Abstract
Objective: Perfluorooctanoic acid (PFOA) is widely used in consumer products and detected in human serum. Our study meant to elucidate the uncovered molecular mechanisms underlying the PFOA induced colorectal cancer cell DLD-1 invasion and matrix metalloproteinases (MMP) expression. Methods and results: Trans-well filter assay appeared that PFOA treatment stimulated DLD-1 cells invasion significantly. Meanwhile, the results of luciferase reporter, quantitative real-time PCR, western blotting, and gelatin zymography showed that PFOA induced MMP-2/-9 expression and enzyme activation levels consistently (P < 0.05 each). Subsequently, western blotting and immunofluorescence assay demonstrated that PFOA could enhance nuclear factor kappaB (NF-κB) activity by stimulating NF-κB translocation into nuclear in DLD-1 cells. Furthermore, JSH-23, a well-known NF-κB inhibitor, could reverse the PFOA induced colorectal cancer cell invasion and MMP-2/-9 expression. Conclusions: Our study confirmed that PFOA could induce colorectal cancer cell DLD-1 invasive ability and MMP-2/-9 expression through activating NF-κB, which deserves more concerns on environmental pollutant-resulted public health risk.
Keywords: Perfluorooctanoic acid, colorectal cancer, invasion
Introduction
Perfluorooctanoic acid (PFOA, or C8), is one of perfluorinated compounds (PFCs), which are man-made persistent organic pollutants. Since 1950s, it has been widely used in industrial and consumer products, including the manufacture of fluoropolymers, non-stick coating on cookware, stain-resistant coatings on fabric, and so on, for its stain-resistant and water-repellant characteristics [1]. It has attracted more concerns because of its widespread occurrence in the environmental matrices, wildlife, bio-accumulate in food chains, even in drinking water and human populations [2]. Over past decades, amounts of epidemiological studies have demonstrated that the dosage of PFOA dissolved in drinking water varied from pg/l to μg/l [3], meanwhile, environmental contamination of PFOA has been increased on an average of 7~11% per year [4]. Besides occupational exposure, drinking water is one of the main exposure pathways for human group. Contamination of human serum attracted more attention about side effects in the long term, the data showed that half-life in human is about 3.8 years for PFOA [5]. Recently, several cohort studies implied that contamination of PFOA in human serum attained on average of 28.2 ng/ml (approximately 68.1 nM) [6], and more seriously, PFOA has been linked to kinds of tumorigenesis, including pancreatic cancer, liver cancer, testicular cancer, and so on [7]. While, there is little information related to the contact between PFOA exposure and cancer metastasis, since cancer metastasis is the harmful section during carcinoma progression, which might cause the majority of patients’ treatment failure and poor prognosis.
Cancer metastasis composes by a series of steps, involving migration, invasion, cell attachment, angiogenesis, and cell proliferation [8]. Cancer cells need to digest surrounding tissue barriers, and then migrate through to escape from the primary site, finally form the distant organic colonization. Therefore, degradation of extracellular matrix (ECM) and basement membranes is the initial step of metastasis process [9]. This process requires amounts of cellular proteolytic enzymes, for example matrix metalloproteinases (MMPs), which are members of proteinases contributed to ECM degradation and rebuilding. MMPs family consists of over 20 zinc-binding endopeptidases, which can degrade most of ECM components [10,11]. MMPs are synthesized broadly in most cells and immediately secreted into the ECM [12]. They have been demonstrated to play important roles in various biological processes involving wound healing, cancer invasion, embryogenesis, arthritis, cardiovascular disease and inflammation [13]. Two members of MMPs family, MMP-2 (72 kDa gelatinase) and MMP-9 (92 kDa gelatinase), which can efficiently degrade native collagen types I/IV, fibronectin, entactin, and elastin, are well known to play critical roles in colorectal cancer progression, such as peripheral invasion and distant organic metastasis [14,15]. Meanwhile, MMP-2/-9 overexpression is closely related to poor prognosis in most cancer patients [16].
The MMP-2/-9 expression could be adjusted by nuclear factor kappaB (NF-κB) [17]. As a multi-functional transcription factor, NF-κB plays important roles in types of cellular processes, including development, cancer cell invasion, differentiation, inflammation [18], apoptosis, and survival [19]. It could initiate the target genes transcription when the activated NF-κB dimer transferred to nuclei from cytoplasm [20]. NF-κB has been found contributing to intensified invasive ability of tumor cells through activate MMP-2/-9 expression [21,22]. In the year 2011, it has been demonstrated that PFOA could regulated LPS-induced MMP-9 release via regulating NF-κB phosphorylation level [3]. Hence, we supposed that PFOA might influence the cancer invasive malignancy, and probably through NF-κB activated MMP-2/-9 expression. Recently, Zhang et al., has confirmed that microcystin-LR, a kind of water pollutants, could enhance melanoma cancer cells invasion [23]. However, there is still [3] little information about the effect of environmental pollutants on colorectal cancer cell invasion. In this study, we aimed to investigate the potential effect of PFOA on colorectal cancer cell DLD-1 invasion. In addition, the undergoing mechanism was also explored. We have used a wild range of PFOA concentrations, which had relatively little effect on the viability of the cells.
