to poor five-year survival (16% of the overall survival rate [4]). Tobacco smoke is the single most significant risk factor in developing lung cancer [5,6], although certain population, like Chinese females with a lower prevalence of tobacco smoke (less than 4% adult smokers [7]) is more frequently developing lung cancer than that of certain European counterparts with an 20% adult tobacco smoke rate [8]. Lung cancer risk also occurs in different ethnic groups [9,10]. These data indicate that gene-environment interaction played an important role in lung carcinogenesis. Thus, research focuses on individual genetic susceptibility could help us to better understand mechanism of lung cancer development.
Indeed, to date, a great number of studies have shown that genetic susceptibility is the most important factor in lung cancer development and contributes to individual lung cancer risk [11-13]. Our research has been focused on tankyrase 1 (TNKS1), which is a multifunctional poly (ADP-ribose) polymerase (PARP) that utilizes NAD+ as a substrate to transfer ADP-ribose polymers onto protein acceptors, including itself [14,15]. Previous studies demonstrated that tankyrase 1 was able to regulate telomere length [16] and apoptosis [17] in cells, overexpression of which occurred in NSCLC tissue specimens [18]. Knockdown of TNKS1 expression using shRNA was shown to reduce lung cancer cell growth and repressed formation and growth of lung cancer cell xenografts in a murine lung cancer model [19]. tankyrase 1 as well as tankyrase 2 is also key mediators of the Wnt-signaling [20,21] and the latter signaling pathway has been used as a potential therapeutic target for lung cancer [22-25]. Thus, detection of TNKS1 genetic polymorphisms could help us to evaluate and identify whether TNKS1 is a lung cancer susceptibility. Therefore, in this study, we recruited 500 each of NSCLC patients and healthy controls to genotype 24 TNKS1 SNPs for association with a risk in developing NSCLC in a Chinese population.
Patients and methods
Study population
This study obtained 500 each of NSCLC patients and healthy controls, as described in our previous study [10]. Specifically, 500 each of NSCLC patients and unrelated age-matched healthy controls were recruited from Zhejiang Cancer Hospital between March 2011 and April 2012. All patients were histologically diagnosed as NSCLC and any patients with history of primary cancer, other than lung cancer, were excluded from this study. The controls were independent of any lung-related diseases to avoid any probable interference from overlapping genes. All control subjects were matched to these patients by sex and age. Tobacco smoking was recorded and defined as one who had smoked more than ten packs of tobacco in life time and a current or former smoker was defined as a smoker who was still smoking in the current year or in a year before participation of this study according to a previous study [26]. This study was approved by the ethical committee of Zhejiang Cancer Hospital. All subjects provided n informed consent before participation of this study.
Selection and genotyping of TNKS1 SNPs
TNKS1 SNPs were selected by using the SNP Browser version 3.5 (http://www.allsnps. com/snp browser) based on previously published criteria [27], i.e., pairwise r2 (≥ 0.8) among all common SNPs with minor allele frequency (MAF) ≥ 0.15 using the Tagger program implemented in Haploview version 4.1 (http://www.broad.mit.edu/mpg/ haploview). After that, we utilized the Chinese HapMap database to select TNKS1 SNPs for genotyping in this study, i.e., population = CHB (Han-Chinese, Beijing), resulting in 24 TNKS1 Tag SNPs.
To genotype these 24 SNPs, we extracted genomic DNA from whole blood of these participants using the AxyPrep Blood Genomic DNA Miniprep kit (Axygen Biosciences, Union City, CA) and then genotyped these 24 SNPs using the SEQUENOM MassARRAY matrix-assisted laser desorption ionization-time of flight mass spectrometry platform (Sequenom, San Diego, CA). PCR primers and single base extension primers were designed using the Assay Designer’s software version 3.0 (Sequenom) and synthesized by Sangon Biotech (Shanghai, China). A total of 10% samples were randomly selected and repeated PCR amplification; PCR data showed 100% of repeatability.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL). Hardy-Weinberg equilibrium (HWE) was performed to analyze TNKS1 allele and genotype frequencies between cases and control using χ2 test and a P < 0.001 value was selected for a HWE discrepancy. We also performed the χ2 test to assess frequencies of the selected allele and genotype for association with lung cancer risk. The association between SNPs and NSCLC risk and link between tobacco smoke and lung cancer risk were evaluated by computing the odds ratio (OR) and 95% confidence interval (CI) from multivariate unconditional logistic regression analysis. Haploview software version 4.1 was to analyze association between haplotypes and NSCLC. A two-sided p value less than 0.05 was considered statistically significant.
Results
Clinical characteristics of our study population
The clinical characteristics of patients and controls are show in Table 1. Specifically, the mean age of patients was 56.6 years (ranged between 33 and 80 years) and 350 males and 150 females with 115 stage I/II and 385 stage III/IV diseases. There were 381 adenocarcinoma and 169 squamous cell carcinoma and 301 tobacco smokers and 199 non-smokers. In contrast, the mean age of the healthy controls was 50.7 years (ranged between 29 and 73 years) and 259 males and 240 females with 203 tobacco smokers and 296 non-smokers.
Table 1.
