Abstract
Background: Lung adenocarcinoma is often composed of a complex and heterogeneous mixture of histological subtypes. Invasive adenocarcinomas are now classified by their predominant pattern, using the comprehensive histological subtyping of the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) classifications. This study aimed to determine whether the expression levels of predictive chemotherapy biomarkers are associated with the histological subtypes proposed by the IASLC/ATS/ERS classification. Materials and Methods: We reviewed data on representative tissue samples from 27 patients who received surgical resection and the expression of excision repair cross complementation group 1 (ERCC1), class III β-tubulin, thymidylate synthase (TS), ribonucleotide reductase M1 (RRM1), and c-Met were examined using immunostaining on tumor tissue slides. We assessed immunohistochemical H-scores, as calculated from the intensity and distribution of intratumor expression, according to the IASLC/ATS/ERS histological subtype. Results: The expression levels of predictive chemotherapy biomarkers varied according to histological subtype. The H-scores of TS and class III β-tubulin expression levels were higher in solid-type components than they were in lepidic-type components Tumors with solid predominant histology tended to recur earlier than non-solid predominant tumors. However, none of the H-scores in histologically predominant tissues was significantly associated with staging or overall survival. Conclusions: Immunohistochemical H-scores of the predictive chemotherapy biomarkers were strongly associated with histological subtype. The presence of a solid subtype, which was associated with poor outcomes, might be assessed by measuring these biomarkers in mixed subtype adenocarcinomas.
Keywords: Thymidylate synthase, excision repair cross complementation group 1, Class III β-tubulin, ribonucleotide reductase M1, c-Met, immunohistochemistry
Introduction
Lung cancer is the most common cause of cancer death worldwide and adenocarcinoma is the most common histological type of lung cancer [1]. More than half of patients with lung adenocarcinoma have developed metastasis at the time of diagnosis, and chemotherapy is the most effective treatment modality. After the complete resection of localized non-small cell lung cancer (NSCLC), adjuvant chemotherapy is associated with an absolute survival advantage of approximately 5% at 5 years, but a relatively high risk of relapse remains, even for early-stage NSCLC [2]. Although the number of treatment options for lung adenocarcinoma is increasing every year, drug resistance remains a persistent obstacle. It is quite difficult to identify any drugs that have equal effects on tumors of the same histology. Indeed, the individual’s genetic characteristics often determine the response to treatment. If the expression of predictive chemotherapy biomarkers could be analyzed immunohistochemically with a robust scoring system, its use would be possible at most pathology institutions.
A new lung adenocarcinoma classification has been published by the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) [3]. Lung adenocarcinomas are often composed of a complex and heterogeneous mixture of histological subtypes. Of diagnosed invasive adenocarcinomas, 90% show a histologically mixed subtype. Invasive adenocarcinomas are now classified by their predominant pattern, based on the following comprehensive histological subtypes: lepidic, acinar, papillary, solid, and micropapillary. Histological subtype is classified semi-quantitatively by estimating the percentages of the various subtypes that are present in 5% increments.
Since the IASLC/ATS/ERS classification was published, a growing number of studies of resected lung adenocarcinomas have demonstrated the utility of this classification scheme for identifying significant prognostic subsets and molecular correlates of the predominant pattern. Regarding early-stage invasive adenocarcinomas, a favorable prognosis is found for lepidic-predominant tumors, while poor prognoses are found for micropapillary- and solid-predominant tumors, and intermediate survival is observed for acinar- and papillary-predominant adenocarcinomas [4,5]. Hence, histological subtyping is an important and independent prognostic variable in lung adenocarcinoma.
On the other hand, experimental and clinical studies have revealed that certain molecular markers have been associated with NSCLC patients’ sensitivity to chemotherapy. Thymidylate synthase (TS), a target enzyme of fluorouracil (5-FU), catalyzes the degradation of 5-FU. Meta-analyses have shown that better responses to antifolate drugs, including pemetrexed, are usually observed in NSCLC patients with lower TS expression [6]. Excision-repair cross-complementation type 1 (ERCC1) is a component of the nucleotide excision repair pathway, which is associated with cellular resistance to platinum compounds. In cases of NSCLC, patients with a low level of ERCC1 exhibit prolonged survival after treatment with platinum-based chemotherapy, as compared with patients with a high level of ERCC1 [7]. The over expression of ribonucleotide reductase subunit M1 (RRM1) has been linked to gemcitabine resistance in a retrospective assessment. Preliminary findings indicate that a subset of gemcitabine/cisplatin-treated patients with low ERCC1 and RRM1 mRNA levels survived significantly longer than patients with higher level of ERCC1 and RRMI mRNA [8]. A recent meta-analysis of more than 1000 patients with NSCLC revealed both higher response rates (P < 0.0001) and improved survival in those patients with cancers harboring low RRM1 (P < 0.001) [9]. Taxanes bind to β-tubulin, which is one of the major components of microtubules, and exert their growth-inhibitory effects, arresting the growth of tumor cells at the G2-M phase. Over expression of class III β-tubulin has been associated with resistance to these drugs, and studies have shown that over expression of class III β-tubulin is correlated with poor prognosis in NSCLC [7]. C-Met over expression had also been reported to be associated with poor clinical outcomes in NSCLC [10,11]. Influential studies have revealed that MET gene amplification is associated with resistance to second-generation tyrosine kinase inhibitors (TKIs) in patients with NSCLC who harbor epidermal growth factor receptor (EGFR) and are initially responsive to EGFR-TKI treatment [12,13]. The c-Met protein could be a significant therapeutic target for patients with NSCLC and acquired resistance to TKI.