Materials and methods
Reagents and antibodies
The PFOA (purity ≥ 95%, by HPLC) was obtained from Sigma (USA). Human colorectal cancer cell line DLD-1 was purchased from the American Type Culture Collection. Cell culture medium RPMI-1640 was purchased from Gibco (Invitrogen China Limited, China). All antibodies used in this study were provided by Cell Signaling Technology (USA). Other reagents were all provided by Sigma-Aldrich Inc. (USA) unless otherwise mentioned.
Cell culture and PFOA exposure
Human colorectal cancer cells DLD-1 were maintained in RPMI-1640 medium containing 100 U/ml streptomycin and 100 U/ml penicillin supplemented with 10% fetal bovine serum (FBS). Cells were cultured in a humidified atmosphere of 5% carbon dioxide (CO2) at 37°C. PFOA was dissolved by sterile deionized water to make a 2 mM stock solution and added to the culture medium to reach different doses (0, 1, 10, 100, 1000, and 10000 nM). In order to explore the role of NF-κB in the molecular pathway mediating the promoted cell invasion and MMP-2/-9 expression, JSH-23 was dissolved in dimethyl sulfoxide (DMSO) and added to the culture media with final concentration of 10 μM [24] (equal DMSO added to culture medium as solvent control groups).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
MTT experiment was performed to judge the effect of PFOA on cell proliferation viability [25]. Human colorectal cancer cells DLD-1 were seeded in a 96-well plate with serum-free medium, 2 thousand cells per well, and 24 h later, cells were treated with different doses of PFOA. At certain time points (72 h), the cells were incubated with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) in growth medium at 37°C for 4 h and lysed in 100 μL of dimethyl sulfoxide at room temperature for 15 min. The absorbance in each well was measured at 490 nm by Synergy 2 Absorbance Microplate Reader (Biotek, USA). Cell growth inhibition rate was expressed as a fraction of absorbance compared to control.
Cell invasion ability assay
After PFOA exposure, trans-well filter assays were conducted to investigate DLD-1 invasion ability by using 24-well ECM-coated trans-well inserts [26]. Briefly, the upper surface of the filter (8.0 μm in pore size) was coated with 100 μg ECM gel before air-dried overnight at 37°C. Approximately 6 × 104 cells were added to the upper chamber, cultured in serum-free RPMI-1640 culture medium and RPMI-1640 with 10% FBS was added to the lower chamber. For drug inhibition experiments, JSH-23 (10 μM) was added to the upper filters prior to invasion assays, and equal DMSO was added to culture medium of another group as solvent control. After 72 h incubation with different doses of PFOA in an environment of 5% CO2 at 37°C, cells degraded the ECM and adhered to the opposite surface of the filter. The filters were then fixed with 4% paraformaldehyde (dissolved in PBS) and stained with crystal violet before the cells on the upper surface were removed completely with a cotton swab. The cells, which invaded from the upper to the lower side of the filter, were observed under light microscopy at a magnification of × 100. Cells were then lysed by cell lysis buffer and collected for the detection of optical density (OD) at 570 nm. OD values of the cells which were treated with different concentrations of PFOA were normalized to those of the control cells (without PFOA exposure). Human colorectal cancer cells DLD-1 invasive ability was defined as the mean OD volume of cells.
RNA extraction, reverse transcription PCR, and quantitative real time PCR
We employed Quantitative real time PCR (qRT-PCR) to detect the mRNA expression levels of homo-mmp-2/-9, with homo-β-actin as housekeeping genes [26]. After cells were treated with different doses of PFOA for 24 h, total RNA was isolated from colorectal cancer cells with Trizol reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). The purified total RNA (1 μg) was then reversely transcribed using the First Strand cDNA Synthesis Kit (TaKaRa, Japan). The qRT-PCR primers of mmp-2 gene were: forward 5’-GAG AAC CAA AGT CTG AAG AG-3’ and reverse 5’-GGA GTG AGA ATG CTG ATT AG-3’; the primers of mmp-9 gene were: forward 5’-TGC CCG GAC CAA GGA TAC AG-3’ and reverse 5’-TCA GGG CGA GGA CCA TAG AG-3’; PCR primers set of the internal control gene β-actin were: forward 5’-TGA CGT GGA CAT CCG CAA AG-3’ and reverse 5’-CTG GAA GGT GGA CAG CGA GG-3’. Reactions were conducted in 96-well plates with a final volume of 20 µl including 10 µl SYBR Green PCR Master Mix (Invitrogen, Carlsbad, CA), plus 1 µl each primer (2 µM), 1 µl template DNA, and 7 µl ddH2O. Thermal cycling and fluorescence detection were conducted on a Applied Biosystems ViiATM7 (Life Technologies, USA), using the following protocol: 95°C for 5 min, followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. Each reaction was run in triplicate. The levels of mmp-2/-9 gene expression were normalized to β-actin levels using the method of 2-^^ct. As assessed by electrophoresis of PCR products, no primer-dimer was observed for both the target genes and β-actin, and the specificity of the products was also confirmed by melt curve analysis (data not shown).
