Abstract
Non-small cell lung cancer (NSCLC) is a leading cause of cancer-related death and often has a poor prognosis. Investigation of NSCLC cancer cell migration, invasion and development of strategies to block this process is essential to improve the disease prognosis. In this study, we tested our hypothesis that Grb2-associated binder 2 (Gab2) regulate NSCLC cancer cell H1975 malignant biological behaviors, and silencing Gab2 reduced H1975 cellular colony forming ability, migration and invasion. Moreover, silenced cells present defects in phosphatidylinositol 3-kinase (PI3K)-serine/threonine kinase (Akt) signaling, and reduced expression/activity of matrix metallopeptidase (MMP)-2/9. Furthermore, in Gab2 siRNA-transfected cells, we detected a decrease in signal transducer and activator of transcription 3 (STAT3) phosphorylation and nuclear translocation. In vivo, Gab2 siRNA cells inoculated subcutaneously in nude mice demonstrated decreased tumor growth and PI3K-Akt signaling inhibition. These results indicate that Gab2 is a key factor in H1975 tumor migration, invasion, suggesting that Gab2 can be a novel therapeutic target in NSCLC.
Keywords: Gab2, invasion, STAT3, non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death worldwide and more than 80% of lung cancers are non-small cell lung carcinoma (NSCLC) [1]. This cancer has a very poor prognosis, principally because it is most commonly diagnosed during its later stages, and it is often characterized by aggressive local invasion, early metastasis and poor response to chemotherapy. In the last few decades, our understanding of the molecular biology of NSCLC metastasis has increased tremendously. Future, continue to identify the key molecules that control tumor deteriorate and development of novel treatments than can block or inhibit invasion and/or metastasis is important for improving the prognosis of NSCLC [2].
The Grb2-associated binder (Gab) proteins including mammalian Gab1, Gab2 and Gab3, comprise a family of scaffolding or docking adaptor proteins. Gab proteins are recruited to activated receptors by direct or indirect mechanisms, mostly indirectly via Gab2 [3]. Recent studies provide evidence that Gab2 plays a critical role in human cancer. Gab2 cooperates with receptor tyrosine kinases and promotes an invasive phenotype in breast tumorigenesis. Gab2 (-/-) breast cancer cells exhibited decreased migration and impaired extracellular signal-regulated kinases (Erk) activation, suggesting its role in promoting mammary tumor metastasis [4]. In addition to breast tumorigenesis, Gab2 confers an invasive phenotype and drives progression of primary melanoma cells and enhances their metastatic capability via activation of the PI3K-Akt pathway [5]. Activation of PI3K has been linked to mitogenesis, differentiation, survival, migration, invasion, and actin cytoskeletal reorganization. The PI3K-Akt pathway is a major regulator of STAT3, which can promote oncogenesis by being constitutively active through various pathways, and MMP-2/9 activity. Collectively, these results suggest that endogenous Gab2 regulates cell metastasis in a variety of cancers [6]. In contrast, many of the functions of Gab2 in NSCLC progression, invasion, and metastasis remain unclear.
In the present study, to investigate how Gab2 regulates NSCLC cancer migration and invasion, we examined effects of transient Gab2 silencing by RNA interference on NSCLC carcinoma cell line H1975. We found that silencing Gab2 decreased the H1975 cells migration and invasion abilities, and reduced the phosphorylated PI3K (p-PI3K), phosphorylated Akt (p-Akt) and STAT3 transcriptional activation. Furthermore, silencing of Gab2 decreased MMP-2/9 levels and activity in H1975. These effects are consequence of a down-regulation of PI3K-Akt signaling pathway, and eventually lead to an impairment of in vivo tumorigenesis.
Materials and methods
Cell culture and transfections
The human NSCLC lines (H1650, H1975, A549, HCC827, H1299) and SV40-immortalized non-tumorigenic human bronchial epithelial cells BEAS-2B used as control were obtained from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (CBTCCCAS, Shanghai, China). H1975 cells were transfected with Gab2 siRNA or control siRNA (Santa Cruz Biotechnology), respectively, using the Lipofectamine RNAi MAX reagent (Invitrogen), following the manufacturer’s instructions. After 48 h of transfection, the culture medium was replaced by RPMI-1640 Medium and was incubated for tests [7].
Cell proliferation assay
The cell viability was determined by CellTiter 96® Aqueous One Solution cell proliferation assay (Promega). H1975 cells (2.5 × 103 cells/well) transfected with either control- or Gab2-siRNA and were seeded in 96-well cell culture plates and treated with indicated agents. After incubation for indicated times, 20 μL of One Solution reagent were added to each well and incubation was continued for additional 4 h. The absorbance was measured at 490 nm using Synergy™ HT Multi-Mode Microplate Reader (Bio-Tek). The effect of siRNA Gab2 on cell viability was assessed as the percent of cell viability compared with untreated control cells, which were arbitrarily assigned 100% viability [8]. Experiment was performed in triplicate.