We hypothesized that the expression of predictive chemotherapy biomarkers may be associated with clinical outcomes for patients with mixed subtypes of invasive lung adenocarcinoma, and that these associations may differ according to the histological subtype in tumor tissues. In the current study, we measured the H-scores of TS, ERCC1, RRM1, class III β-tubulin, c-Met, and phospho-Met (Tyr1234/1235) assayed by immunohistochemistry in pathologically diagnosed invasive adenocarcinoma. Further, we evaluated correlations between protein expression levels (in terms of H-scores) and clinicopathological parameters. Finally, we investigated whether the H-scores had clinical relevance according to histological subtype.
Materials and methods
Patient characteristics
In this retrospective study, we examined 27 invasive adenocarcinomas treated at a single institution (Juntendo University Hospital, Tokyo, Japan). Twenty-one patients had received various adjuvant chemotherapies after surgical complete resection of the primary lung tumors. Three patients underwent surgical resection of remaining lung tumor as a salvage operation in advanced stages. Tumor response was examined using computed tomography and was evaluated according to the Response Evaluation Criteria in Solid Tumors. The majority of patients were male (65%). The median age of the patients was 61 years (range, 34-75 years). Twenty-one patients (87.5%) received adjuvant chemotherapy. Details of the patients’ clinical characteristics are presented in Table 1. Of the pathological diagnoses, the acinar-predominant pattern was most common (37%). In tumor tissue, the acinar subtype was found with the highest frequency (67%), as compared with other patterns in the heterogeneous mixture of histological subtypes. The ethics Committee of Juntendo University School of Medicine approved this study (No. 2013068). All subjects provided written informed consent to participate in this experimental procedure according to our institutional guidelines.
Table 1.
Clinicopathological characteristics for each investigated tumor
| Parameters | No. | Percentage |
|---|---|---|
| Patient age (y) | 61.2 ± 9.7 | |
| Patient gender (M/F) | ||
| Male | 18 | 66.7 |
| Female | 9 | 33.3 |
| Patient smoking (Brinkman Index) | 513 ± 108 | 59.3 |
| Predominant histological type | ||
| Solid | 9 | 33.3 |
| Acinar | 11 | 40.7 |
| Papillary | 3 | 11.1 |
| Lepidic | 4 | 14.8 |
| Pathological stage | ||
| I | 9 | 33.3 |
| II | 8 | 29.7 |
| III | 6 | 22.2 |
| IV | 4 | 14.8 |
| Adjuvant chemotherapy | 21 | 77.8 |
| Tegafur-uracil | 10 | 37 |
| Cisplatin and Docetaxel | 5 | 18.5 |
| Cisplatin and Vinorelbine | 4 | 14.8 |
| Others (Carboplatin and Gemcitabine, Tegafur-gimeracil-oteracil potassium) | 2 | 7.4 |
| Overall survival (5 y) | 7 | 25.9 |
| Median overall survival (mo) | 47.7 ± 4.8 | (4.5-78) |
| Median recurrence-free period (mo) | 29.8 ± 3.7 | (9.5-105.5) |
Quantitative values are summarizes as mean ± standard deviation.