Luciferase assay
Luciferase reporter assay experiment was conducted to perform the effect of PFOA on the gene promoter activity of mmp-2/-9 [27]. DLD-1 cells were grown on a 24-well plate for 24 h to reach approximately 70% confluence prior to transfection. The cells in each well were co-transfected with 0.4 μg of mmp-2/-9-promotor site-derived luciferase reporter plasmid and 0.1 μg of β-galactosidase (β-gal) expression plasmid using Lipofectamine 2000 (Invitrogen, China Limited, China) according to the manufacturer’s protocol. After transfection for 6 h, cells were treated with different doses of PFOA, 24 h later, Cell lysates were prepared with Reporter Lysis Buffer (Promega, Madison, WI) following the manufacturer’s instructions, and luciferase activity was measured with Luciferase Assay Reagent 10-Pack (Promega). The β-gal activity of the cells was measured as follows: 1 μL of Mg solution (0.1 M MgCl2, 4.5 M beta-mercaptoethanol), 22 μL of 4 mg/mL O-nitrophenol-β-D-galactoside solution (O-nitrophenyl-beta-D-galactopyranoside) (in 0.1 M phosphate buffer [pH 7.5]), and 57 μL of 0.1 M phosphate buffer (pH 7.5) were added into the obtained cell extract solution (20 μL) to reach a total volume of 100 μL. As control, the optical density (OD) volume of β-gal at 450 mm was measured in parallel.
Western blotting
After the cells’ protein components were collected, a standard Western blotting analysis was used to investigate the MMP-2/-9 and NF-κB protein expression levels. Briefly, nuclear and cellular protein extracts were prepared, 72 h after treated with different doses of PFOA, JSH-23 (10 μM) or both JSH-23 (10 μM) and PFOA (1 μM ) (equal DMSO was added to culture medium of another group as solvent control), by using KEYGEN Protein Extraction Kit (KEYGEN, Nanjing). For detecting the ratio of nuclei (p65): cytosol (p65), cytosol protein and nucleus protein were dissolved in equal volume lysis buffer of each group cells. Protein lysates were boiled in SDS-sample buffer for 5 min and then subjected to 8% SDS-polyacrylamide gel electrophoresis and transferred to PVDF (polyvinylidene fluoride) membranes (Bio-Rad). Membranes were then blocked for 2 h in 5% milk-Tris-Buffered Saline Tween-20 (TBST) at room temperature, and incubated overnight at 4°C either with the monoclonal antibodies (Cell Signaling Technology, USA) of anti-GAPDH, anti-Lamin B, anti-MMP-2, anti-MMP-9 or anti-NF-κB. Membranes were washed four times in TBST and incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. Blots were visualized by enhanced chemiluminescence (Thermo, USA) and analyzed using a scanning densitometer with the molecular analysis software FluorChem M system (Protein simple, USA).
Gelatin zymography
The enzyme activities of MMP-2 and MMP-9 were analyzed by gelatin zymography. Cells (4 × 104) were seeded into a 24-well plate and allowed to adhere for 6-8 h with the presence of serum. Subsequently, the culture medium was replaced by serum-free DMEM medium (400 μl per well) with different doses of PFOA (0, 1, 10, 100, and 1000 nM). After incubation for 72 h, the medium in each well was gathered and centrifuged for 10 min at 2,000 rpm to remove cell debris. The supernatant was collected and mixed with sample loading buffer containing no DTT (dithiothreitol) before 8% SDS-polyacrylamide gel (containing 1 mg/ml gelatin) electrophoresis. Then the gel was soaked in 2.5% Triton-X-100 for 30 min twice to remove SDS, and transferred to bath buffer containing 50 mM Tris (pH 8.0), 5 mM CaCl2 and 2 mM ZnCl2 at 37°C for 16 h. The gel was then stained with 0.1% Coomassie blue in 45% methanol and 10% acetic acid. The visualized bands indicating the presence of a protein with gelatinolytic activity were analyzed by the FluorChem M system (Protein simple, USA).
Immunofluorescence
A standard immunostaining procedure was employed to analyze NF-κB nuclear translocation activity. After 24-h treatment with PFOA at the indicated concentrations (0, 1, 10, 100, and 1000 nM), cells grown on thick slides were washed with phosphate buffered saline (PBS), fixed by immersion at room temperature with 4% polyformaldehyde for 20 min, and permeabilized with 0.1% Triton-X-100 in PBS at 4°C for 10 min. Slides were then washed with PBS and blocked with blocking buffer consisting of 4% bovine serum albumin (BSA) in PBS for 30 min at room temperature. The cells were then incubated with primary anti-NF-κB monoclonal antibody (Cell Signaling Technology, USA) (diluted 1:25) in blocking buffer overnight at 4°C, followed by incubation with the secondary anti-rabbit Fluorescein Isothiocyanate (FITC) antibody (Cell Signaling Technology, USA) (diluted 1:100) in blocking buffer at room temperature for 1 h. Subsequently, cells were stained with 5 μg/ml 4’,6-diamidino-2-phenylindole (DAPI) for 2 min and washed with PBS. Coverslips and stained cells were captured and analyzed by a confocal laser scanning microscopy system using a 400 × magnification (LSM710, Carl Zeiss, Germany).
