Abstract
Autophagy plays a complicated role in tumorigenesis in a variety of cancers. Recently, many studies report that some autophagy-related markers could be detected in several types of human tumors. However, fewer studies have been conducted to evaluate the relationship between autophagy and lung cancer, especially in non-small cell lung cancer (NSCLC). In this study, the expression levels of autophagy-related markers Beclin 1 and p62 were detected by Western blot analysis and cell immunofluorescence staining in three human NSCLC cell lines A549, H1299 and HCC827. Then, tissue microarray and immunohistochemical staining were used to determine Beclin 1 and p62 expression in 104 NSCLC specimens collected from patients. Beclin 1 and p62 were observed to primarily distribute in the cytoplasm of the cells. Beclin 1 was expressed more predominantly in male and heavy-smoker and its expression was significantly correlated with the differentiation and lymph node metastasis. p62 expression was negatively correlated with TNM stage and lymph node metastasis. Univariate Cox regression analysis revealed that low expression of Beclin 1 and high expression of p62 were significantly associated with shorter survival. Meanwhile, multivariate Cox regression analysis indicated that Beclin 1 and p62 were independent risk factors related to overall survival for patients with NSCLC. Collectively, our study suggests that Beclin 1 and p62 could serve as potential indicators for the prognosis of patients with NSCLC.
Keywords: Beclin 1, p62, non-small cell lung cancer, prognosis, autophagy
Introduction
Lung cancer is one of the leading causes of cancer-related mortality. Non-small cell lung cancer (NSCLC) accounts for 75-80% of all lung cancer [1]. Despite significant advances in medication and surgery, the prognosis of lung cancer is still poor. Acquired resistance often leads to transient responses and eventual disease progression, and the 5-year survival rate in patients with NSCLC remains only 15% [2]. Therefore, it is very important for the early diagnosis and accurate evaluation of the prognosis of NSCLC. However, there are very few biological markers available to predict recurrence after surgery and assess the need for adjuvant treatment.
Autophagy refers to basic homeostatic and catabolic process that delivers cytoplasmic components to the lysosomes for subsequent degradation or recycles long-lived proteins and organelles in order to sustain cellular metabolism. More than 30 autophagy specific genes (Atg) have been identified to regulate autophagy. Beclin 1, a key indicator and regulator of autophagy, together with Atg14/barkor and p150/Vps15, constitute Vps34, which is required for autophagy-regulating macromolecular complex (PI3K complex) [3]. Studies have shown that different Beclin 1 binding proteins have contradictory functions. The binding of ambra-1, UVRAG, and bif-1 can induce autophagy, while the binding of Beclin 1 to Bcl-2 or Bcl-XL leads to the inhibition of autophagy [4]. The aberrant expression of Beclin 1 has been found in many human malignancies. The expression of Beclin 1 was significantly lower in NSCLC tissues compared with the adjacent normal tissues, and negatively associated with tumor recurrence rate [5]. Furthermore, knockdown of Beclin 1 in the mice resulted in the inhibition of autophagy and subsequent development of spontaneous tumors, including lymphoma, liver and lung cancer. Previous studies have shown that Beclin 1 may be a predictive factor of favorable prognosis in gastric and breast cancers [6]. p62 is another selective substrate for autophagy, in contrast to Beclin 1, it always accumulates when autophagy is decreased. p62 deficiency leads to increased reactive oxygen species (ROS) levels, enhanced cell death and reduced tumorigenicity of Ras [7]. In addition, p62 can inhibit Fas/Cav-1 complex formation which accounts for the cleavage of Beclin 1, releasing a C-terminal Beclin 1 peptide that is translocated to the mitochondria and initiates apoptosis [8]. Previous studies have reported that p62 expression was increased in many diseases including metabolic diseases and tumors. However, the clinical significance and prognostic value of p62 in NSCLC remain unclear.