Clinical characteristics of cases and controls
| Population | Gender, n | Age (years) | TNM stage, n | ADC, n | SCC, n | Smoking status, n | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| Smoking | Non-smoking | |||||||||||||||
|
|
||||||||||||||||
| Male | Female | Average | Range | I | II | III | IV | Male | Female | Male | Female | Male | Female | Male | Female | |
| NSCLC patients | 350 | 150 | 56.66 | 33-80 | 42 | 73 | 138 | 247 | 189 | 142 | 161 | 8 | 280 | 21 | 70 | 129 |
| Controls | 259 | 240 | 50.75 | 29-73 | - | - | - | - | - | - | - | - | 189 | 14 | 70 | 226 |
TNKS1 polymorphisms between NSCLCs and healthy controls
In this study, we selected and genotyped 24 TNKS1 SNPs (rs10109026, rs1055328, rs12541709, rs4840426, rs7459728, rs4075359, rs10111376, rs11991621, rs7006406, rs13249683, rs17150478, rs10095667, rs2048656, rs11774818, rs4626603, rs12681705, rs7015700, rs7018399, rs11984585, rs12542420, rs2048655, rs6601328, rs17734024, and rs10503380) in both cases and controls. We found that all of these 24 SNPs were within the Hardy-Weinberg equilibrium between cases and controls. However, frequency of rs6601328 A allele was 79.0% and G allele was 21.0% in female controls, whereas they were 72.7% and 27.3%, and 72.5%% and 27.5% in Female NSCLCs and ADCs, respectively (P=0.04). Logistic regression analysis revealed that rs6601328 A allele associated with a reduced risk in developing NSCLC and ADC (OR=0.71; 95% CI, 0.51-0.99 and OR=0.70; 95% CI, 0.50-0.99, respectively) in females. Similar, frequency of rs11991621 T and C allele was 29.2% and 70.8%, and 22.2% and 77.8% in female controls and female ADC, respectively (P=0.03). Logistic regression analysis revealed that rs11991621 C allele associated with a greater risk in developing ADC (OR=1.44; 95% CI, 1.03-2.03) in females (Table 2).
Table 2.
TNKS1 allele frequency between female NSCLC patients and healthy controls
| Gene allele | Female controls | Female NSCLC patients | P value | OR (95% CI) | Female ADC | P value | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| rs6601328 | |||||||
| A | 379 (79.0) | 218 (72.7) | 206 (72.5) | ||||
| G | 101 (21.0) | 82 (27.3) | 0.04 | 0.71 (0.51-0.99) | 78 (27.5) | 0.04 | 0.70 (0.50-0.99) |
| rs11991621 | |||||||
| C | 340 (70.8) | 231 (77.0) | 221 (77.8) | ||||
| T | 140 (29.2) | 69 (23.0) | 0.06 | 1.37 (0.99-1.92) | 63 (22.2) | 0.03 | 1.44 (1.03-2.03) |
Genotype analysis showed that rs6601328 A/A genotype was associated with an increased NSCLC risk in males (59.4% vs. 51.4% between male NSCLC and controls, OR=1.39; 95% CI, 1.00-1.92; P=0.047). In contrast, A/A genotype was associated with a reduced NSCLC risk in females (51.3% vs. 62.5% between female NSCLC and controls, OR=0.63; 95% CI, 0.42-0.96; P=0.03; Table 3). Furthermore, rs11991621 C/C and rs10503380 G/G genotypes had a 1.56- and 1.65-fold increased risk in developing female NSCLC (95% CI, 1.03-2.35; P=0.03 and 95% CI, 1.09-2.50, P=0.02, respectively; Table 3). However, rs12541709 C/C genotype did have a protective role to reduce ADC risk in both male and female (OR, 0.75; 95% CI, 0.56-0.99; P=0.04; Table 4). In addition, rs7015700 G/G genotype had 1.51-fold risk in developing male ADC (95% CI, 1.04-2.21; Table 4). Similarly, rs11991621 C/C and rs10503380 G/G genotypes had 1.64-fold (95% CI, 1.08-2.50) and 1.69-fold (95% CI, 1.11-2.58) risk in developing female ADC, respectively (Table 4). In contrast, rs6601328 A/A genotype had 0.64-fold reduction of risk in developing female ADC (95%, 0.42-0.97; Table 4).
Table 3.