Lactate dehydrogenase (LDH) toxicity assay
The LDH released into cell cultures is an index of cytotoxicity and evaluation of the permeability of cell membrane. H1975 cells were transfected with Gab2 siRNA or control siRNA, respectively, and were seeded in 96-well plate at a density of 3 × 103 cells per well. Other two groups of H1975 cells were incubated with vehicle (0.1% DMSO, as untreated cells ) or 1% Triton X-100 (as positive group) for 24 h, and, all group cell supernatants were collected and analyzed for LDH activity using LDH cyto-toxicity assay kit from Keygen biotech [9].
Anchorage-independent cell growth in soft agar
After transfection, H1975 cells (5 × 104) suspended in 2 ml medium, 0.3% agar, and 10% FBS were plated over the bottom agar pre-solidified with 3 ml medium containing 0.6% agar and 10% FBS. Colony formation was then carried out by photographing through the microscope after 14 days [10]. Five random fields under microscope were taken and colony formation unities were counted.
Wound healing assay
We examined the migration of control- and Gab2-siRNA-transfected H1975 using a wound-healing assay. Briefly, the transfected cells were each grown on 3.5-cm plates with their respective culture media. After the growing cell layers had reached confluence, we inflicted a uniform wound (i.e., in a straight line) in each plate using a pipette tip, and washed the wounded layers with PBS to remove all cell debris. Then, we evaluated the closure at 48 h using bright-field microscopy (Nikon) [11]. All experiments were performed in triplicate.
Migration assay
H1975 motility was measured using 48-well BioCoat Cell Culture Inserts (BD Biosciences). Briefly, complete medium was placed in each lower chamber, which was separated from the upper chamber by a membrane with 8-μm pores. A single-cell suspension of H1975 cancer cells (5 × 104) in serum-free medium was placed in each upper chamber. After incubation for 24 h, the cells were fixed with 70% ethanol and stained with crystal violet [12]. Cell counting was then carried out by photographing the membrane through the microscope. Five random fields under microscope were taken.
Invasion assay
H1975 invasion assays were performed with Matrigel-coated chambers from a BioCoat Matrigel Invasion Chamber Kit (BD Biosciences) according to the manufacturer’s instructions. In brief, 1 × 105 control- and Gab2-siRNA-transfected H1975 with 500 µl in serum-free medium were added into the upper chamber and complete medium was added into the lower chamber. After incubation for 24 h, the non-invasive cells in the upper surface of the membrane were removed by “scrubbing” with cotton tipped swab and the cells invasion to the lower surface of the membrane were fixed and stained with DiO (3,3’-dioctadecyloxacarbocyanine perchlorate) for 20 min. Cell counting was then carried out by photographing the membrane through the microscope [12] and five random fields were taken.
Western blot analysis
All cells were harvested after indicated control- and Gab2-siRNA treatments. Whole-cell lysates were prepared with RIPA buffer containing protease and phosphatase inhibitors. Nuclear and cytoplasmic cell extracts were prepared using the NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo). Equal amounts of cell lysates (25 μg) were loaded on 10% SDS-PAGE and transferred onto PVDF membranes. After membranes were blocked, they were incubated with monoclonal antibody against Gab2 (1:1000, Santa Cruz), MMP-2 and MMP-9 (1:1000, CST), PI3k p85 and phosphor-PI3K p85 Tyr458 (1:10000, CST), STAT3 and phosphor-STAT3 Tyr705 (1:1000, CST), Akt and phosphor-Akt Thr308 (1:1000, CST), GPADH (1:5000, Bioworld Technology) and Lamin A/C (1:5000, Epitomics) followed by incubation with horseradish peroxidase-conjugated IgGs (1:10000, Bioworld Biotechnology). Target proteins were detected by the ECL system (Millipore) and visualized with the ChemiDoc XRS system (Bio-Rad) [12].
Matrix metalloprotein (MMP) activity assay
The activity of MMP-9 and MMP-2 were determined by QuickZyme MMPs activity assay (QucikZyme BioSciences) according to the manufacturer’s instructions [7]. Briefly, after transfection for 48 h, H1975 were washed with fresh medium and replaced with serum-free medium. After additional 24 h, the medium was collected and centrifuged at 10000 g for 10 min. Respective supernatant was added to the 96-well strip coated with MMP-9 antibody or MMP-2 antibody and incubated at 4°C overnight. After washing with wash buffer for 3 times, 50 µl assay buffer was added into the well, followed by adding 50 µl detection reagent. After incubation at 37°C for 1 h, OD405 was measured with Microplate Reader (Bio-Tek).