To the best of our knowledge, few studies have investigated Beclin 1 and p62 expression concurrently in NSCLC and estimated their associations with clinical characteristics. In the current study, we examined the expression of Beclin 1 and p62 in NSCLC and evaluated their potential diagnostic or prognostic value in NSCLC.
Materials and methods
Patients and tissue samples
In this study, a total of 128 NSCLC specimens were collected from patients who accepted surgical resection at Department of Chest Surgery, the Second Affiliated Hospital of Soohow University from October 2006 to December 2009. All patients in this study received no chemotherapy, radiotherapy or adjuvant treatment before surgery. We collected the patient-specific data, including age, gender, smoking index (multiply the number of cigarettes smoked per day by years of smoking), history of COPD, histological type, differentiation, lymphoid node metastasis, TNM stage and status of CEA. The TNM stage and histological type were assessed according to Tumor-Node-Metastasis (TNM) system of cancer staging by WHO and the WHO classification of lung carcinomas [9]. COPD was estimated by pulmonary function tests. After surgery, the patients with positive lymphoid node metastasis accepted four to six cycles of first-line standard chemotherapies. The adjuvant radiotherapy after surgery was not used. The patients were followed up by telephone contact and consulting their case documents. The patients lost to follow-up were not included in our study. Totally 104 patients were selected based on the inclusion criteria, including 70 men and 34 women. All their clinicopathologic data were summarized in Table 1. The survival data were updated to December 2014. The mean postoperative follow-up time was 48.5 months (range from 3 to 96.5 months). This study was approved by the Ethics committee of Soohow University and informed consent was provided from all patients before surgery.
Table 1.
Correlation of Beclin 1 and p62 expression with clinicopathologic variables in 104 NSCLC patients
| Characteristics | Total | Beclin 1 | P | p62 | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| High | Low | High | Low | ||||
| All cases | 104 | 44 | 60 | 51 | 53 | ||
| Gender | |||||||
| Male | 70 | 36 | 34 | 32 | 38 | ||
| Female | 34 | 8 | 26 | 0.007* | 19 | 15 | 0.331 |
| Age (years) | |||||||
| <60 | 36 | 17 | 19 | 14 | 22 | ||
| ≥60 | 68 | 27 | 41 | 0.460 | 37 | 31 | 0.132 |
| Smoking indexa | |||||||
| <400 | 48 | 15 | 33 | 22 | 26 | ||
| ≥400 | 56 | 29 | 27 | 0.035* | 29 | 27 | 0.545 |
| History of COPD | |||||||
| Yes | 46 | 15 | 31 | 26 | 20 | ||
| No | 58 | 29 | 29 | 0.075 | 25 | 33 | 0.174 |
| Histological type | |||||||
| Squamous | 54 | 20 | 34 | 29 | 25 | ||
| Adenocarcinoma | 39 | 18 | 21 | 18 | 21 | ||
| Other | 11 | 6 | 5 | 0.443 | 4 | 7 | 0.520 |
| Differentiation | |||||||
| Well | 15 | 10 | 5 | 9 | 6 | ||
| Moderately | 52 | 25 | 27 | 24 | 28 | ||
| Poorly | 37 | 9 | 28 | 0.010* | 18 | 19 | 0.639 |
| TNM stage | |||||||
| I | 36 | 20 | 16 | 11 | 25 | ||
| II | 21 | 7 | 14 | 10 | 11 | ||
| III | 28 | 9 | 19 | 18 | 10 | ||
| IV | 19 | 8 | 11 | 0.215 | 12 | 7 | 0.029* |
| Lymph node metastasis | |||||||
| Yes | 46 | 10 | 36 | 29 | 17 | ||
| No | 58 | 34 | 24 | 0.000* | 22 | 36 | 0.011* |
| CEA (ng/ml) | |||||||
| ≤5 | 49 | 23 | 26 | 22 | 27 | ||
| >5 | 55 | 21 | 34 | 0.367 | 29 | 26 | 0.425 |
Sort by 400 cigarettes/years.
P<0.05.