TNKS1 SNP genotypes between NSCLC patients and controls
| Genotypes | NSCLC Controls | p value | OR (95% CI) | M NSCLC M Controls | p value | OR (95% CI) | F NSCLC F Controls | p value | OR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| N=500 n (%) | N=500 n (%) | N=350, n (%) | N=259, n (%) | N=150 n (%) | N=240 n (%) | |||||||
| rs6601328 | ||||||||||||
| AA | 285 (57.0) | 284 (56.8) | 208 (59.4) | 133 (51.4) | 77 (51.3) | 150 (62.5) | ||||||
| AG | 187 (37.4) | 192 (38.4) | 123 (35.1) | 113 (43.6) | 64 (42.7) | 79 (32.9) | ||||||
| GG | 28 (5.6) | 24 (4.8) | 0.83 | 19 (5.4) | 13 (5.0) | 0.10 | 9 (6.0) | 11 (4.6) | 0.09 | |||
| AG+GG | 215 (43.0) | 216 (43.2) | 0.95 | 1.01 (0.79-1.30) | 142 (40.6) | 126 (48.6) | 0.047 | 1.39 (1.00-1.92) | 73 (48.7) | 90 (37.5) | 0.03 | 0.63 (0.42-0.96) |
| rs11991621 | ||||||||||||
| CC | 267 (53.4) | 265 (53.0) | 178 (50.9) | 14 8(57.1) | 89 (59.3) | 16 (48.3) | ||||||
| TT | 35 (7.0) | 28 (5.6) | 27 (7.7) | 12 (4.6) | 8 (5.3) | 16 (6.7) | ||||||
| CT | 198 (39.6) | 207 (41.4) | 0.61 | 145 (41.4) | 99 (38.2) | 0.16 | 53 (35.3) | 108 (45.0) | 0.11 | |||
| TT+CT | 233 (46.6) | 235 (47.0) | 0.90 | 1.02 (0.79-1.30) | 172 (49.1) | 111 (42.9) | 0.12 | 0.78 (0.56-1.07) | 61 (40.7) | 124 (51.7) | 0.03 | 1.56 (1.03-2.35) |
| rs10503380 | ||||||||||||
| GG | 216 (43.2) | 197 (39.4) | 144 (41.1) | 110 (42.5) | 72 (48.0) | 86 (35.8) | ||||||
| AG | 213 (42.6) | 244 (48.8) | 155 (44.3) | 116 (44.8) | 58 (38.7) | 128 (53.3) | ||||||
| AA | 71 (14.2) | 59 (11.8) | 0.13 | 51 (14.6) | 33 (12.7) | 0.81 | 20 (13.3) | 26 (10.8) | 0.02 | |||
| AG+GG | 284 (56.8) | 303 (60.6) | 0.22 | 1.17 (0.91-1.51) | 206 (58.9) | 149 (57.5) | 0.74 | 0.95 (0.68-1.31) | 78 (52.0) | 154 (64.2) | 0.02 | 1.65 (1.09-2.50) |
M, male; F, female.
Table 4.
TNKS1 SNP genotypes between ADC patients and controls
| Genotypes | ADC Controls | p value | OR (95% CI) | M ADC M Controls | p value | OR (95% CI) | F ADC F Controls | p value | OR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| N=331 n (%) | N=500 n (%) | N=189 n (%) | N=259 n (%) | N=142 n (%) | N=240 n (%) | |||||||
| rs12541709 | ||||||||||||
| CC | 128 (38.7) | 229 (45.8) | 76 (40.2) | 120 (46.3) | 52 (36.6) | 108 (45.0) | ||||||
| GG | 38 (11.5) | 55 (11.0) | 23 (12.2) | 35 (13.5) | 15 (10.6) | 20 (8.3) | ||||||
| CG | 165 (49.8) | 216 (43.2) | 0.12 | 90 (47.6) | 104 (40.2) | 0.27 | 75 (52.8) | 112 (46.7) | 0.26 | |||
| GG+CG | 203 (61.3) | 271 (54.2) | 0.04 | 0.75 (0.56-0.99) | 113 (59.8) | 139 (53.7) | 0.20 | 0.78 (0.53-1.14) | 90 (63.4) | 132 (55.0) | 0.11 | 0.76 (0.46-1.08) |
| rs7015700 | ||||||||||||
| GG | 167 (50.5) | 236 (47.2) | 99 (52.4) | 109 (42.1) | 68 (47.9) | 126 (52.5) | ||||||
| AA | 26 (7.9) | 42 (8.4) | 12 (6.3) | 20 (7.7) | 14 (9.9) | 22 (9.2) | ||||||
| AG | 138 (41.7) | 222 (44.4) | 0.66 | 78 (41.3) | 130 (50.2) | 0.09 | 60 (42.3) | 92 (38.3) | 0.68 | |||
| AA+AG | 164 (49.5) | 264 (52.8) | 0.36 | 1.14 (0.86-1.50) | 90 (47.6) | 150 (57.9) | 0.03 | 1.51 (1.04-2.21) | 74 (52.1) | 114 (47.5) | 0.38 | 0.83 (0.55-1.26) |