Real-time PCR
Total RNA was isolated using TRIzol according to the manufacturer’s instructions (Invitrogen, USA) and the concentration of total RNA was detected by spectrophotometry at OD260. Reverse transcription was carried out using superscript III reverse transcriptase (Invitrogen) as described in the manufacturer’s manual [13]. The real-time PCR was performed on ABI Prism 7500 Sequence detection system (Applied Biosystems) with the KAPA SYBR® qPCR Kit (KAPA Biosystems) according to the manufacturer’s instructions. The conditions for real-time PCR amplification were as follows: 2 min at 50°C, 1 min at 95°C, 40 cycles at 95°C for 3 s and 60°C for 1 min, followed by melting curve analysis at the end of each run from 60°C to 95°C. The primers used were as follows: Gab2 (Forward: 5’-ACAGTACCTACGACCTCCCC-3’, Reverse: 5’-CTGGGCGTCTTGAAGGTGTA-3’), β-actin (Forward: 5’-GCT CTT TTC CAG CCT TCCTT-3’, Reverse: 5’-TGA TCC ACA TCT GCT GGAAG-3’). The target mRNA level of control cells normalized to the level of β-actin mRNA, was defined as 1. Results were obtained from three independent experiments.
Immunocytochemistry
H1975 were seeded onto four-chamber slides, fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X for 20 min, and blocked with 3% BSA for 30 min. Subsequently, the cells were immunostained by incubating with rabbit monoclonal antibody against phosphor-STAT3 Tyr705 (1:500) overnight at 4°C. After being washed with PBS, cells were incubated with FITC-conjugated goat anti-rabbit secondary antibody (1:60, Boster Biotechnology). Nuclei were stained with 4’, 6-diamino-2-phenylindole (DAPI; Invitrogen) [14]. Images were taken and analyzed using the ZEN 2011 imaging software on a Zeiss invert microscope (CarlZeiss).
Subcutaneous xenograft models
After transfected with either control- or Gab2-siRNA, H1975 cells (3 × 106) were subcutaneously implanted into female, BALB/c nude mice to build non-small cell lung cancer xenograft. Tumor volume and mice body weight were measured every 3 days [15]. Tumor volume was calculated as mm3 =0.5 × length (mm)3 width (mm)2. After sacrificing mice on day 25, tumors tissues will be harvested for western blotting. Deparaffinized tumor sections were stained with specific antibodies including p-PI3K p85Tyr458 and p-AKTThr308. Detection was done with avidin-biotin-HRP complex (Thermo scientific) and diaminobenzidine as chromogen. Nuclei were counterstained with hematoxylin. p-PI3K p85Tyr458 and p-AKTThr308 positive cells were counted in five random high-power fields per section and were reported as a percentage of positive cells in each cellular compartment [12]. All animal experiments were carried out in compliance with the Guidelines for the Institute for Experimental Animals, Center of Hunan University Of Chinese Medicine.
Statistical analysis
The data were presented as mean ± SD. Differences in the results of two groups were evaluated using either two-tailed Student’s t test or one-way ANOVA followed by post-hoc Dunnett’s test. The differences with P < 0.05 were considered statistically significant.
Results
Gab2 silencing reduced clone formation of non-small cell lung cancer cell
To investigate the expression of Gab2, we performed RT-PCR and western blot analysis using five non-small cell lung cancer cell lines (H1650, H1975, A549, HCC827, H1299) and human bronchial normal epithelial cells BEAS-2B. Gab2 mRNA and protein expression status were up-regulated in all NSCLC cell lines, especially in H1975 cells compared with that in BEAS-2B (Figure 1A and 1B). To determine the temporal pattern of Gab2 gene silencing, we transfected H1975 with either Gab2- or control-siRNA for comparison. As shown in Figure 1C, Gab2 silencing achieved significantly knockdown at 48 h after transfection in H1975 cells. Then, we observed cell proliferation of Gab2 siRNA, control-siRNA transfected H1975 and untreated cells for 24 h and 48 h, respectively, and detected significantly growth inhibition in Gab2-siRNA-transfected cells (Figure 1D). To validate whether Gab2 siRNA would result in toxicity effects on H1975, LDH cytotoxicity assay was carried out. As shown in Figure 1E, 0.1% Triton X-100 significantly increased LDH release and Gab2 siRNA transfection resulted in little toxic effects on H1975 cells when compared to untreated control cells. In anchorage-independent cell growth assay, only untreated and control-siRNA transfected H1975 cells could form more colonies in soft agar, an in vitro hallmark of cell transformation. Strikingly, however, the ability of H1975 to form colonies was completely abrogated by Gab2 silencing (Figure 1F).
Figure 1.