Statistical analysis
The differences between the treated and the control cells were analyzed by applying one way ANOVA (analysis of variance) method followed by post-hoc Tukey’s analysis. A P-value of less than 0.05 was considered statistically significant, and each experiment was performed independently at least triple times. Computer-based calculations were conducted using SPSS version 20.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Effects of PFOA exposure on colorectal cancer cells invasive ability
On the purpose of detecting the invasive ability of the colorectal cancer cells DLD-1 after exposure to different doses of PFOA, a standard trans-well filter invasion assay was carried out. First, we assessed the effect of PFOA on DLD-1 cells viability. The viability of DLD-1 cells could be inhibited dominantly by PFOA treatment (> 1 μM, P < 0.05), but no cell viability inhibition was observed after treatment with lower concentrations (≤1 μM) (P > 0.05) (Figure 1A). More important, we found that the invasion ability of DLD-1 cells, which were stained by crystal violet, was strengthened by PFOA (≤1 μM) (Figure 1B), and subsequently confirmed by detecting the OD value of the invaded cells (Figure 1C). Compared to control group, the cell invasiveness was enhanced by 1.2, 1.45, 2.29, and 3.25 fold after PFOA exposure at 1, 10, 100, 1000 and 10000 nM separately. The colorectal cancer cells DLD-1 invasion was stimulated by PFOA in a dose-dependent manner (R2 = 0.9042).
Figure 1.
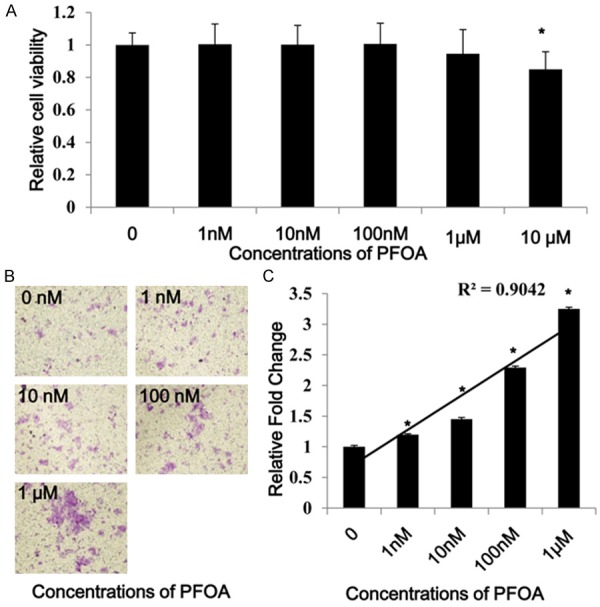
PFOA enhanced DLD-1 cells invasion. The MTT result showed that PFOA only inhibited DLD-1 cells viability at the concentration of 10 μM (P < 0.05), but not affect the cell viability at the concentrations less than 1 μM (P > 0.05) (A). Excluded the influence on cell vitality, PFOA stimulated the invasiveness of the crystal violet stained DLD-1 cells (B), which was subsequently confirmed by determining the OD value of the invaded cells (C). (Each experiment was performed independently at least triple times).
Induced MMP-2/-9 expression and activation levels in PFOA-treated DLD-1 cells
To explore the effect of PFOA exposure on MMP-2/-9 expression, we performed luciferase assay, qRT-PCR, and western blot to detect the gene promoter activity, mRNA and protein expression levels of MMP-2/-9 in DLD-1 cells separately. After 24 h treatment with different doses of PFOA, luciferase assay results demonstrated that the mmp-2/-9 gene promoter activity was stimulated in a dose-dependent manner (mmp-2, R2 = 0.8706; mmp-9, R2 = 0.8) (Figure 2A). qRT-PCR data showed that PFOA exposure also up-regulated the mRNA expression level of gene mmp-2/-9 in a dose-dependent manner (mmp-2, R2 = 0.9471; mmp-9, R2 = 0.9311) (Figure 2B), which was further confirmed by the protein expression level of MMP-2/-9 in each group via western blotting (Figure 2C). Meanwhile, gelatin zymography analysis clarified that the activation levels of MMP-2/-9 were enhanced after PFOA treatment (Figure 2D). Results of luciferase reporter, qRT-PCR, Western blotting, and gelatin zymography results revealed that PFOA exposure could evidently up-regulate both expression and activation levels of MMP-2/-9 in colorectal cancer cells DLD-1, which may be subsequently included in the cell invasion process.
Figure 2.
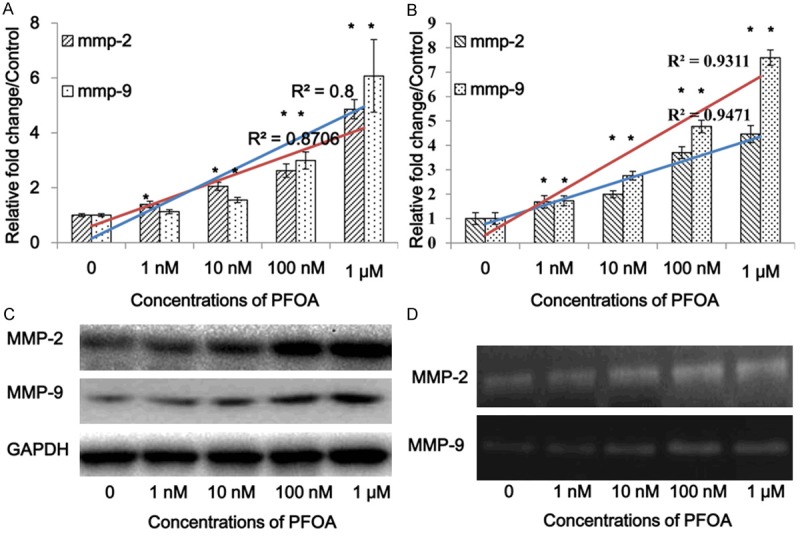
PFOA increased MMP-2/-9 expression and activity levels. The luciferase reporter assay showed that PFOA exposure promoted gene mmp-2/-9 transcription (A), which is consistent with the changes of mRNA level (B). Western blotting data appeared that PFOA also up-regulate the protein levels of MMP-2/-9 (C). Furthermore, PFOA treatment increased MMP-2/-9 activation process by performing gelatin zymography analysis (D). (Each experiment was performed independently at least triple times).