| rs11991621 | ||||||||||||
| CC | 180 (54.4) | 265 (53.0) | 94 (49.7) | 148 (57.1) | 86 (60.6) | 116 (48.3) | ||||||
| TT | 22 (6.6) | 28 (5.6) | 15 (7.9) | 12 (4.6) | 7 (4.9) | 16 (6.7) | ||||||
| CT | 129 (39.0) | 207 (41.4) | 0.70 | 80 (42.3) | 99 (38.2) | 0.17 | 49 (34.5) | 108 (45.0) | 0.07 | |||
| TT+CT | 151 (45.6) | 235 (47.0) | 0.70 | 1.06 (0.80-1.40) | 95 (50.3) | 111 (42.9) | 0.12 | 0.74 (0.51-1.08) | 56 (39.4) | 124 (51.7) | 0.02 | 1.64 (1.08-2.50) |
| rs6601328 | ||||||||||||
| AA | 187 (56.5) | 284 (56.8) | 114 (60.3) | 133 (51.4) | 73 (51.4) | 150 (62.5) | ||||||
| GG | 17 (5.1) | 24 (4.8) | 8 (4.2) | 13 (5.0) | 9 (6.3) | 11 (4.6) | ||||||
| AG | 127 (38.4) | 192 (38.4) | 0.98 | 67 (35.4) | 113 (43.6) | 0.17 | 60 (42.3) | 79 (32.9) | 0.10 | |||
| GG+AG | 144 (43.5) | 216 (43.2) | 0.93 | 0.99 (0.75-1.31) | 75 (39.7) | 126 (48.6) | 0.06 | 1.44 (0.99-2.11) | 69 (48.6) | 90 (37.5) | 0.03 | 0.64 (0.42-0.97) |
| rs10503380 | ||||||||||||
| GG | 145 (43.8) | 197 (39.4) | 76 (40.2) | 110 (42.5) | 69 (48.6) | 86 (35.8) | ||||||
| AA | 43 (13.0) | 59 (11.8) | 24 (12.7) | 33 (12.7) | 19 (13.4) | 26 (10.8) | ||||||
| AG | 143 (43.2) | 244 (48.8) | 0.29 | 89 (47.1) | 116 (44.8) | 0.88 | 54 (38.0) | 128 (53.3) | 0.01 | |||
| AA+AG | 186 (56.2) | 303 (60.6) | 0.21 | 1.20 (0.91-1.59) | 113 (59.8) | 149 (57.5) | 0.63 | 0.91 (0.62-1.33) | 73 (51.4) | 154 (64.2) | 0.01 | 1.69 (1.11-2.58) |
Synergy of TNKS1 polymorphisms in association with NSCLC risk
We next utilized Haploview software to identify the Haplotype Blocks and then performed logistic regression analyses to associate haplotype blocks with NSCLC risk. We found that rs11991621 and rs4840426 were identified a Block 1 (the length was 2kb) in males and that their CT haplotype was associated with a decreased risk in developing NSCLC (OR, 0.78; 95%, 0.62-0.99; P=0.04), more obviously in reduction of ADC risk (Block 1 in Table 5 and Figure 1). Similarly, CTG haplotype was associated with increased risk in developing female NSCLC (OR, 1.47; 95%, 1.06-2.06; P=0.02), especially in developing female ADC (OR, 1.49; 95%, 1.06-2.09; P=0.02). Furthermore, TTA haplotype was associated with decreased risk in developing ADC (OR, 0.70; 95%, 0.49-0.99; P=0.04) in Block 2 (the length was 3kb, including rs11991621, rs4840426, and rs10503380; Table 5 and Figure 2). GAAGG haplotype was associated with increased risk of developing male ADC (OR, 1.48; 95%, 1.06-2.07; P=0.02) and SCC (OR, 1.52; 95%, 1.05-2.21; P=0.03), but more obviously in developing female SCC (OR, 3.08; 95%, 0.95-9.93; P=0.048) in Block 3 (the length was 91 kb, including rs12542420, rs11774818, rs4075359, rs7015700, and rs7006406; Table 5 and Figures 1, 3 and 4).
Table 5.
Haplotype distribution and formation between patients and controls for association of haplotype blocks with NSCLC risk