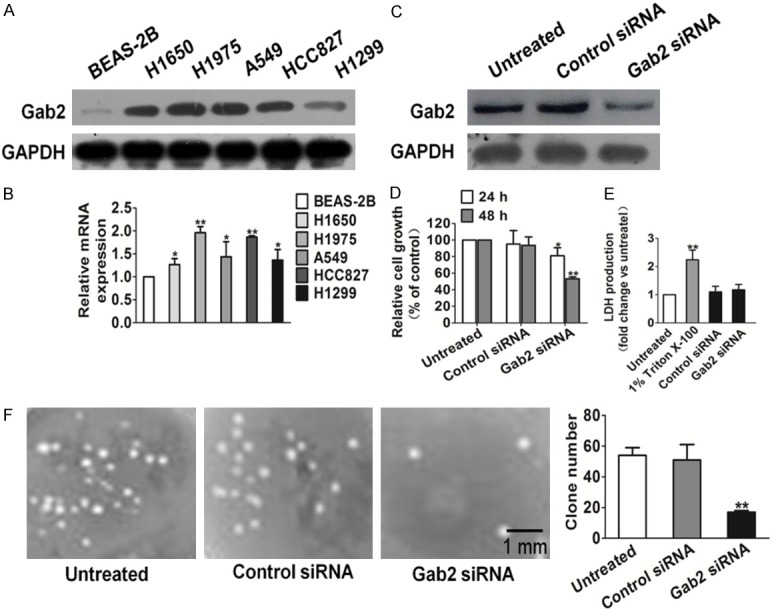
Effects of Gab2 silencing on H1975 cells clone formation. A. Gab2 protein expression in non-small cell lung cancer cell lines and human bronchial epithelial cells BEAS-2B. B. Gab2 mRNA level in five NSCLC cancer cell lines and BEAS-2B. (Data are presented as means ± SD, n = 3, *P < 0.05, ** P < 0.01 versus BEAS-2B). C. H1975 cells were transfected with either Gab2 siRNA or control-siRNA, or were left untreated. Gab2 expression levels were detected by western blotting. D. Proliferation assays indicated that Gab2-siRNA exerted apparent inhibition on H197 cells growth in vitro. E. Gab2 siRNA did not result in LDH release, indicating Gab2 siRNA and control siRNA brought little toxic effects on H1975. F. Clone formation assay indicated that Gab2 siRNA inhibited H1975 cell colony-forming effectively. Scale bars: 1 mm. (Data are presented as means ± SD, n = 6, **P < 0.01 versus untreated cells).
Gab2 silencing reduced migration and invasion of H1975
Cellular invasion, a characteristic of metastatic tumors, involves cell attachment to extracellular matrix (ECM), ECM degradation and cell migration. To determine if loss function of Gab2 affects cell migration, wound-healing assay was performed. Compared to untreated cells, disruption of Gab2 significantly reduced cell wound healing (Figure 2A). Then we performed Transwell assay to confirm the inhibitory effect of Gab2 silencing on cell migration. In contrast with untreated H1975, Gab2 silencing decreased the migration of H1975 (Figure 2B). Furthermore, we determined that Gab2 silencing affected cell invasion similarly to migration of the NSCLC cancer line. Consistently, there were less Gab2-silenced H1975 that invasive across the Matrigel-coated chamber compared to the control cells (Figure 2C).
Figure 2.
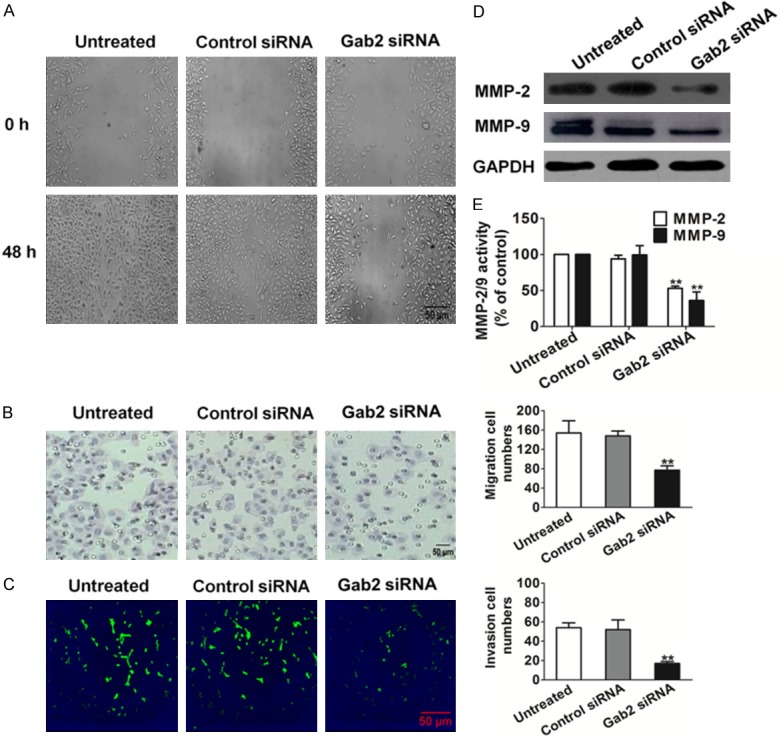
Effects of Gab2 silencing on H1975 cell motility. A. Representative examples of wounding experiment results from control and siRNA-Gab2 H1975 cells. Representative pictures of the wound distance were taken at each time point as indicated. Scale bars: 50 µm. B. H1975 motility was also estimated by Transwell assay. Representative pictures were taken after staining with crystal violet. Scale bars: 50 µm. C. H1975 cells that invaded through the pores to the lower surface of Matrigel-coated chambers were labeled with green a fluorescent probe DiOC18(3). Invaded cells transfected with control- or Gab2-siRNA were evaluated on the basis of the mean values from five fields for each group. Scale bars: 50 µm. D. Total cell lysates were isolated after control- or Gab2-siRNA transfection and MMP-2/9 expression levels were detected by western blotting. E. Quantification of MMP-2/9 activity. Bars are represented as the mean ± SD, n = 5, **P < 0.01 versus untreated cells.