Cell culture
Three human NSCLC cell lines A549, H1299 and HCC827, were cultured to exponential phase in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (Gibco, USA). The cells were collected after 24-h incubation.
Western blot analysis
Cells were lysed in radio-immunoprecipitation assay (RIPA) buffer and the lysates were collected after high speed centrifugation. The protein concentration was determined by a BCA kit (Biorad, USA), and the proteins were isolated by 12% Sodium dodecyl sulfate-polycrylamide gel (SDS-PAGE) electrophoresis and transferred to 0.2 μm polyvinylidene fluoride (PVDF) membrane (Millipore, USA). The membrane was blocked overnight in 5% skim milk with Tris-buffered saline Tween-20 (TBST), then incubated with antibody for beclin1 (1:500, Cell Signal Technology, USA), p62 (1:1000, Abcam, USA), or β-actin (1:10000, Sigma-Aldrich, USA) overnight at 4°C. Next, the membrane was incubated with secondary antibodies (1:5000, Santa Cruz, USA) at room temperature for 60 min. The protein bands were observed on X films after incunation with ECL reagents (Millipore, USA).
Immunofluorescence staining
NSCLC cells were seeded into 24-well plates at 1×105 cells/well. The next day the cells were fixed by 4% formaldehyde with phosphate buffer saline (PBS) for 30 min, and blocked by 1% bovine serum albumin (BSA) with PBST (0.1% Triton X-100 in PBS) for 60 min at room temperature. The cells were then incubated overnight with rabbit anti-Beclin 1 (1:150, Cell Signal Technology, USA) and anti-p62 antibodies (1:200, Abcam, USA), and incubated with the Alexa Fluor 488 tagged anti-rabbit IgG (1:500, Abcam, USA) at room temperature for 1 h in the dark. 4’6-diamidino-2-phenylindole dihydrochloride (DAPI) was used to stain the nuclei for 10 min. The cells were observed under the inverted fluorescence microscope (Observer A1, Zeiss, Germany).
Tissue microarray and immunohistochemical staining
All the NSCLC tissues were formalin-fixed and paraffin embedded. Under the guidance of hematoxylin and eosin-stained slides, representative core tissue biopsies were selected and marked. With biopsy needles, 3 mm-in-diameter tissue cores were punched from the selected tissue areas and placed on a recipient block. One core per case was arranged. Then immunohistochemistry was performed on the tissue microarray (TMA) of NSCLC using streptavidin-biotin-peroxidase complex method. Briefly, after deparaffinization and rehydration, antigen retrieval was accomplished by using the boiling citric acid buffer (pH=6.0) in a water bath for 20 min. Then 3% hydrogen peroxide was used to block endogenous peroxidase, and 5% bovine serum albumin (BSA) was used to prevent non-specific straining. The slides were then incubated with rabbit anti-beclin1 (1:80, Cell Signal Technology, USA) and anti-p62 antibody (1:200, Abcam, USA) overnight at 4°C. The slides were incubated with biotin-labeled anti-rabbit secondary antibodies at room temperature for 30 min, and detected with 3,3-diaminobenzidine (DAB). After counterstaining with hematoxylin, the slides were dehydrated and mounted with coverslips. For negative controls PBS was used to replace primary antibody.
Evaluation of immunohistochemical staining
All slides were evaluated by two pathologists who had no prior knowledge of the patients’ clinicopathological information. The pathologists gave scores and then come to consensus after discussion. The staining intensity and extent of Beclin 1 and p62 were scored as follows. Staining intensity was defined as the depth of shade of the protein and graded as 0 (no staining), 1 (mild staining), 2 (moderate staining), 3 (strong staining). Staining extent was defined as the percentage of tumor cells with positive brown-red staining and graded as follows: 0 (<10%), 1 (10%-49%), 2 (≥50%). Then sum index of the two variables was obtained and a total score of 4 was considered as the point to categorize the staining as high (≥4) or low (<4) [10].