| Block | loci | Cases/controls | Haplotype | Frequency (%) | OR (95% CI)# | P value# | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Case | Control | ||||||
| Block 1 | rs11991621 and rs4840426 | Male NSCLC patients Male controls | CT | 31.9 | 37.5 | 0.78 (0.62-0.99) | 0.04 |
| CG | 39.7 | 38.8 | 1.04 (0.82-1.31) | 0.75 | |||
| TT | 28.4 | 23.5 | 1.29 (0.99-1.67) | 0.06 | |||
| Male ADC patients Male controls | CT | 31.0 | 37.5 | 0.75 (0.57-0.99) | 0.04 | ||
| CG | 39.9 | 38.7 | 1.05 (0.80-1.38) | 0.73 | |||
| TT | 29.0 | 23.5 | 1.33 (0.99-1.80) | 0.06 | |||
| Block 2 | rs11991621, rs4840426 and rs10503380 | Female NSCLC patients Female controls | CTG | 28.0 | 20.9 | 1.47 (1.06-2.06) | 0.02 |
| TTA | 22.2 | 28.0 | 0.73 (0.52-1.03) | 0.07 | |||
| CGG | 38.5 | 40.5 | 0.92 (0.69-1.24) | 0.59 | |||
| CTA | 9.6 | 9.2 | 1.06 (0.65-1.73) | 0.83 | |||
| Female ADC patients Female controls | TTA | 21.4 | 28.0 | 0.70 (0.49-0.99) | 0.04 | ||
| CTG | 28.2 | 20.9 | 1.49 (1.06-2.09) | 0.02 | |||
| CGG | 38.6 | 40.5 | 0.92 (0.68-1.25) | 0.61 | |||
| CTA | 10.2 | 9.2 | 1.12 (0.69-1.84) | 0.65 | |||
| Block 3 | rs12542420, rs11774818, rs4075359, rs7015700 and rs7006406 | Male ADC patients Male controls | GAAGG | 22.7 | 16.6 | 1.48 (1.06-2.07) | 0.02 |
| AAGGA | 38.3 | 37.3 | 1.04 (0.79-1.37) | 0.76 | |||
| AGAAA | 18.9 | 21.4 | 0.86 (0.62-1.20) | 0.37 | |||
| GAAGA | 10.1 | 9.5 | 1.07 (0.68-1.67) | 0.77 | |||
| AAAAA | 6.7 | 9.2 | 0.71 (0.43-1.17) | 0.18 | |||
| GAAAA | 0.8 | 1.4 | 0.58 (0.15-2.27) | 0.44 | |||
| AGAGA | 0.9 | 1.2 | 0.70 (0.19-2.68) | 0.61 | |||
| SCC patients Controls | GAAGA | 13.9 | 9.6 | 1.52 (1.05-2.21) | 0.03 | ||
| AAGGA | 36.5 | 37.7 | 0.95 (0.74-1.23) | 0.71 | |||
| AGAAA | 21.2 | 20.5 | 1.04 (0.77-1.41) | 0.78 | |||
| GAAGG | 17.1 | 18.3 | 0.92 (0.66-1.27) | 0.61 | |||
| AAAAA | 5.8 | 8.5 | 0.67 (0.40-1.11) | 0.12 | |||
| AAAGA | 1.1 | 1.5 | 0.73 (0.23-2.31) | 0.59 | |||
| AGAGA | 1.9 | 0.9 | 2.13 (0.77-5.86) | 0.15 | |||
| Female SCC patients Female controls | GAAGA | 25.0 | 9.8 | 3.08 (0.95-9.93) | 0.048 | ||
| AAGGA | 37.5 | 37.8 | 0.99 (0.35-2.76) | 0.98 | |||
| AGAAA | 18.7 | 19.9 | 0.93 (25.9-3.32) | 0.91 | |||
| GAAGG | 12.5 | 20.1 | 0.57 (0.13-2.54) | 0.46 | |||
| AAAAA | 0.0 | 7.6 | - | 0.25 | |||
| AAAGA | 0.0 | 1.9 | - | 0.57 | |||
Compared with other haplotypes.
Figure 1.
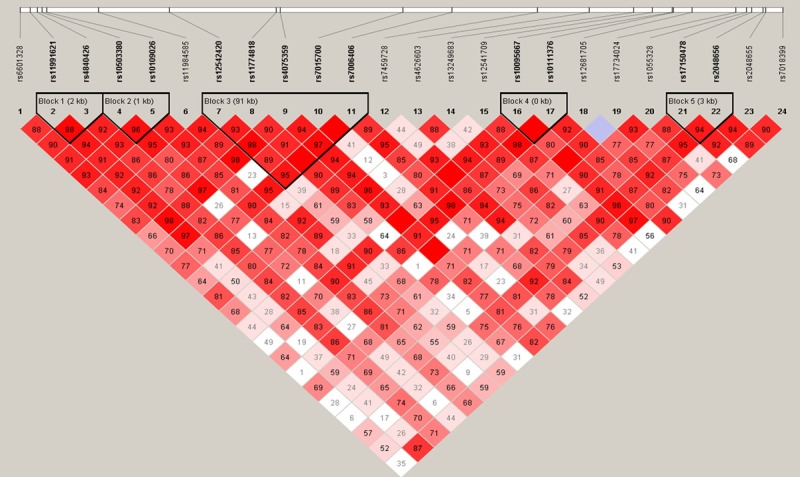
Structure of haplotype block 1, including rs11991621 and rs4840426 among male NSCLC, ADC, and healthy controls. Haplotype structure of Block 3, including rs12542420, rs11774818, rs4075359, rs7015700, and rs7006406 between male ADC and healthy controls. The darker color indicates a higher level of the linkage disequilibrium (LD), while the lighter color indicates less LD.
Figure 2.
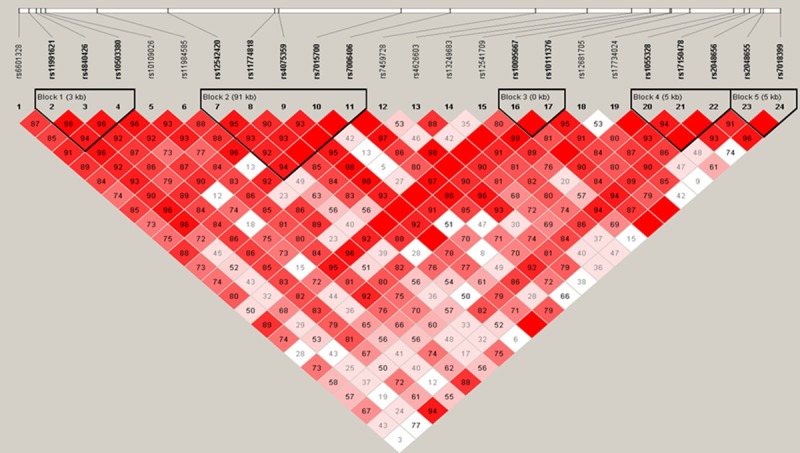
Haplotype structure of rs11991621, rs4840426, and rs10503380 among female NSCLC, ADC, and healthy controls. The darker color indicates a higher level of linkage disequilibrium (LD), while the lighter color indicates less LD.