Tissues, immunohistochemistry and protein expression scoring
Lung tumor tissue samples were fixed in 10% neutral-buffered formalin for 48 h and embedded in paraffin by either pathologist (T.U. or T.H.). Paraffin sections were cut at 4 μm. Endogenous peroxidase activity was quenched by incubation with 0.3% hydrogen peroxide in methanol. The antigen retrieval was carried out in citrate buffer (pH 6.0: TS, ERCC1, phospho-Met; pH 7.0: class III β-tubulin) or in 10 mM EDTA (pH 9.0: RRM1, p-Met) for 10 min. TS and ERCC1 staining were conducted by microwave for 10 min, and the other biomarkers were conducted by autoclave at 100°C for 10 min. These sections were examined on coated slide glass, and then labeled with antibodies against TS (dilution of 1:100, 1 hour at room temperature; Taiho Pharmaceutical Co., Saitama, Japan), mouse monoclonal anti-ERCC1 antibody 8F1 (dilution of 1:100, overnight at 4°C; Novus Biologicals, Littleton, CO, USA), mouse monoclonal anti-RRM1 antibody (dilution of 1:200, 1 hour at room temperature; Rockland Immunochemicals Inc., Gilbertsville, PA, USA), mouse monoclonal anti-class III β-tubulin antibody TU-20 (dilution of 1:100, overnight at 4°C; Cell Signaling Technology, Danvers, MA, USA), c-Met D1C2 (dilution of 1:300, overnight at 4°C; Cell Signaling Technology), and phospho-Met (Tyr1234/1235) D26 (dilution of 1:160, overnight at 4°C; Cell Signaling Technology). Tyr1234/1235 is among the major phosphorylation sites of c-Met. The secondary antibody (Dako Real Envision/HRP, K5027) for 30-60 min at room temperature. Peroxidase activity was visualized with 3,3’ diaminobenzidine tetrahydrochloride solution, and counter staining was performed with hematoxylin. Mixed subtype adenocarcinomas were defined according to their predominant subtype, following the IASLC/ATS/ERS classification of lung adenocarcinoma. All of the immunostained sections were reviewed separately by 2 observers (S.T. and K.S.) under a microscope at magnification × 400. These reviews were performed without knowledge of the patient’s clinical data. Sections with discrepant results were jointly confirmed by consulting either pathologist (T.U. or T.H.) and then re-evaluated until a consensus was reached (with observers S.T. and K.S.). Immunochemical staining for TS, ERCC1, RRM1, class III β-tubulin, c-Met, and phospho-Met were scored in a manner that reflected both the intensity of staining and the percentage of cells with staining at each intensity. Staining intensity was classified as 0 (no staining), +1 (weak staining), +2 (distinct staining), or +3 (strong staining). The percentage of positive cells was graded as 0 (0%), 0.1 (1% to 9%), 0.5 (10% to 49%), or 1.0 (50% and greater). Normal lymph node endothelial cells have been used by many researchers as an external control with staining intensity 2 for ERCC1. Appropriate positive controls for other biomarkers were run and evaluated according to the manufacturers’ instructions and as previously described [14]. A summary value, designated the H-score, was then obtained as Σ (I×PC), where I and PC indicate the staining intensity and the percentage of cells that stained at each intensity, respectively (and as previously described [7]).
Statistical analysis
Expression levels of TS, ERCC1, RRM1, class III β-tubulin, c-Met, and phospho-Met were compared between groups using the Mann-Whitney U test. The recurrence-free period was assessed from the date of resection to the date of objective disease progression. The correlations between clinicopathological parameters and the H-scores were analyzed by Pearson’s correlation analysis. Values of P < 0.05 were considered to indicate statistical significance. Mean values are reported as ± 1 standard deviation values. All statistical analyses were performed (S.T.) with GraphPad prism software for Macintosh (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Immunohistochemical assessment according to histological subtype
The expressions of TS, ERCC1, RRM1, class III β-tubulin, c-Met, and phospho-Met proteins were assessed by immunohistochemistry (Figure 1). Immunoreactivities for all predictive chemotherapy biomarkers were consistently absent in non-tumorous lung tissue with a normal appearance, except for faint immunoreactivity in bronchial, bronchiolar epithelia, and type II pneumocytes. Expression of ERCC1 protein was detected in the nuclei of cancer cells. The c-Met receptors were evident on the cell surface and the other proteins were detected in cytoplasm.
Figure 1.
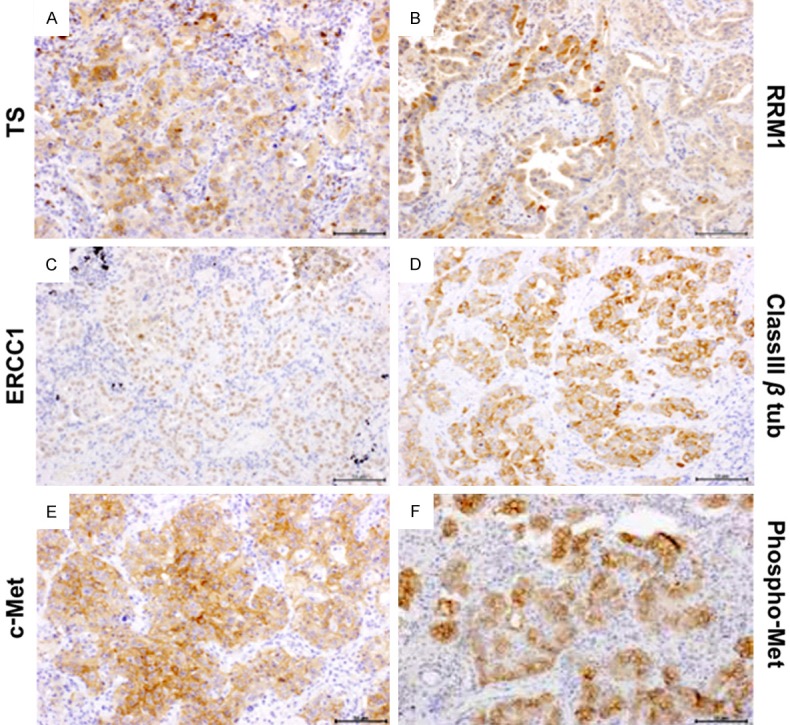
Immunohistochemical staining of human non-small cell lung cancer tissue. Representative sections of carcinomas with high levels of expression of (A) TS, (B) ERCC1, (C) RRM1, (D) class III β-tubulin, (E) c-Met, and (F) phosphor-Met proteins in lung cancer tissues. Examples of the expression of each protein are shown. Expression of ERCC1 protein was detected in the nuclei of cancer cells, and other proteins were present in the cytoplasm. Original magnification: × 400; Scale bars: 100 μm; TS, thymidylate synthase; ERCC1, excision repair cross complementation group 1; RRM1, ribonucleotide reductase M1.