Statistical analysis
All data analysis was conducted using SPSS version 17.0 (SPSS, Inc, USA). A chi square test or Fisher’s exact test were used to analyze the relationship of Beclin 1 and p62 expression with clinicopathological variables. These tests were also used to evaluate whether there was any significant relationship between Beclin 1 and p62. Kaplan-Meier survival curves were constructed to estimate the association between Beclin 1 and p62 expression and the patients’ overall survival (OS). Moreover, statistical significance was checked using the log-rank test. The univariate and multivariate Cox proportional hazards models were used to calculate the hazard ratios and 95% confidence intervals for patients’ outcome. P value of less than 0.05 was considered statistically significant.
Results
Beclin 1 and p62 expression in NSCLC cell lines
Western blot analysis showed similar expression levels of Beclin 1 and p62 in all three NSCLC cell lines (Figure 1A). Immunofluorescence staining demonstrated that Beclin 1 and p62 were primarily distributed in the cytoplasm of the cells, and that some perinuclear membrane staining was also detected (Figure 1B).
Figure 1.
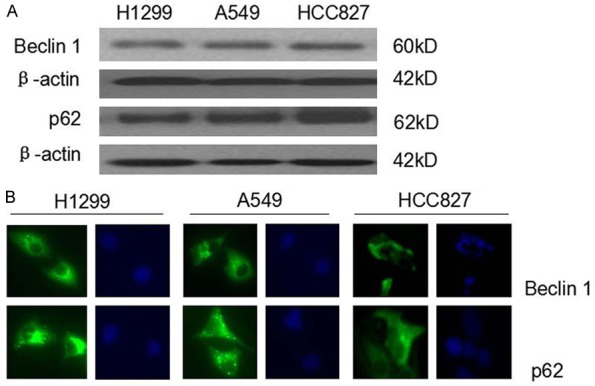
The expression of Beclin 1 and p62 in NSCLC cell lines. A. Western blot analysis of Beclin 1 and p62 in H1299, A549 and HCC827 cells. B. Immunofluorescence staining of Beclin 1 and p62 in H1299, A549 and HCC827 cells. The bright green fluorescence indicated positive expression of Beclin 1 and p62. The blue indicated the nuclei stained with DAPI under the same field (×400).
Correlations of Beclin 1 and p62 expression with clinicopathologic characteristics
Immunohistochemical staining was conducted to evaluate the expression of Beclin 1 and p62 in NSCLC tissues. Immunoreactivity of Beclin 1 and p62 was detected mainly in the cytoplasm (Figure 2). Although some nuclear staining was found, we only examined cytoplasmic expression of Beclin 1 and p62 with the clinical features.
Figure 2.
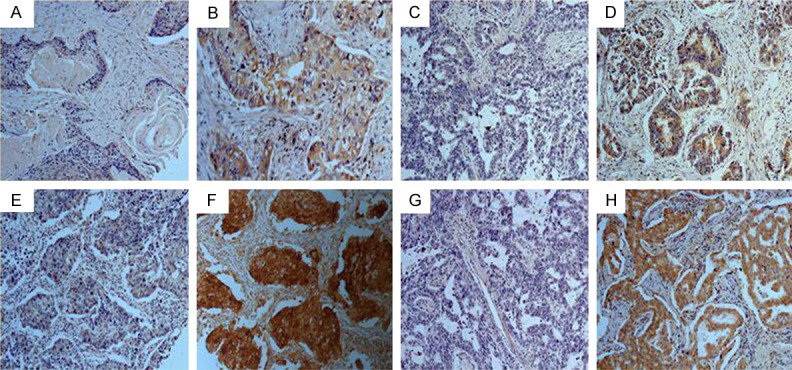
Representative immunohistochemical staining of Beclin 1 and p62 in NSCLC tissues. Beclin 1 low expression cases (A and C); Beclin 1 high expression cases (B and D); p62 low expression cases (E and G); p62 high expression cases (F and H); Squamous cell carcinoma (A, B, E, F); Adenocarcinoma (C, D, G, H). Original magnification ×200.