Figure 3.
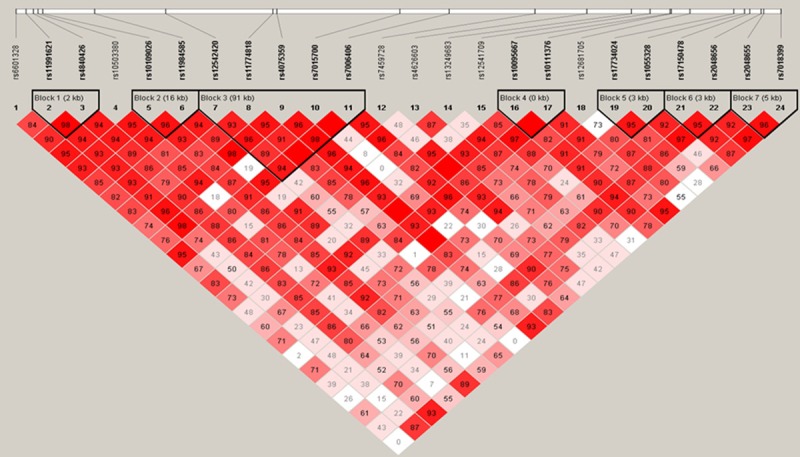
Haplotype structure of all markers among SCC patients and controls. The darker color indicates a higher level of linkage disequilibrium (LD), while lighter color indicates less LD.
Figure 4.
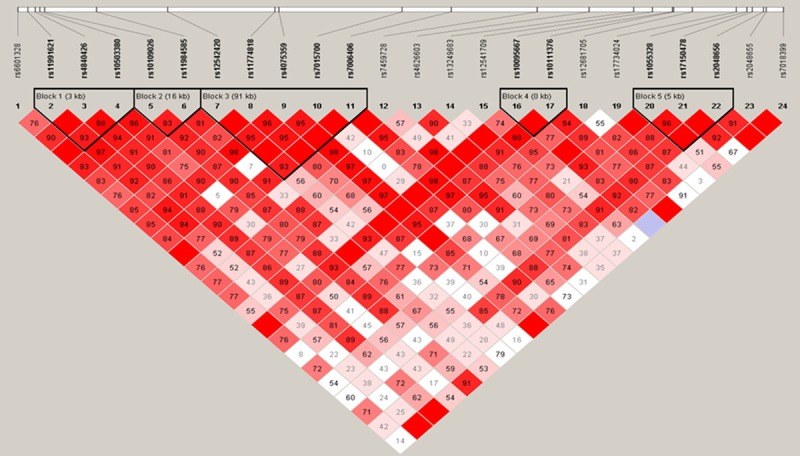
Haplotype structure of all markers between female SCC and healthy controls. The darker color indicates a higher level of linkage disequilibrium (LD), while lighter color indicates less LD.
Discussion
The gene-environment interaction plays a role in pathogenesis of human diseases and genetic susceptibility is the most important factor in lung cancer development and contributes to individual lung cancer risk [28]. In the current study, we selected and analyzed 24 TNKS1 SNPs between NSCLC and healthy control for association with NSCLC risk in a Chinese population. We found that TNKS1 rs6601328 reduced the risk in developing NSCLC and adenocarcinomas (ADC) in females but increased NSCLC risk in males. Moreover, TNKS1 rs11991621 and rs10503380 were associated with a increased risk in developing NSCLC and ADC in females, while TNKS1 rs7015700 had an increased risk in developing ADC in males and rs12541709 C/C has a protective effect to reduce ADC risk in both male and female. These data indicate that different TNKS1 SNPs could affect TNKS1 transcription, protein expression, or enzymatic activity, and therefore, differently modulate NSCLC risk. Furthermore, our current data also showed that TNKS1 SNPs associated differently with male and female risk in developing NSCLC, suggesting that different environmental risk factors interact TNKS1 for genetic susceptibility in male and female lung cancer development.
Indeed, previous studies showed a different gene-environment interaction of histological type of NSCLC and gender [29,30]. Molecular studies can better characterize genetic effects on disease risk and increase our understanding of the apparent heterogeneity of effects across sex and histological type [30]. In our current study, we found that TNKS1 rs6601328 SNPs had a protective role in females but was a risk factor for male in developing NSCLC. To the best our knowledge, up to date, there was no such a report in the literature; however, the underlying molecular mechanism remains unclear. There maybe have two reasons, i.e., firstly, the sample size is moderate and this association of NSCLC risk in males was just passed statistical threshold; thus, further confirmation studies are needed to verify our current data, and secondly, this is the first genetic polymorphism study of such phenomenon and future study will disclose TNKS1 SNPs for TNKS1 transcription and Tankyrase 1 expression and enzymatic activity for the gene-environment interaction, such as tobacco smoke.