We examined the expression levels of TS, ERCC1, RRM1, class III β-tubulin, c-Met, and phospho-Met according to histological subtype in tumor sections. The H-scores of TS expression levels were higher in solid areas than in papillary or lepidic areas. TS expression levels in the papillary and lepidic areas were 2.12-fold and 2.07-fold lower than the expression level in solid areas, respectively (papillary vs. solid: P = 0.009; lepidic vs. solid: P = 0.015) (Figure 2A). The H-score of TS was significantly greater in solid areas (1.36 ± 0.26) than it was in non-solid areas (solid vs. any other histological subtype: 1.36 ± 0.26 vs. 0.92 ± 0.12; P = 0.05) (Figure 2A). The H-scores of class III β-tubulin expression levels were higher in solid areas than they were in lepidic areas. Class III β-tubulin expression in lepidic areas was 5.8-fold lower than the expression in solid areas (P = 0.022) (Figure 2D). For ERCC1 (Figure 2B) and RRM1 (Figure 2C), we observed no statistically significant differences in expression levels according to histological subtype.
Figure 2.
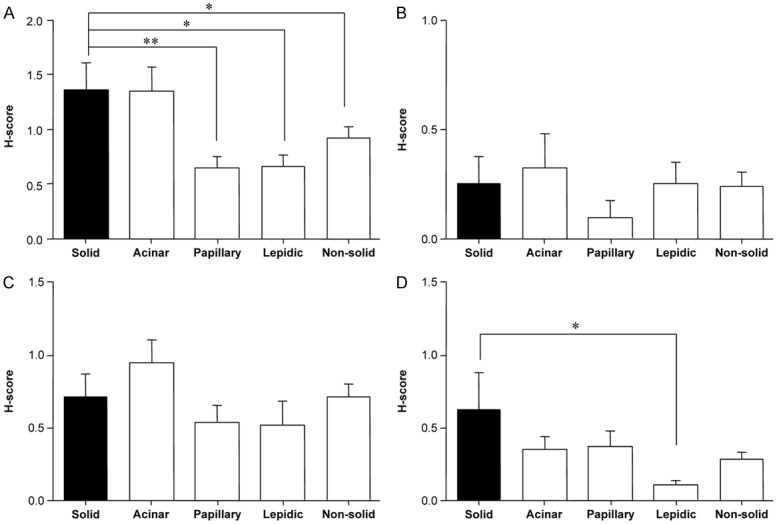
Expression levels of predictive chemotherapy biomarkers according to histological subtypes. A: TS, B: ERCC1, C: RRM1, D: class III β-tubulin. Quantitative estimation of immunohistochemical staining was summarized by the H-score, as described in the methods. Vertical axis: the value of the H-score in both predominant and non-predominant tissue. Horizontal axis: histological subtypes. Non-solid refers to the combination of all subtype groups except solid. *P < 0.05 and **P < 0.01, as compared with H-scores for solid components. TS, thymidylate synthase; ERCC1, excision repair cross complementation group 1; RRM1, ribonucleotide reductase M1.
We additionally analyzed the expression of c-Met and phospho-Met (Tyr1234/1235) according to histological subtype. C-Met expression is widely recognized to be a key protein that influences chemoresistance to TKI and the prognosis of NSCLC. The H-scores of both c-Met and phospho-Met expression levels tended to be higher in solid areas than in areas of other histological subtypes; however, the differences were not statistically significant (Figure 3).
Figure 3.
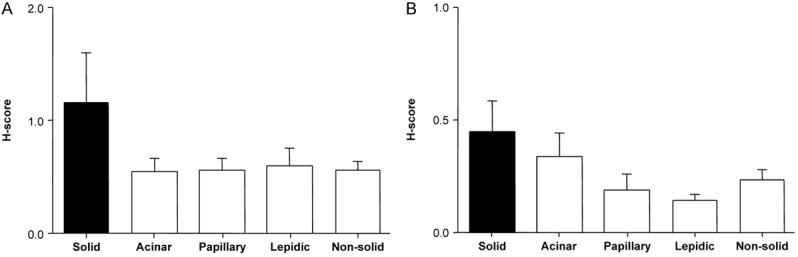
c-Met expression levels according to histological subtypes. A: c-Met, B: phospho-Met (Tyr1234/1235). Quantitative estimation of immunohistochemical staining was summarized by the H-score, as described in the methods. Vertical axis: the value of the H-score in both predominant and non-predominant tissue. Horizontal axis: histological subtypes. Non-solid refers to the combination of all subtype groups except solid. *P < 0.05, as compared with H-scores for solid components.