Stimulation of NF-κB activation in PFOA-treated DLD-1 cells
In order to clarify the potential molecular mechanisms of MMP-2/-9 overexpression after PFOA treatment, we further examined the level of NF-κB Activation in DLD-1 cells. After 72 h PFOA treatment, the cytosol and nuclear proteins of DLD-1 cells were isolated for western blotting of p65 separately (Figure 3A). The grayscale value of each band was analyzed, and the data showed that compared to the control group, the ratio of nuclear/cytosol protein level of p65 was elevated dominantly with PFOA exposure (Figure 3B). Subsequently, immunofluorescence assays showed that PFOA treatment accelerated nuclear translocation of NF-κB (p65) in a dose-dependent manner (P < 0.05) (Figure 3C), which agrees with the result of western blotting (Figure 3A).
Figure 3.
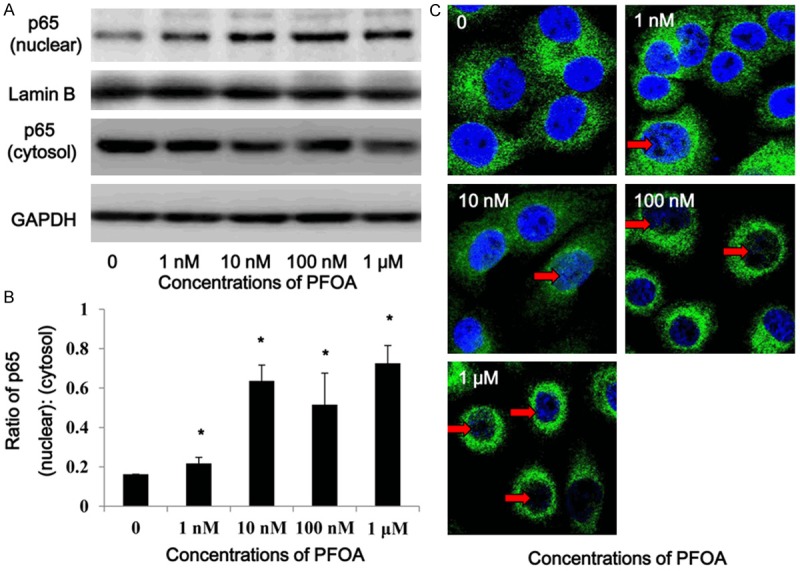
PFOA promoted NF-κB translocation into nuclear from cytosolic. The cytosol and nuclear proteins were isolated from the PFOA-treated DLD-1 cells for Western blotting of p65 (A). After PFOA treatment, the nucleus/cytosol ratio of NF-κB was elevated significantly (B). Meanwhile, immunofluorescence assay showed that green dyes labelled-p65 (indicated by red arrow) accumulated in the blue nuclear (C). (Each experiment was performed independently at least triple times).
Mediation of NF-κB in MMP-2/-9 expression and cell invasion
We carried out the drug inhibition experiment to confirm the role of NF-κB activation in the MMP-2/-9 enhancement and cell invasive ability stimulation. After NF-κB inhibitor JSH-23 (10 μM) pre-incubation, the nuclear/cytosol ratio of p65 was confirmed to be decreased by 16% compared to the control, and only increased approximately 11% with PFOA (1 μM) co-treatment. On the other hand, the nuclear/cytosol ratio of p65 was up-regulated 39% only with PFOA (1 μM) exposure, which indicated that JSH-23 partially inhibited PFOA-induced NF-κB activation (Figure 4A). Then the effect of JSH-23 on PFOA-induced DLD-1 invasiveness was certified by trans-well filter assay. The result appeared that PFOA-induced cell invasiveness was blocked by JSH-23 pre-treatment (Figure 4B), which suggested that PFOA could stimulate colorectal cancer cell invasion through increasing NF-κB activation. Furthermore, JSH-23 could reverse the stimulation effect of PFOA on gene mmp-2/-9 promoter activity (Figure 4C), which was consistent with the western blotting result (Figure 4D).
Figure 4.

NF-κB mediated PFOA-induced MMP-2/-9 expression and DLD-1 invasion. Drug inhibition experiment was carried out to confirm the role of NF-κB in PFOA-induced MMP-2/-9 stimulation and DLD-1 invasion promotion. After pre-treatment with NF-κB inhibitor JSH-23 (10 μM) for 1 h, western blotting data confirmed that the ratio of NF-κB (nucleus: cytosol) level was decreased significantly (A), and the same effect was observed in the DLD-1 cells invasion process (B). Meanwhile, luciferase reporter assay data indicated that JSH-23 could reverse the stimulation effect of PFOA on gene mmp-2/-9 promoter activities (C), which was agreed with the changes of protein expression level (D). (Each experiment was performed independently at least triple times).