As shown in Table 1, high expression levels of Beclin 1 and p62 were detected in 42.31% (44 of 104) and 49.03% (51 of 104) of NSCLC tissues, respectively. Furthermore, high expression of Beclin 1 was primarily detected among male patients (P=0.007) and the patients with a smoking index greater than 400 (P=0.035). Besides, Beclin 1 expression was correlated significantly with the differentiation (P=0.01) and lymph node metastasis (P=0.000), while p62 expression was correlated significantly with TNM stage (P=0.029) and lymph node metastasis (P=0.011). There was no correlation between Beclin 1 and p62 expression with other clinicopathological variables. In addition, to evaluate the relationship between Beclin 1 and p62, we investigate their expression using chi square test. As listed in Table 2, Beclin 1 expression was negatively correlated with p62 (P=0.001).
Table 2.
Relationship between the expression of Beclin 1 and p62
| Beclin 1 expression | p62 expression | P value | |
|---|---|---|---|
|
|
|||
| High | Low | ||
| High | 13 | 31 | 0.001* |
| Low | 38 | 22 | |
P<0.05.
Prognostic factors determined by univariate and multivariate Cox regression analysis
Next we performed univariate and multivariate Cox proportional hazard regression analysis on the main factors related to patient overall survival. Univariate Cox regression analysis revealed that low expression of Beclin 1 and high expression of p62 were significantly associated with shorter survival (all P<0.001) (Table 3; Figure 3A, 3B). The median survival for patients with high expression of Beclin 1 and p62 was 82.50 months and 20.30 months, while it was 31.75 months and 86.30 months for patients with low expression of Beclin 1 and p62. Then we examined the relation of the combination of Beclin 1 and p62 expression status with the patients’ clinical outcome. We found that Beclin 1 high/p62 low cases had longer survival than other cases (P<0.0001, Figure 4). Furthermore, history of COPD and high TNM stage were related to inferior OS (P=0.014, P<0.001, P<0.001, respectively, Table 3). There was no correlation between other variables and patient clinical outcome.
Table 3.
Univariate Cox proportional hazard regression analysis for clinical outcome in 104 NSCLC patients
| Characteristics | For death | P value |
|---|---|---|
|
| ||
| Hazard Ratio (95% confidence interval) | ||
| Gender (Male VS. Female) | 1.078 (0.652-1.782) | 0.769 |
| Age (years) (<60 VS. ≥60) | 0.713 (0.432-1.178) | 0.187 |
| Smoking index (<400 VS. ≥400) | 0.913 (0.573-1.454) | 0.702 |
| History of COPD (No VS. Yes) | 0.557 (0.350-0.888) | 0.014* |
| Histological type | ||
| Squamous | 1.732 (0.733-4.093) | 0.211 |
| Adenocarcinoma | 1.396 (0.574-3.397) | 0.462 |
| Other | 1 | |
| Differentiation | ||
| Well | 0.655 (0.307-1.394) | 0.272 |
| Moderately | 0.873 (0.530-1.439) | 0.596 |
| Poorly | 1 | |
| TNM stage | ||
| I | 0.230 (0.114-0.465) | 0.000* |
| II | 0.468 (0.230-0.952) | 0.036* |
| III | 0.907 (0.484-1.701) | 0.761 |
| IV | 1 | |
| CEA (ng/ml) (≤5 VS. >5) | 0.924 (0.581-1.469) | 0.738 |
| Beclin 1 (Low VS. High) | 2.854 (1.715-4.750) | 0.000* |
| p62 (Low VS. High) | 0.161 (0.096-0.272) | 0.000* |
P<0.05.
Figure 3.