Associations between the recurrence-free period after surgical resection and histologically predominant subtypes
We investigated the association between the histological predominant subtypes based on IASLC/ATS/ERS classification and the recurrence-free period. The histological solid-predominant tumors tended to recur earlier than other tumors with other predominant subtypes. When we compared the solid-predominant and non-solid-predominant tumors, the recurrence-free period was significantly shorter for solid-predominant tumors under the same clinical characteristics, although the number of samples was limited (solid vs. non-solid: 17.3 ± 5.3 months vs. 36.1 ± 4.1 months; P = 0.031) (Figure 4).
Figure 4.
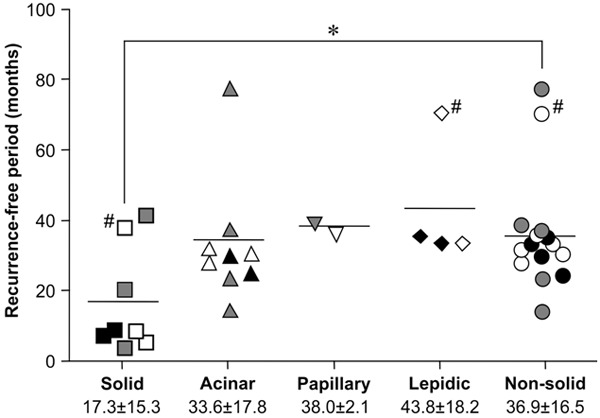
Association between the recurrence-free period after surgical resection and histological subtype-predominant tumors. Vertical axis: duration of the recurrence-free period. Horizontal axis: tumors grouped by histological predominant subtype. Non-solid refers to the combination of all subtype groups except solid. Open, gray, and closed symbols indicate Stage I, Stage II, and Stage III, respectively. Each symbol indicates an individual study subject. *P < 0.05, as compared with the recurrence-free period in the solid group. # indicates subjects who did not received adjuvant chemotherapy.
Associations between the immunohistochemical assessment of histologically predominant subtype and clinical parameters
For each tumor tissue sample, we treated the H-scores of the histological predominant tissue as being representative of the overall expression levels of TS, ERCC1, RRM1, class III β-tubulin, c-Met, and phospho-Met. Additionally, we analyzed the correlations between clinicopathological parameters and the H-scores of these predictive chemotherapy biomarkers in the histological predominant tissue. Overall survival was significantly associated with stage and recurrence-free period. However, we observed no significant associations between overall survival and any of the predominant-tissue H-scores for TS, ERCC1, RRM1, class III β-tubulin, or c-Met (Table 2). The H-score for ERCC1 had a weak tendency towards higher expression levels in stage I disease (P = 0.075). However, none of the predominant-tissue H-scores differed significantly between stage I disease and stage II or greater disease (Table 3).
Table 2.
Correlations between overall survival (OS) and the H-score in the histologically predominant subtype of adenocarcinoma
| Characteristic | Range | p value |
|---|---|---|
| Age (y) | 34-75 | 0.349 |
| Stage | IA-IV | 0.032 |
| Recurrence-free period (mo) | 4.5-78.0 | 0.017 |
| H-score | ||
| TS | 0.3-3.6 | 0.865 |
| ERCC1 | 0-1.75 | 0.658 |
| RRM1 | 0.1-2.3 | 0.081 |
| Class III β-tub | 0-2.3 | 0.492 |
| tMet | -4.5 | 0.258 |
| pMet | 0-1.5 | 0.971 |
| pMet/tMet | 0-10 | 0.424 |
TS: thymidylate synthase; ERCC1: excision repair cross complementation group 1; RRM1: ribonucleotide reductase M1; Class III β-tub: class III β-tubulin.
Table 3.
Associations between H-scores in predominant subtypes of adenocarcinoma and staging
| Stage I vs. Stage II-IV | Mean ± SEM | p value | |
|---|---|---|---|
|
|
|||
| H-score | Stage I | Stage II-IV | |
| TS | 1.33 ± 0.38 | 1.35 ± 0.19 | 0.519 |
| ERCC1 | 0.51 ± 0.23 | 0.13 ± 0.09 | 0.075 |
| RRM1 | 0.99 ± 0.29 | 0.78 ± 0.13 | 0.793 |
| Class III β-tub | 0.26 ± 0.15 | 0.56 ± 0.87 | 0.157 |
| tMet | 0.50 ± 0.10 | 0.64 ± 0.13 | 0.691 |
| pMet | 0.21 ± 0.07 | 0.35 ± 0.12 | 0.878 |
| pMet/tMet | 0.69 ± 0.21 | 1.03 ± 0.81 | 0.518 |
SEM: standard error of the mean; TS: thymidylate synthase; ERCC1: excision repair cross complementation group 1; RRM1: ribonucleotide reductase M1; Class III β-tub: class III β-tubulin.