Discussion
This is the first study revealing that the environmental pollutant PFOA can stimulate colorectal cancer cell invasion. Recent years, amounts of evidence have demonstrated that PFOA can initiate tumor occurrence probably through inducing cell oxidative stress and DNA damages [28,29]. However, there is little information reported about the linkage between PFOA exposure and colorectal cancer invasion, although Zhang et al. have demonstrated PFOA could stimulate breast cancer cell MDA-MB-231 invasiveness [26]. The relation between environmental pollutant and tumor initiation has been investigated for decades, while, recent concerns of physicochemical factors have been focused on the stimulation of cancer cell migration and invasion process, for example extracellular calcium [30], progesterone receptor [31], bacterial peptidoglycan [32], glutamate [33], microcystin-LR [23] and so on, but the relationship between environmental pollutant and colorectal cancer invasion is still omitted.
Effects of environmental pollutant on cell viability and invasiveness are cell-/time-/dose-dependent, since previous studies have indicated the contrasting cellular responses to PFOA [34,35]. In our present study, the MTT data demonstrated that certain degree of PFOA exposure (≤1 μM) could not influence colorectal cancer cells DLD-1 viability, while higher dose treatment (> 1 μM) could inhibit cells vitality (Figure 1A). In order to exclude the influence on cell viability, we chose the certain dose groups (0-1 μM) to clarify the effect and molecular mechanism of PFOA on colorectal cancer cell invasion. The trans-well filter assay results showed that PFOA could enhance the colorectal cancer cells DLD-1 invasive ability significantly (Figure 1B, 1C). Our data appeared accordance with Zhang et al. study, which demonstrated PFOA could stimulate breast cancer cells invasion.
MMP-2/-9, as important members of MMPs family, has been identified to play critical roles in colorectal invasive and metastasis process for their ability of degrading extra-cellular matrix. On the purpose of elucidating the covered molecular mechanisms of PFOA induced colorectal cancer cell invasion, we first confirmed the effect of PFOA on the promoter activity, mRNA expression, protein expression, and enzyme activation levels of MMP-2/-9 in DLD-1 cells. The results appeared that PFOA could not only stimulate MMP-2/-9 expression levels, but also activate enzyme activation process in colorectal cancer cells (Figure 2). High-expression levels of MMPs has been clarified to be related to tumor metastasis, and based on this point, much effort has been devoted to screening and developing drugs to reduce MMPs expression [36]. Nevertheless, a growing number of researches have demonstrated that environmental pollutants could induce MMPs expression in kinds of cancers. For example, Zhang et al. documented that microcystin-LR, a well-known water pollutant, could stimulate MMP-2/-9 expression via activating NF-κB in human melanoma cells [23]. Beside chemicals, physical factors including radiation can also up-regulate MMP-9 expression level and catalytic activity through inducing NF-κB activation in hepatocellular carcinoma cells [37]. PFOA has been confirmed to induce MMP-2/-9 expression via NF-κB in breast cancer cells [26], while, effect of environmental pollutant, even gene over-expression on cell invasiveness is cell-dependent. Corsini et al. found that PFOA inhibited LPS-induced MMP-9 expression via reducing NF-κB activation level [3]. In this study, we uncovered that PFOA (≤1 μM) induced NF-κB translocation into nuclei, which might be involved in PFOA regulated MMP-2/-9 over-expression (Figure 3).
In order to elucidate the proposed mechanism that PFOA might up-regulate MMP-2/-9 expression through NF-κB, we carried out the JSH-23 suppression assay, which is a well-established NF-κB inhibition method in cancer cells [38]. In the present study, PFOA induced MMP-2/-9 over-expression and strengthened cell invasion were reversed by JSH-23 in colorectal cancer cells (Figure 4), which suggested that NF-κB mediated PFOA induced MMP-2/-9 over-expression and cell invasiveness in colorectal cancer cell DLD-1. NF-κB has been explained well to be a key transcriptional factor regulating cell apoptosis and survival process [19], meanwhile, appropriated increased activity of NF-κB could only promote MMPs expression and colorectal cancer cell invasion, but not affect cell vitality in this study, which is supported by previous studies [39-41]. Lee et al. clarified that sulforaphane could decrease TPA-induced mmp-9 gene expression through inhibiting NF-κB activity in MCF-7 cells [42]. Shih et al. also identified that NF-κB could regulate mmp-2 transcription and enzyme activation through stimulating MT1-MMP expression [43]. In this study, PFOA has been confirmed to induce NF-κB activation in colorectal cancer cell DLD-1, which resulted in MMP-2/-9 over-expression and increased DLD-1 invasiveness. PFOA is widely used in industrial and consumer products, including the manufacture of fluoropolymers, non-stick coating on cookware, and stain-resistant coatings on fabric for its stain-resistant and water-repellant characteristics. Contamination of human serum attracted more attention about side effects in the long term; the data showed that contamination of PFOA in human serum attained on average of 28.2 ng/ml (approximately 68.1 nM), and the half-life in human is about 3.8 years for PFOA. Our findings pointed the importance of assessing the induced public health risks in our surrounding environments.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (81302304) and Shanghai Municipal Health Bureau Key Disciplines Grant (ZK2012A05).