Taking into account that MMPs such as MMP-2 and MMP-9 can be involved in the development of several human malignancies, as degradation of collagen IV in basement membrane and extracellular matrix facilitates tumor progression, including invasion, metastasis, and angiogenesis, we analyzed their levels and activity. A significant drop in MMP-2 and MMP-9 expression levels were observed in Gab2 silenced cells (Figure 2D). Quantification of MMP-2 and MMP-9 activities using a fluorogenic assay showed a significantly decrease in extracellular MMP-2 and MMP-9 activity in H1975 Gab2 siRNA cells compared to control-siRNA or untreated cells (Figure 2E). In summary, disruption of Gab2 led to reduced cell migration and significantly impaired the invasion of H1975 cells.
Gab2 silencing induced degradation of STAT3 and suppressed phosphorylation of both PI3K and Akt
It has been reported that Gab2 plays a role in breast, colon, and gastric cancer cells by interacting with PI3K/Akt. Accordingly, we investigated the signaling pathway that was mediated by Gab2 and that was associated with cell motility. Specifically, we first confirmed whether Gab2 modulates PI3K/Akt phosphorylation in H1975 cells by western blotting. We found that the expression levels of both proteins remained unchanged after silencing Gab2, whereas the phosphorylated forms of PI3K and Akt were strongly reduced by this transfection (Figure 3A).
Figure 3.
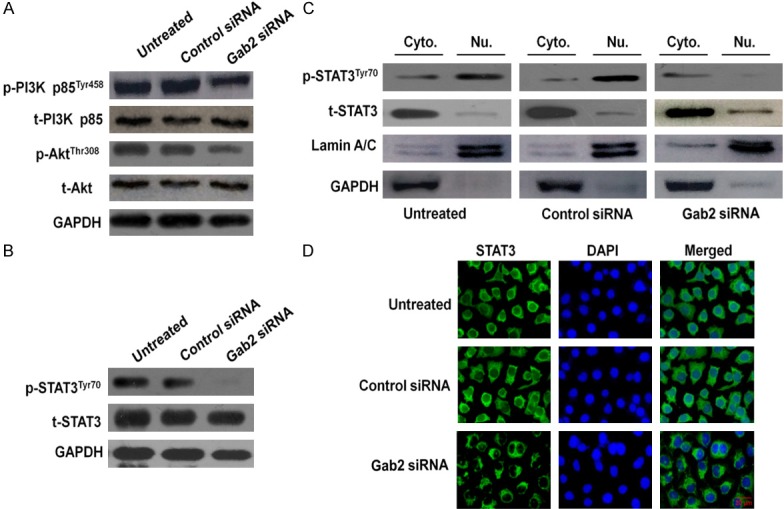
Effects of Gab2 silencing on the PI3K/Akt pathway. A. Expression of PI3K and Akt in Gab2-silenced cells. After transfection of cells with either control- or Gab2-siRNA for 48 h (or cells were left untreated), the total cells were isolated and PI3K/Akt expression levels were detected by western blotting. GAPDH was used as a loading control. B. H1975 cells transfected using the same conditions as described above and total protein was extracted, the total and phosphorylated (p) STAT3 were detected. C. After Gab2 silencing, cytoplasmic (Cyto.) and nuclear (Nu.) extracts were prepared and then subjected to western blot for measuring protein level of phosphor-STAT3Tyr705 and total-STAT3 respectively. D. H1975 cells were transfected, then cells were fixed and incubated with primary antibody against STAT3. H1975 cells were immunostained with anti-rabbit FITC-conjugated secondary antibody and then stained with DAPI. The specimens were visualized and photographed using a fluorescence microscope. Scale bars: 20 µm. Blue depicts the nucleus and green depicts localization of STAT3.