Discussion
In this study, we have shown that immunohistochemically assessed expression levels of predictive chemotherapy biomarkers differed according to the histological subtype of adenocarcinoma. The H-scores of both TS and class III β-tubulin expression levels were higher in solid-type components than they were in samples from lepidic-type components. Furthermore, we observed that tumors with solid predominant histology tended to recur earlier than non-solid predominant tumors.
Immunohistochemistry is a widely applicable and reliable method of evaluating the protein expression level of biomarkers in tumor tissue. Considering the significant biomarker expression in non-neoplastic cells, quantitative mRNA analyses would require precise and time-consuming microdissection of the analyzed tissues to avoid significant contamination of the assay by non-neoplastic tissue components. To obtain precise tissue samples that are limited to a single histological subtype would require further time-consuming microdissection. In contrast, immunohistochemical signals on neoplastic and non-neoplastic cells can be visually differentiated, including according to histological subtype.
Previous reports have shown than mRNA and protein expression levels of predictive chemotherapy biomarkers are inconsistent; indeed, significant correlations have not always been found between mRNA and protein expression. Biomarkers assessed using immunohistochemistry are significantly predictive of overall survival in patients with NSCLC involving ERCC1-negative and class III β-tubulin-negative tumors [15]. However, no significant predictive ability was found for real-time reverse-transcription polymerase chain reaction assessments of biomarkers [15].
Tantraworasin et al. [16] performed a retrospective cohort study of 247 patients with completely resected NSCLC. Among these patients, ERCC1 and RRM1 expression did not demonstrate prognostic value for tumor recurrence or overall survival [16]. Indeed, certain molecular markers, such as ERCC1 and RRM1 have been associated with the sensitivity of NSCLC tumors to cisplatin-based regimens, although there is currently insufficient evidence to recommend their routine clinical use [17].
The within-tumor sensitivity of TS staining has varied considerably among previous studies, and there is no standardized immunohistochemical scoring system for NSCLC. Reported rates of TS positivity range from 29.6% to 72.5% [18-24]. Many factors could be responsible for this significant heterogeneity, including differences in stages, previous treatments, pathological subtype, treatment regimens, treatment cycles, and performance status. The histological subtypes of pulmonary adenocarcinoma are extremely complicated and highly variable. Overall, 90% of progressive adenocarcinomas are classified as the mixed type. Hence, we suggest that intratumor histological heterogeneity may explain the inconsistent results of previous immunohistochemical scoring systems. Therefore, this intratumor heterogeneity may directly influence results in studies investigating the therapeutic impact of predictive biomarkers in NSCLC [25].
In recent years, biomarkers have been measured following the IASLC/ATS/ERS classification of lung adenocarcinoma. EGFR mutations were significantly associated with adenocarcinoma in situ, minimally invasive adenocarcinoma, lepidic-predominant adenocarcinoma, and papillary-predominant adenocarcinoma [26]. KRAS mutations were associated with adenocarcinomas with mucinous tumor subtypes [26]. Furthermore, the expressions of thyroid transcription factor 1 and cancer stem cell-related markers also vary according to histological subtype. These reports suggested that the comprehensive histological subtyping approach in the IASLC/ATS/ERS classification provides new molecular biology insights into the genesis of lung adenocarcinoma, allowing predictions of chemosensitivity [27,28].
MET gene amplification is an independent unfavorable prognostic factor in NSCLC [29], and is reported to be associated with 20% of lung adenocarcinomas with acquired resistance against EGFR–TKIs [12,30]. C-Met protein is associated with advanced tumor stages and poor clinical outcomes [29,31,32]. Phospho-Met (Tyr1235) over expression is correlated with the papillary or micropapillary histology of lung adenocarcinomas [29,33]. However, we found that the phospho-Met expression level tended to be higher in solid subtype tumors than in lepidic subtype tumors (P < 0.1).
No previous study has focused on the expression levels of predictive chemotherapy biomarkers according to the histological subtype of lung adenocarcinoma. We demonstrated that the expression levels of some predictive chemotherapy biomarkers tended to be higher in solid components than they were in non-solid components, suggesting that the different expression of predictive chemotherapy biomarkers in different histological subtypes might influence responses to chemotherapy. In this cohort of 24 patients, the solid-predominant tumors recurred significantly earlier than the non-solid-predominant tumors. Several recent studies of early-stage adenocarcinomas have shown that lepidic-predominant tumors have favorable prognosis, including 86% to 90% 5-year disease-free survival [34]. Subtyping invasive adenocarcinomas according to the predominant subtype has prognostic value, with a generally favorable prognosis for lepidic-predominant tumors, poor prognoses for micropapillary- and solid-predominant tumors, and intermediate survival for acinar- and papillary-predominant adenocarcinomas [4,5].