Disclosure of conflict of interest
None.
References
- 1.Vieira V, Hoffman K, Fletcher T. Assessing the Spatial Distribution of Perfluorooctanoic Acid Exposure via Public Drinking Water Pipes Using Geographic Information Systems. Environ Health Toxicol. 2013;28:e2013009. doi: 10.5620/eht.2013.28.e2013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013;121:318–323. doi: 10.1289/ehp.1205829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corsini E, Avogadro A, Galbiati V, Marinovich M, Galli CL, Germolec DR. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs) Toxicol Appl Pharmacol. 2011;250:108–116. doi: 10.1016/j.taap.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Holmström KE, Järnberg U, Bignert A. Temporal trends of PFOS and PFOA in guillemot eggs from the Baltic Sea, 1968-2003. Environ Sci Technol. 2005;39:80–84. doi: 10.1021/es049257d. [DOI] [PubMed] [Google Scholar]
- 5.Costa G, Sartori S, Consonni D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med. 2009;51:364–372. doi: 10.1097/JOM.0b013e3181965d80. [DOI] [PubMed] [Google Scholar]
- 6.Steenland K, Jin C, MacNeil J, Lally C, Ducatman A, Vieira V, Fletcher T. Predictors of PFOA levels in a community surrounding a chemical plant. Environ Health Perspect. 2009;117:1083–1088. doi: 10.1289/ehp.0800294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA) Environ Health Perspect. 2010:1100–1108. doi: 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10(Suppl 1):S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dijk M, Göransson SA, Strömblad S. Cell to extracellular matrix interactions and their reciprocal nature in cancer. Exp Cell Res. 2013;319:1663–1670. doi: 10.1016/j.yexcr.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Tallant C, Marrero A, Gomis-Rüth FX. Matrix metalloproteinases: fold and function of their catalytic domains. Biochim Biophys Acta. 2010;1803:20–28. doi: 10.1016/j.bbamcr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 13.Sbardella D, Fasciglione GF, Gioia M, Ciaccio C, Tundo GR, Marini S, Coletta M. Human matrix metalloproteinases: an ubiquitarian class of enzymes involved in several pathological processes. Mol Aspects Med. 2012;33:119–208. doi: 10.1016/j.mam.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Baker EA, Leaper DJ. Measuring gelatinase activity in colorectal cancer. Eur J Surg Oncol. 2002;28:24–29. doi: 10.1053/ejso.2001.1179. [DOI] [PubMed] [Google Scholar]
- 15.Waas ET, Lomme RM, DeGroot J, Wobbes T, Hendriks T. Tissue levels of active matrix metalloproteinase-2 and -9 in colorectal cancer. Br J Cancer. 2002;86:1876–1883. doi: 10.1038/sj.bjc.6600366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and-9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie. 2005;87:287–297. doi: 10.1016/j.biochi.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Adya R, Tan BK, Chen J, Randeva HS. Nuclear factor-κB induction by visfatin in human vascular endothelial cells its role in MMP-2/9 production and activation. Diabetes Care. 2008;31:758–760. doi: 10.2337/dc07-1544. [DOI] [PubMed] [Google Scholar]
- 18.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 19.Karin M, Lin A. NF-κB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 20.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799:775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babykutty S, S PP, J NR, Kumar MA, Nair MS, Srinivas P, Gopala S. Nimbolide retards tumor cell migration, invasion, and angiogenesis by downregulating MMP-2/9 expression via inhibiting ERK1/2 and reducing DNA-binding activity of NF-κB in colon cancer cells. Mol Carcinog. 2012;51:475–490. doi: 10.1002/mc.20812. [DOI] [PubMed] [Google Scholar]
- 22.Suboj P, Babykutty S, Valiyaparambil Gopi DR, Nair RS, Srinivas P, Gopala S. Aloe emodin inhibits colon cancer cell migration/angiogenesis by downregulating MMP-2/9, RhoB and VEGF via reduced DNA binding activity of NF-κB. Eur J Pharm Sci. 2012;45:581–591. doi: 10.1016/j.ejps.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XX, Fu Z, Zhang Z, Miao C, Xu P, Wang T, Yang L, Cheng S. Microcystin-LR promotes melanoma cell invasion and enhances matrix metalloproteinase-2/-9 expression mediated by NF-κB activation. Environ Sci Technol. 2012;46:11319–11326. doi: 10.1021/es3024989. [DOI] [PubMed] [Google Scholar]