To further investigate the signaling pathway that mediated STAT3 expression, we assessed the expression of total and phosphorylated STAT3 in cells transfected with either control-or Gab2-siRNA by western blotting. We found that the STAT3 total expression level remained unchanged after Gab2 silencing, whereas STAT3 phosphorylation was inhibited by this transfection (Figure 3B).
Additionally, the activity of STAT3 was also regulated by subcellular localization; we therefore attempted to explore the effect of Gab2-siRNA in different cell fractions. The experiment showed that the nuclear STAT3 phosphorylation in H1975 cells was evidently abrogated by Gab2-siRNA (Figure 3C). We also examined the subcellular distribution of STAT3 in both control- and Gab2-siRNA-transfected H1975 cells by immunofluorescence staining and confocal microscopy. As illustrated in Figure 3D, treatment with Gab2-siRNA hardly decreased broad nuclear translocation of STAT3, while untreated and control-siRNA transfection rendered STAT3 stability and general nuclear distribution.
Gab2 siRNA impaired the growth of H1975 in vivo
To further investigate the role of Gab2 in tumoral growth in vivo, Gab2-siRNA, control-siRNA, and un-transfected H1975 cells were injected subcutaneously into the backs of female nude mice, respectively (6 mice in each group), and tumor growth was monitored for 25 days (Figure 4A). According to our in vitro findings, the mean tumoral volume (Figure 4B) and weight (Figure 4C) of the Gab2-siRNA group was significantly smaller compared to the control-siRNA and untreated. Furthermore, there was no significant difference in weight between control group and Gab2-siRNA treated groups (Figure 4D). Gab2 expression was checked on tumor tissue after H175 injection into nude mice to confirm that Gab2 remained silenced (Figure 4E). Immunohistochemistry staining (IHC) of p-PI3K p85Tyr458 and p-AktThr308 with tumoral tissues in vivo clearly showed decreased immunostaining for PI3K and Akt activity in the Gab2-siRNA derived tumors compared with control-siRNA transfected H1975 injected mice (Figure 4F). In addition, Gab2-siRNA also resulted in down-regulation of PI3K-Akt downstream molecules including MMP-2 and MMP-9 (Figure 4G). At the same time, we observed a significant decrease in the phosphorylation of STAT3 in the mice injected Gab2-siRNA H1975 (Figure 4G), which was consistent with the results of H1975 cells in vitro.
Figure 4.
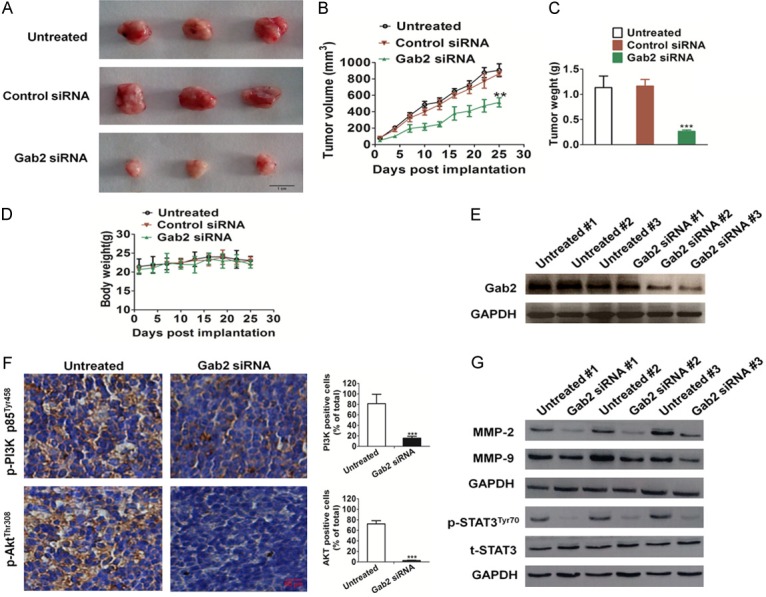
In vivo tumorigenicity of Gab2-siRNA H1975 and untreated H1975 cells. (A) Representative pictures of tumor masses from mice were taken after 25 days of implanted with H1975 cells or with siRNA-Gab2 H1975 cells. Scale bars: 1 cm (B). Gab2 silencing resulted in significantly H1975 tumor growth inhibition versus mice injected untreated H1975 cells. (C) After surgical excision, significant differences were also evident in tumor weight. (D) There was no significant difference in body weight in the three groups. (E) Total tumor tissue lysates were isolated and Gab2 expression levels were detected by western blotting. #, representatives mice number. (F) Tumor tissues were prepared for immunohistochemistry detection with antibodies against p-PI3K p85Tyr458 and p-AKTThr308, and the p-PI3K p85Tyr458 and p-AKTThr308 positive cells were statistical analyzed. Scale bars: 50 µm (G) Shows western blot of MMP-2/MMP-9 expression and phosphorylated STAT3 in tumor tissues. (Bars are represented as the mean ± SD, n = 6, **P < 0.01, ***P < 0.001 versus mice injected untreated H1975 cells).