At the chromosome level, TNKS1 is localized at the centrosome of chromosome 8, function of which is to prevent over-elongation of centrioles and ensure a proper centrosome function by promoting degradation of centrosomal P4.1-associated protein (CPAP) in early G1 phase of cell cycle [31]. Thus, silence of Tankyrase 1 expression was able to promote human cells to apoptosis and inhibit cell proliferation [32]. In lung cancer, Tankyrase 1 was highly expressed [18], indicating that Tankyrase 1 enzyme could associate with lung cancer development or progression. Indeed, previous studies demonstrated that function of Tankyrase 1 protein is to maintain cell telomere length and cell division capacity through Tankyrase 1-mediated PARsylation because Tankyrase 1 protein contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, tankyrase-binding protein (TAB182), which will promote TRF1 release from the telomeres, allowing telomerase access to telomeres and enhancing telomere elongation [33]. Indeed, a previous study reported by Riffell JL et al showed that Tankyrase-1 inhibitors were able to increase a risk of critically shortened telomere and promote the crisis and mortality of lung cancer cells [34]. Tankyrase 1 or tankyrase 2 is a key mediator in regulation of the Wnt signaling [20,21]. Tankyrase-1-mediated PARsylation of Axin 1 and 2 (regulators of the Wnt-signaling pathway) resulted in their degradation through the ubiquitin-proteasome pathway [20]. Moreover, the Wnt pathway proteins were activated or overexpressed in the resected NSCLC tissue samples, while aberrant methylation of various Wnt pathway components or downregulation of Wnt pathway inhibitors were associated with poor prognosis of lung cancer [35]. Thus, these data indicate that TNKS1 SNPs may affect TNKS1 transcription and tankyrase 1 expression and enzymatic activity and in turn lose the ability to block the Wnt-signaling during lung carcinogenesis, depending further investigation for confirmation.
However, our current study does have some limitations; for example, there were many more cases of male smokers than nonsmokers, which led to a difficulty to stratify and analyze the data according to smoking status. Overall, it is just a proof-of-principle study and our data showed interesting findings: TNKS1 SNPs associated with risk in developing NSCLC and TNKS1 SNPs could affect TNKS1 transcription, protein expression, or enzymatic activity, and therefore, differently modulate NSCLC risk. Furthermore, our current data also showed that but further studies will need to confirm our current data and applicability to other populations. For sure, future investigation of tankyrase 1 and its role in lung cancer development and progression could help us to better understand TNKS1 SNPs in regulation of TNKS1 transcription and tankyrase 1 expression and enzymatic activity and therefore, disclose TNKS1 interaction with environmental risk factors (tobacco smoke or gender difference) in modulation of NSCLC risk.
Acknowledgements
This study was supported in part by grants from Zhejiang Provincial Science Department Public Technology Application Research Fund (#2013C33184) and Zhejiang Provincial Natural Science fund (#Y14H270049).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone A, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) based on November 2011SEER data submission. Bethesda, MD: National Cancer Institute; 2012. Available at: http://seer.cancer,gov/csr/1975_2009_pops09. [Google Scholar]
- 5.Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl 5):e1S–29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian J, Govindan R. lung cancer in never smokers: a review. J. Clin. Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 7.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 8.Mackay J, Eriksen M, Shafey O. The tobacco atlas. 2nd edn. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 9.Zhang Y, Hua S, Zhang A, Kong X, Jiang C, Deng D, Wenlong B. Association between polymorphisms in COMT, PLCH1, and CYP17A1, and non-small-cell lung cancer risk in Chinese nonsmokers. Clin Lung Cancer. 2013;14:45–49. doi: 10.1016/j.cllc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Zhang Y, Gu C, Bao W, Bao Y. Neuronal acetylcholine receptor subunit alpha-9 (CHRNA9) polymorphisms are associated with NSCLC risk in a Chinese population. Med Oncol. 2014;31:932. doi: 10.1007/s12032-014-0932-5. [DOI] [PubMed] [Google Scholar]
- 11.Wang HM, Zhang XY, Jin B. TERT genetic polymorphism rs2736100 was associated with lung cancer: a meta-analysis based on 14,492 subjects. Genet Test Mol Biomarkers. 2013;17:937–941. doi: 10.1089/gtmb.2013.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Zhang YJ, Kong XM. No association of XRCC1 and CLPTM1L polymorphisms with non-small cell lung cancer in a non-smoking Han Chinese population. Asian Pac J Cancer Prev. 2013;14:5171–5174. doi: 10.7314/apjcp.2013.14.9.5171. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Bao S, Xu X, Bao Y, Zhang Y. Polymorphisms of CHRNA5-CHRNA3-CHRNB4 Gene Cluster and NSCLC Risk in Chinese Population. Transl Oncol. 2012;5:448–452. doi: 10.1593/tlo.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao SJ, Smith S. Tankyrase function at telomeres, spindle poles, and beyond. Biochimie. 2008;90:83–92. doi: 10.1016/j.biochi.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly (ADP- ribose) polymerase at human telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 16.Rippmann JF, Damm K, Schnapp A. Functional characterization of the poly (ADP-ribose) polymerase activity of tankyrase 1, a potential regulator of telomere length. J Mol Biol. 2002;323:217–224. doi: 10.1016/s0022-2836(02)00946-4. [DOI] [PubMed] [Google Scholar]