- 24.Gaultier A, Arandjelovic S, Niessen S, Overton CD, Linton MF, Fazio S, Campana WM, Cravatt BF, Gonias SL. Regulation of tumor necrosis factor receptor-1 and the IKK-NF-κB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood. 2008;111:5316–5325. doi: 10.1182/blood-2007-12-127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Yao D, Zhao S, He C, Ding N, Li L, Long F. MiR-1246 promotes SiHa cervical cancer cell proliferation, invasion, and migration through suppression of its target gene thrombospondin 2. Arch Gynecol Obstet. 2014;290:725–32. doi: 10.1007/s00404-014-3260-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Wang F, Xu P, Miao C, Zeng X, Cui X, Lu C, Xie H, Yin H, Chen F. Perfluorooctanoic acid stimulates breast cancer cells invasion and up-regulates matrix metalloproteinase-2/-9 expression mediated by activating NF-κB. Toxicol Lett. 2014;229:118–125. doi: 10.1016/j.toxlet.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Xu P, Zhang XX, Miao C, Fu Z, Li Z, Zhang G, Zheng M, Liu Y, Yang L, Wang T. Promotion of melanoma cell invasion and tumor metastasis by microcystin-LR via phosphatidylinositol 3-kinase/AKT pathway. Environ Sci Technol. 2013;47:8801–8808. doi: 10.1021/es4007228. [DOI] [PubMed] [Google Scholar]
- 28.Yao X, Zhong L. Genotoxic risk and oxidative DNA damage in HepG2 cells exposed to perfluorooctanoic acid. Mutat Res. 2005;587:38–44. doi: 10.1016/j.mrgentox.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Klaunig JE, Hocevar BA, Kamendulis LM. Mode of Action analysis of perfluorooctanoic acid (PFOA) tumorigenicity and Human Relevance. Reprod Toxicol. 2012;33:410–418. doi: 10.1016/j.reprotox.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Joeckel E, Haber T, Prawitt D, Junker K, Hampel C, Thüroff JW, Roos FC, Brenner W. High calcium concentration in bones promotes bone metastasis in renal cell carcinomas expressing calcium-sensing receptor. Chemotherapy. 2014;4:5. doi: 10.1186/1476-4598-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu XD, Goglia L, Sanchez AM, Flamini M, Giretti MS, Tosi V, Genazzani AR, Simoncini T. Progesterone receptor enhances breast cancer cell motility and invasion via extranuclear activation of focal adhesion kinase. Endocr Relat Cancer. 2010;17:431–443. doi: 10.1677/ERC-09-0258. [DOI] [PubMed] [Google Scholar]
- 32.Xie W, Huang Y, Xie W, Guo A, Wu W. Bacteria peptidoglycan promoted breast cancer cell invasiveness and adhesiveness by targeting toll-like receptor 2 in the cancer cells. PLoS One. 2010;5:e10850. doi: 10.1371/journal.pone.0010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herner A, Sauliunaite D, Michalski CW, Erkan M, Oliveira TD, Abiatari I, Kong B, Esposito I, Friess H, Kleeff J. Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int J Cancer. 2011;129:2349–2359. doi: 10.1002/ijc.25898. [DOI] [PubMed] [Google Scholar]
- 34.Hu XZ, Hu DC. Effects of perfluorooctanoate and perfluorooctane sulfonate exposure on hepatoma Hep G2 cells. Arch Toxicol. 2009;83:851–861. doi: 10.1007/s00204-009-0441-z. [DOI] [PubMed] [Google Scholar]
- 35.Florentin A, Deblonde T, Diguio N, Hautemaniere A, Hartemann P. Impacts of two perfluorinated compounds (PFOS and PFOA) on human hepatoma cells: Cytotoxicity but no genotoxicity? Int J Hyg Environ Health. 2011;214:493–499. doi: 10.1016/j.ijheh.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 37.Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene. 2006;25:7009–7018. doi: 10.1038/sj.onc.1209706. [DOI] [PubMed] [Google Scholar]
- 38.Lin G, Tang Z, Ye YB, Chen Q. NF-κB activity is downregulated by KRAS knockdown in SW620 cells via the RAS-ERK IκBα pathway. Oncol Rep. 2012;27:1527. doi: 10.3892/or.2012.1669. [DOI] [PubMed] [Google Scholar]
- 39.Zhao M, Gao Y, Wang L, Liu S, Han B, Ma L, Ling Y, Mao S, Wang X. Overexpression of integrin-linked kinase promotes lung cancer cell migration and invasion via NF-κB-mediated upregulation of matrix metalloproteinase-9. Int J Med Sci. 2013;10:995. doi: 10.7150/ijms.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee EJ, Lee SJ, Kim S, Cho SC, Choi YH, Kim WJ, Moon SK. Interleukin-5 enhances the migration and invasion of bladder cancer cells via ERK1/2-mediated MMP-9/NF-kappaB/AP-1 pathway: involvement of the p21WAF1 expression. Cell Signal. 2013;25:2025–2038. doi: 10.1016/j.cellsig.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh MJ, Lin CW, Yang SF, Chen MK, Chiou HL. Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of NF-κB and AP-1 activity in human liver cancer cells. Brit J Pharmacol. 2014;171:3037–3050. doi: 10.1111/bph.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YR, Han JH, Hwang BM, Lee SH, Youn HJ, Kim JS. Sulforaphane controls TPA-induced MMP-9 expression through the NF-κB signaling pathway, but not AP-1, in MCF-7 breast cancer cells. BMB Rep. 2013;46:201–206. doi: 10.5483/BMBRep.2013.46.4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shih YW, Chien ST, Chen PS, Lee JH, Wu SH, Yin LT. Alpha-mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via alphavbeta3 integrin/FAK/ERK and NF-kappaB signaling pathway in human lung adenocarcinoma A549 cells. Cell Biochem Biophys. 2010;58:31–44. doi: 10.1007/s12013-010-9091-2. [DOI] [PubMed] [Google Scholar]