Discussion
Gab2, a member of the family of Gab scaffolding adaptors, is frequently over-expression in human primary tumors and transmits, amplifies the signals from receptor tyrosine kinases [16]. Great deals of research demonstrate that Gab2 is over-expressed in ovarian, non-small cell lung cancer, gastric cancer and acute myeloid leukemia, and Gab2 is an oncogenic protein [17]. The Gab2 is increasingly implicated in PI3K/Akt signaling and Gab2 can stimulate Akt signaling through its interactions with p85 subunit of PI3K [18]. Through stimulate PI3K signaling, Gab2 contribute to the initiation of several signaling pathways promoting tumor cellular growth, migration, invasion, and metastasis [19]. However, the role of Gab2 in non-small cell lung cancer has not been reported.
In this work, we firstly reported a novel role for Gab2 in the migration and invasion of non-small cell lung cancer cells. We found that the tumor colony forming units of H1975 was significantly inhibited, when the Gab2 gene was disrupted by siRNA. Further investigation with gene silencing of Gab2 showed that the migration and invasion of H1975 was significantly decreased as revealed by the wound healing assay and Transell assay. Here, we also examined the key factors and signaling pathways that are mediated by Gab2 and that are associated with H1975 cancer cell motility. In this study we examined whether Gab2 silencing affected MMP-2/9 expression in H1975. We found that Gab2-siRNA-transfected cells from H1975 had drastically reduced MMP-2/9 levels and activity compared with corresponding control cells.
Gab2 undergoes tyrosine phosphorylation, creating a number of docking sites to mediate interactions with the p85 subunit of PI3K [5]. The interaction of Gab2 with p85 subunit of PI3K is crucial in mediating the PI3K-Akt signaling. In this study, using western blotting experiment, we determined that the levels of p-Akt and p-PI3K decreased in siRNA-Gab2 H1975. It has been demonstrated that phosphorylated Akt activates its transcription factor STAT3, thereby activates specific target genes, including those that encode c-Myc, cyclin D1, and MMP-2/9 [20]. STAT3, which acts as transcription activator, is reported plays a role in cell, proliferation, invasion, angiogenesis, and survival. Therefore, we performed immunofluorescence and western blot from control- and Gab2-siRNA-transfected H1975 cells, and found that STAT3 phosphorylation was significantly decreased in Gab2-silenced cells.
More convincingly, Gab2 silencing markedly impaired the growth of H1975 cells in vivo. Histological studies of the tumor sections revealed that Gab2 silencing also significantly reduced PI3K/Akt activity. Meanwhile, western blot results show that Gab2 silencing could obviously attenuate expressions of MMP-2/9 expression in tumor tissue. All these data presented that Gab2 is involved in the growth of NSCLC cancer cell H1975. Due to the important roles of Gab2 in the growth and migration of NSCLC H1975 cells, it may serve as an attractive target for molecular targeting cancer therapy.
Disclosure of conflict of interest
None.
References
- 1.Shtivelman E, Hensing T, Simon GR. Molecular pathways and therapeutic targets in lung cancer. Oncotarget. 2014;5:1392–1433. doi: 10.18632/oncotarget.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forde PM, Ettinger DS. Targeted therapy for non-small-cell lung cancer: past, present and future. Expert Rev Anticancer Ther. 2013;13:745–758. doi: 10.1586/era.13.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi L, Sun X, Zhang J, Zhao C, Li H, Liu Z, Fang C, Wang X, Zhao C, Zhang X, Zhou F, Lu S, Luo R, Zhang B. Gab2 expression in glioma and its implications for tumor invasion. Acta Oncol. 2013;52:1739–1750. doi: 10.3109/0284186X.2012.750032. [DOI] [PubMed] [Google Scholar]
- 4.Fleuren ED, O’Toole S, Millar EK. Overexpression of the oncogenic signal transducer Gab2 occurs early in breast cancer development. Int J Cancer. 2010;127:1486–1492. doi: 10.1002/ijc.25172. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Sheng Q, Spillman MA. Gab2 regulates the migratory behaviors and E-cadherin expression via activation of the PI3K pathway in ovarian cancer cells. Oncogene. 2012;31:2512–2520. doi: 10.1038/onc.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz-Padilla C, Gallego-Ortega D, Browne BC. Functional characterization of cancer-associated Gab1 mutations. Oncogene. 2013;32:2696–2702. doi: 10.1038/onc.2012.271. [DOI] [PubMed] [Google Scholar]