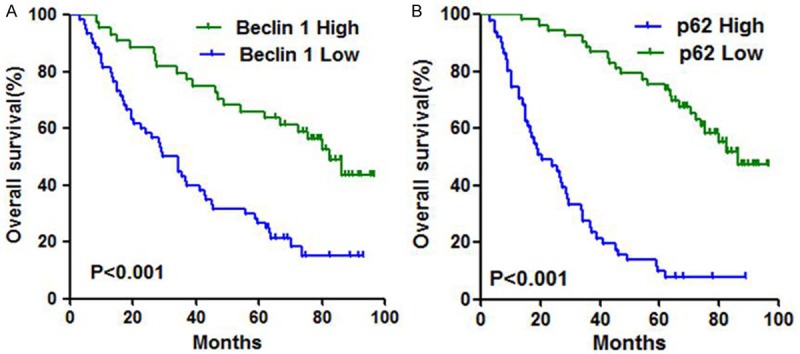
Kaplan-Meier survival curves showing high VS. Low expression of Beclin 1 (A) and p62 (B).
Figure 4.
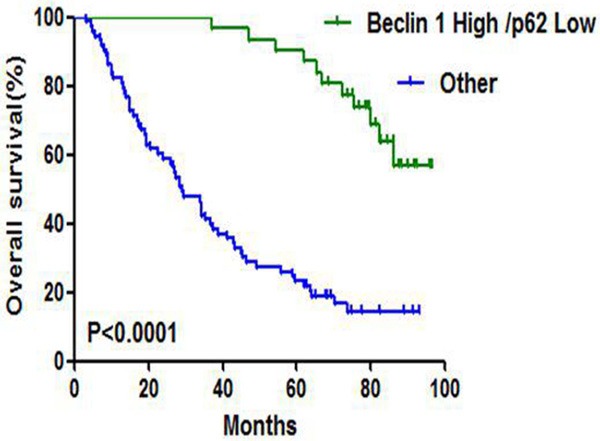
Kaplan-Meier survival curves according to the combination of Beclin 1 and p62.
To adjust confounding factors, multivariate Cox regression analysis was conducted for the significant variables in univariate analysis. Beclin 1 and p62 were independent risk factors related to overall survival for patients with NSCLC (for Beclin 1, hazard ratio, 1.806; 95% confidence interval, 1.035 to 3.151; P=0.037; for p62, hazard ratio, 0.223; 95% confidence interval, 0.128 to 0.386; P<0.001). Moreover, TNM stage was considered as independent prognostic factor for NSCLC (Table 4). However, history of COPD, which was significant in univariate analysis, was not regard as independent prognostic indicator by multivariate analysis.
Table 4.
Multivariate Cox proportional hazard regression analysis of prognostic factor in 104 NSCLC patients
| Characteristics | For death | P value |
|---|---|---|
|
| ||
| Hazard Ratio (95% confidence interval) | ||
| History of COPD (No VS. Yes) | 0.685 (0.419-1.118) | 0.130 |
| TNM stage | ||
| I | 0.229 (0.112-0.468) | 0.000* |
| II | 0.314 (0.149-0.660) | 0.002* |
| III | 0.678 (0.358-1.286) | 0.234 |
| IV | 1 | |
| Beclin 1 (Low VS. High) | 1.806 (1.035-3.151) | 0.037* |
| P62 (Low VS. High) | 0.223 (0.128-0.386) | 0.000* |
P<0.05.
Discussion
Autophagy regulates intracellular homeostatic mechanism via the degradation of long-lived proteins and damaged organelles. Meanwhile, it avoids DNA damage and genome instability caused by ROS. During autophagy initiation and autophagosome formation, autophagy-related protein Beclin 1 binds microtubule-associated protein-1 light chain3 (LC3 I) that is converted to its membrane-bound form (LC3 II) and then interacts with the ubiquitin-binding protein p62/sequestosome 1 (SQSTM1). In our study, we have detected the expression of Beclin 1 and p62 in the three NSCLC cell lines. Moreover, we found that Beclin 1 expression was inversely correlated with p62, in accordance with the previous studies about their roles in autophagy regulation [11]. As a selective substrate, p62 accumulation is a result of autophagy inhibition, and when autophagy was suppressed, Beclin 1, as a key promoter and indicator, will decrease at the same time. Our study was the first one to evaluate the expression of Beclin 1 and p62 concurrently in NSCLC and to investigate the correlations of their expression with clinicopathologic variables and patients’ survival.