In the present study, we immunohistochemically examined that H-scores for some predictive chemotherapy biomarkers were significantly higher in solid components than in the lepidic components. These results suggest that the presence of a solid component may lead to poor outcomes to predict chemosensitivity regardless of whether solid components were predominant. Our findings suggest that the putative predictive value of biomarker expression for response to chemotherapy should be prospectively validated in a further study. Additionally, further study is necessary to identify a reproducible and robust immunohistochemical score. The present immunohistochemical scoring system should be confirmed prospectively to better identify those patients who could benefit from biomarkers of chemotherapy response, and to develop personalized treatment algorithms.
Acknowledgements
The authors thank Taiho Pharmaceutical Co. Ltd. for the generous gift of anti-TS polyclonal antibodies. A part of this work was supported by Leading Center for the Development and Research of Cancer Medicine, Juntendo University Graduate School of Medicine.
Disclosure of conflict of interest
None.
References
- 1.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. New Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496–1504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 5.Warth A, Muley T, Meister M, Stenzinger A, Thomas M, Schirmacher P, Schnabel PA, Budczies J, Hoffmann H, Weichert W. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J. Clin. Oncol. 2012;30:1438–1446. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Yin TJ, Zhou R, Zhou S, Fan L, Zhang RG. Expression of thymidylate synthase predicts clinical outcomes of pemetrexed-containing chemotherapy for non-small-cell lung cancer: a systemic review and meta-analysis. Cancer Chemother Pharmacol. 2013;72:1125–1132. doi: 10.1007/s00280-013-2299-2. [DOI] [PubMed] [Google Scholar]
- 7.Azuma K, Sasada T, Kawahara A, Takamori S, Hattori S, Ikeda J, Itoh K, Yamada A, Kage M, Kuwano M, Aizawa H. Expression of ERCC1 and class III beta-tubulin in non-small cell lung cancer patients treated with carboplatin and paclitaxel. Lung Cancer. 2009;64:326–333. doi: 10.1016/j.lungcan.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Crino L, Danenberg K, Scagliotti G, Bepler G, Taron M, Alberola V, Provencio M, Camps C, De Marinis F, Sanchez JJ, Peñas R. Targeted therapy in combination with gemcitabine in non-small cell lung cancer. Semin Oncol. 2003;30(Suppl 10):19–25. doi: 10.1016/s0093-7754(03)00281-1. [DOI] [PubMed] [Google Scholar]
- 9.Gong W, Zhang X, Wu J, Chen L, Li L, Sun J, Lv Y, Wei X, Du Y, Jin H, Dong J. RRM1 expression and clinical outcome of gemcitabine-containing chemotherapy for advanced non-small-cell lung cancer: A meta-analysis. Lung Cancer. 2012;75:374–380. doi: 10.1016/j.lungcan.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Roth JA, Atkinson EN, Fossella F, Komaki R, Bernadette Ryan M, Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M, Hong WK. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1–6. doi: 10.1016/s0169-5002(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 11.Masuya D, Huang C, Liu D, Nakashima T, Kameyama K, Haba R, Ueno M, Yokomise H. The tumour-stromal interaction between intratumoral c-Met and stromal hepatocyte growth factor associated with tumour growth and prognosis in non-small-cell lung cancer patients. Br J Cancer. 2004;90:1555–1562. doi: 10.1038/sj.bjc.6601718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Jänne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 13.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, Balak M, Chang WC, Yu CJ, Gazdar A, Pass H, Rusch V, Gerald W, Huang SF, Yang PC, Miller V, Ladanyi M, Yang CH, Pao W. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda M, Okamoto I, Hirabayashi N, Kitano M, Nakagawa K. Thymidylate synthase and dihydropyrimidine dehydrogenase expression levels are associated with response to S-1 plus carboplatin in advanced non-small cell lung cancer. Lung Cancer. 2011;73:103–109. doi: 10.1016/j.lungcan.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Vilmar A, Garcia-Foncillas J, Huarriz M, Santoni-Rugiu E, Sorensen JB. RT-PCR versus immunohistochemistry for correlation and quantification of ERCC1, BRCA1, TUBB3 and RRM1 in NSCLC. Lung Cancer. 2012;75:306–312. doi: 10.1016/j.lungcan.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Tantraworasin A, Saeteng S, Lertprasertsuke N, Arayawudhikul N, Kasemsarn C, Patumanond J. The prognostic value of ERCC1 and RRM1 gene expression in completely resected non-small cell lung cancer: tumor recurrence and overall survival. Cancer Manag Res. 2013;5:327–336. doi: 10.2147/CMAR.S52073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds C, Obasaju C, Schell MJ, Li X, Zheng Z, Boulware D, Caton JR, Demarco LC, O’Rourke MA, Shaw Wright G, Boehm KA, Asmar L, Bromund J, Peng G, Monberg MJ, Bepler G. Randomized phase III trial of gemcitabine-based chemotherapy with in situ RRM1 and ERCC1 protein levels for response prediction in non-small-cell lung cancer. J. Clin. Oncol. 