- 7.Santiago-Gómeza A, Barrasaa JI, Olmoa N, Lecona E, Burghardt H, Palacín M, Lizarbe MA, Turnay J. 4F2hc-silencing impairs tumorigenicity of HeLa cells via modulation of galectin-3 and β-catenin signaling, and MMP-2 expression. Biochim Biophys Acta. 2013;1833:2045–2056. doi: 10.1016/j.bbamcr.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Shimura T, Yajima T. Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of β-catenin. Int J Cancer. 2011;129:2775–2786. doi: 10.1002/ijc.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youm I, Yang XY, Murowchick JB, Youan BB. Encapsulation of docetaxel in oily core polyester nanocapsules intended for breast cancer therapy. Nanoscale Res Lett. 2011;6:630. doi: 10.1186/1556-276X-6-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim A, Im M, Yim NH, Ma JY. Reduction of metastatic and angiogenic potency of malignant cancer by Eupatorium fortunei via suppression of MMP-9 activity and VEGF production. Sci Rep. 2014;4:6994. doi: 10.1038/srep06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu K, Pan Q, Jia LQ, Dai Z, Ke AW, Zeng HY, Tang ZY, Fan J, Zhou J. MiR-302c inhibits tumor growth of hepatocellular carcinoma by suppressing the endothelial-mesenchymal transition of endothelial cells. Sci Rep. 2014;4:5524. doi: 10.1038/srep05524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y, Zhang T, Li J, Deng H, Song Y, Zhai D, Peng Y, Lu X, Liu M, Zhao Y, Yi Z. Oridonin Inhibits Tumor Growth and Metastasis through Anti-Angiogenesis by Blocking the Notch Signaling. PLoS One. 2014;9:e113830. doi: 10.1371/journal.pone.0113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Ni Z, Xu X, Xiao J. MiR-32 Functions as a Tumor Suppressor and Directly Targets EZH2 in Human Oral Squamous Cell Carcinoma. Med Sci Monit. 2014;20:2527–2535. doi: 10.12659/MSM.892636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Ren Y, Liu A, Jin R, Jiang Q, Huang Y, Kong L, Wang X, Zhang L. WP1066 Sensitizes Oral Squamous Cell Carcinoma Cells to Cisplatin by Targeting STAT3/miR-21 axis. Sci Rep. 2014;4:7461. doi: 10.1038/srep07461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aftab BT, Dobromilskaya I, Liu JO, Rudin CM. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011;71:6764–6772. doi: 10.1158/0008-5472.CAN-11-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wöhrle FU, Halbach S, Aumann K, Schwemmers S, Braun S, Auberger P, Schramek D, Penninger JM, Laßmann S, Werner M, Waller CF, Pahl HL, Zeiser R, Daly RJ, Brummer T. Gab2 signaling in chronic myeloid leukemia cells confers resistance to multiple Bcr-Abl inhibitors. Leukemia. 2013;27:118–129. doi: 10.1038/leu.2012.222. [DOI] [PubMed] [Google Scholar]
- 17.Hoeben A, Martin D, Clement PM, Cools J, Gutkind JS. Role of GRB2-associated binder 1 in epidermal growth factor receptor-induced signaling in head and neck squamous cell carcinoma. Int J Cancer. 2013;132:1042–1050. doi: 10.1002/ijc.27763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyga R, Pecquet C, Harir N, Gu H, Dhennin-Duthille I, Régnier A, Gouilleux-Gruart V, Lassoued K, Gouilleux F. Activated STAT5 proteins induce activation of the PI3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. Biochem J. 2005;390:359–366. doi: 10.1042/BJ20041523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samanta AK, Lin H. Bcr-Abl regulates c-Myc expression in chronic myelogenous leukemia through a Bcr-Abl/Jak2 network of proteins involving Gab2, PI-3 kinase, NF-κB and GSK3β. Cancer Res. 2006;66:968. [Google Scholar]
- 20.Matsumura T, Sugimachi K, Takahashi Y, Uchi R, Sawada G, Ueda M, Hirata H, Sakimura S, Ueo H, Takano Y, Kurashige J, Shinden Y, Eguchi H, Sudo T, Yamamoto H, Doki Y, Mori M, Mimori K. Clinical Significance of GAB2, a Scaffolding/Docking Protein Acting Downstream of EGFR in Human Colorectal Cancer. Ann Surg Oncol. 2014;21(Suppl 4):S743–9. doi: 10.1245/s10434-014-3889-x. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Han S, Sun Y. An IL6-STAT3 loop mediates resistance to PI3K inhibitors by inducing epithelial-mesenchymal transition and cancer stem cell expansion in human breast cancer cells. Biochem Biophys Res Commun. 2014;453:582–587. doi: 10.1016/j.bbrc.2014.09.129. [DOI] [PubMed] [Google Scholar]