- 17.Bae J, Donigian JR, Hsueh Aaron JW. Tankyrase 1 interacts with Mcl-1 proteins and inhibits their regulation of apoptosis. J Biol Chem. 2003;278:5195–5204. doi: 10.1074/jbc.M201988200. [DOI] [PubMed] [Google Scholar]
- 18.Cha N, Li XY, Zhao YJ, Wang EH, Wu GP. hTERT gene amplification and clinical significance in pleural effusions of patients with lung cancer. Clin Lung Cancer. 2012;13:494–499. doi: 10.1016/j.cllc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Busch AM, Johnson KC, Stan RV, Sanglikar A, Ahmed Y, Dmitrovsky E, Freemantle SJ. Evidence for tankyrases as antineoplastic targets in lung cancer. BMC Cancer. 2013;13:211. doi: 10.1186/1471-2407-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snow GE, Kasper AC, Busch AM, Schwarz E, Ewings KE, Bee T, Spinella MJ, Dmitrovsky E, Freemantle SJ. Wnt pathway reprogramming during human embryonal carcinoma differentiation and potential for therapeutic targeting. BMC Cancer. 2009;9:383. doi: 10.1186/1471-2407-9-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F, Jablons DM. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23:6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- 24.Pacheco-Pinedo EC, Durham AC, Stewart KM, Goss AM, Lu MM, Demayo FJ, Morrisey EE. Wnt/β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J Clin Invest. 2011;121:1935–1945. doi: 10.1172/JCI44871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massagué J. WNT/TCF Signaling through LEF1 and HOXB9 Mediates Lung Adenocarcinoma Metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pesch B, Kendzia B, Gustavsson P, Jöckel KH, Johnen G, Pohlabeln H, Olsson A, Ahrens W, Gross IM, Brüske I, Wichmann HE, Merletti F, Richiardi L, Simonato L, Fortes C, Siemiatycki J, Parent ME, Consonni D, Landi MT, Caporaso N, Zaridze D, Cassidy A, Szeszenia-Dabrowska N, Rudnai P, Lissowska J, Stücker I, Fabianova E, Dumitru RS, Bencko V, Foretova L, Janout V, Rudin CM, Brennan P, Boffetta P, Straif K, Brüning T. Cigarette smoking and lung cancer relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 2012;131:1210–1219. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng D, Zhang Y, Bao W, Kong X. Low-density lipoprotein receptor-related protein 6 (LRP6) rs10845498 polymorphism is associated with a decreased risk of non-small cell lung cancer. Int J Med Sci. 2014;11:685–690. doi: 10.7150/ijms.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Gu C, Shi H, Zhang A, Kong X, Bao W, Deng D, Ren L, Gu D. Association between C3orf21, TP63 polymorphisms and environment and NSCLC in never-smoking Chinese population. Gene. 2012;497:93–97. doi: 10.1016/j.gene.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 29.Park SL, Fesinmeyer MD, Timofeeva M, Caberto CP, Kocarnik JM, Han Y, Love SA, Young A, Dumitrescu L, Lin Y, Goodloe R, Wilkens LR, Hindorff L, Fowke JH, Carty C, Buyske S, Schumacher FR, Butler A, Dilks H, Deelman E, Cote ML, Chen W, Pande M, Christiani DC, Field JK, Bickebller H, Risch A, Heinrich J, Brennan P, Wang Y, Eisen T, Houlston RS, Thun M, Albanes D, Caporaso N, Peters U, North KE, Heiss G, Crawford DC, Bush WS, Haiman CA, Landi MT, Hung RJ, Kooperberg C, Amos CI, Le Marchand L, Cheng I. Pleiotropic associations of risk variants identified for other cancers with lung cancer risk: the PAGE and TRICL consortia. J Natl Cancer Inst. 2014;106:dju061. doi: 10.1093/jnci/dju061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soeiro-De-Souza MG, Bio DS, David DP, Missio G, Lima B, Fernandes F, Machado-Vieira R, Moreno RA. Gender effects of the COMT Val 158 Met genotype on verbal fluency in healthy adults. Mol Med Rep. 2013;8:837–844. doi: 10.3892/mmr.2013.1564. [DOI] [PubMed] [Google Scholar]
- 31.Kim MK, Dudognon C, Smith S. Tankyrase 1 regulates centrosome function by controlling CPAP stability. EMBO Rep. 2012;13:724–732. doi: 10.1038/embor.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu H, Lei Z, Lu Z, Lu Q, Lu C, Chen W, Wang C, Tang Q, Kong Q. Silencing tankyrase and telomerase promotes A549 human lung adenocarcinoma cell apoptosis and inhibits proliferation. Oncol Rep. 2013;30:1745–1752. doi: 10.3892/or.2013.2665. [DOI] [PubMed] [Google Scholar]
- 33.Chang W, Dynek JN, Smith S. TRF1 is degraded by ubiquitin- mediated proteolysis after release from telomeres. Genes. 2003;17:1328–33. doi: 10.1101/gad.1077103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riffell JL, Lord CJ, Ashworth A. Tankyrasetargeted therapeutics: expanding opportunities in the PARP family. Nat Rev Drug Discov. 2012;11:923–936. doi: 10.1038/nrd3868. [DOI] [PubMed] [Google Scholar]
- 35.Stewart DJ. The Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:djt356. doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]