2009;27:5808–5815. doi: 10.1200/JCO.2009.21.9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Chuan Pan C, Rui Yu J, Long Y, Hong Cai X, De Yin X, Qiong Hao L, Li Luo L. Association between TYMS expression and efficacy of pemetrexed-based chemotherapy in advanced non-small cell lung cancer: A meta-analysis. PLoS One. 2013;8:e74284. doi: 10.1371/journal.pone.0074284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceppi P, Volante M, Saviozzi S, Rapa I, Novello S, Cambieri A, Lo Iacono M, Cappia S, Papotti M, Scagliotti GV. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107:1589–1596. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Liu D, Masuya D, Nakashima T, Kameyama K, Ishikawa S, Ueno M, Haba R, Yokomise H. Clinical application of biological markers for treatments of resectable non-small-cell lung cancers. Br J Cancer. 2005;92:1231–1239. doi: 10.1038/sj.bjc.6602481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue K, Takao M, Watanabe F, Tarukawa T, Shimamoto A, Kaneda M, Sakai T, Fukushima M, Shimpo H, Yada I. Role of dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine against non-small cell lung cancer-in correlation with the tumoral expression of thymidylate synthase and dihydropyrimidine dehydrogenase. Lung Cancer. 2005;49:47–54. doi: 10.1016/j.lungcan.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi T, Kondo K, Toba H, Yoshida M, Fujino H, Kenzaki K, Sakiyama S, Takehisa M, Tangoku A. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase expression in tumor tissue, regarding the efficacy of postoperatively administered UFT (tegafur + uracil) in patients with non-small cell lung cancer. Anticancer Res. 2007;27:2641–2648. [PubMed] [Google Scholar]
- 23.Nakagawa T, Tanaka F, Otake Y, Yanagihara K, Miyahara R, Matsuoka K, Takata T, Yamada T, Fukushima M, Wada H. Prognostic value of thymidylate synthase expression in patients with p-stage I adenocarcinoma of the lung. Lung Cancer. 2002;35:165–170. doi: 10.1016/s0169-5002(01)00407-x. [DOI] [PubMed] [Google Scholar]
- 24.Otake Y, Tanaka F, Yanagihara K, Hitomi S, Okabe H, Fukushima M, Wada H. Expression of thymidylate synthase in human non-small cell lung cancer. Jpn J Cancer Res. 1999;90:1248–1253. doi: 10.1111/j.1349-7006.1999.tb00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakobsen JN, Santoni-Rugiu E, Ravn J, Sorensen JB. Intratumour variation of biomarker expression by immunohistochemistry in resectable non-small cell lung cancer. Eur J Cancer. 2013;49:2494–2503. doi: 10.1016/j.ejca.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H, Haga H. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 27.Kadota K, Nitadori J, Sarkaria IS, Sima CS, Jia X, Yoshizawa A, Rusch VW, Travis WD, Adusumilli PS. Thyroid transcription factor-1 expression is an independent predictor of recurrence and correlates with the IASLC/ATS/ERS histologic classification in patients with stage I lung adenocarcinoma. Cancer. 2013;119:931–938. doi: 10.1002/cncr.27863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada Y, Saji H, Nomura M, Matsubayashi J, Yoshida K, Kakihana M, Kajiwara N, Ohira T, Ikeda N. Cancer stem cell-related marker expression in lung adenocarcinoma and relevance of histologic subtypes based on IASLC/ATS/ERS classification. Onco Targets Ther. 2013;6:1597–1604. doi: 10.2147/OTT.S52353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura Y, Niki T, Goto A, Morikawa T, Miyazawa K, Nakajima J, Fukayama M. c-Met activation in lung adenocarcinoma tissues: an immunohistochemical analysis. Cancer Sci. 2007;98:1006–1013. doi: 10.1111/j.1349-7006.2007.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onitsuka T, Uramoto H, Nose N, Takenoyama M, Hanagiri T, Sugio K, Yasumoto K. Acquired resistance to gefitinib: the contribution of mechanisms other than the T790M, MET, and HGF status. Lung Cancer. 2010;68:198–203. doi: 10.1016/j.lungcan.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Ichimura E, Maeshima A, Nakajima T, Nakamura T. Expression of c-met/HGF receptor in human non-small cell lung carcinomas in vitro and in vivo and its prognostic significance. Jpn J Cancer Res. 1996;87:1063–1069. doi: 10.1111/j.1349-7006.1996.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, Hansen M, Schaefer E, Naoki K, Lader A, Richards W, Sugarbaker D, Husain AN, Christensen JG, Salgia R. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 33.Koga K, Hamasaki M, Kato F, Aoki M, Hayashi H, Iwasaki A, Kataoka H, Nabeshima K. Association of c-Met phosphorylation with micropapillary pattern and small cluster invasion in pT1-size lung adenocarcinoma. Lung Cancer. 2013;82:413–419. doi: 10.1016/j.lungcan.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, Park BJ, Rusch VW, Travis WD. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: Prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]