Recently, the dysfunction in autophagy pathway has been reported to be associated with an increasing number of human diseases, including cancer. As a key regulator of cancer, autophagy is involved in carcinogenesis, tumor progression and affects the effects of therapeutic interventions [12]. Abnormal DNA methylation and monoallelic deletion of Beclin 1 have been found in human breast and ovarian cancers [13]. Some clinical studies have been conducted to evaluate the correlation of Beclin 1 expression with patients’ survival. However, the results were conflicting. Some studies demonstrated that low expression of Beclin 1 was related to tumor progression and poor prognosis [14,15], while others indicated that high expression of Beclin 1 was associated with inferior OS [16,17]. Based on these disparate outcomes in a variety of cancers, we may assume that the association of Beclin 1 expression with tumor progressions and patient OS may rely on the different tumor types and various phases of tumor growth.
In this study, we found that high expression of Beclin 1 was significantly correlated with well differentiation and negative lymph node metastasis. Also, it significantly related to patient longer survival. The reason that Beclin 1 was related to favorable clinicopathologic variables and longer patient survival is still obscure. Compelling evidences showed that Beclin 1 forms a complex with epidermal growth factor receptor (EGFR) and was negatively regulated via some downstream regulators of EGFR signaling pathway, such as PI3K, Akt and mTOR through Beclin 1 tyrosine phosphorylation [18]. Reduced expression of Beclin 1 or a tyrosine phosphomimetic Beclin 1 mutant may result in decreased autophagy, rapid tumor growth, dedifferentiation of tumor cells, and some resistance to therapy [19]. Furthermore, Beclin 1 may delay cell cycle progression and increase the ration of G1-phase cells via mitogenic signaling, leading to tumor growth inhibition [20]. In addition, our study revealed that high expression of Beclin 1 was detected mainly among the male and patients with heavy smoking. It is well-known that cigarette smoke is prevalent in men and often causes lung inflammation and squamous epithelium metaplasia, which is critical to tumor initiation. Accordingly, autophagy was initiated to respond to smoke-induced oxidative damage, ischemic injury and swollen mitochondrion, which can play a protective role in the early stage of tumorigenesis [21]. In this regard, we may consider that autophagy has a dual role in tumor cells.
p62, another key autophagy-related protein, in opposition to Beclin 1, was associated with unfavorable clinicopathologic features and inferior prognosis in our study. We have found that p62 accumulation was correlated with high TNM stage and positive lymph node metastasis, which indicates that p62 may be involved in tumor progression. As a selective substrate, p62 accumulation has been detected in autophagy defect models, and the elimination of p62 can result in cancer suppression [22]. Consistent with our results, the accumulation of p62 has been found in prostate and gastrointestinal cancers [23,24]. Additionally, as an ubiquitin-binding protein, besides being involved in autophagy regulated by mTOR, p62 is important in a wide variety of cell signal transduction and regulation. NRF2-Keap1 signaling is the central pathway for human body to resist oxidative stress, and excess expression of p62 can competitively combine with Keap1 and result in the stabilization of NRF2, which would lead to tumor progression [25]. Furthermore, p62 can activate NF-кB, a potential regulate metastasis-associated gene, leading to cancer metastasis via NFкB-dependent transcription [7]. Moreover, p62 may affect the function of mitochondrion and regulate cell mitosis [8,26]. All these evidences may explain our current results on p62.
In conclusion, our study has detected the expression of Beclin 1 and p62 in NCSLC cell lines and tissues, demonstrating that Beclin 1 expression was inversely correlated with p62 in NSCLC and they had opposite roles in tumorigenesis and cancer progression. High expression of Beclin 1 is associated with favorable clinicopathologic features and good prognosis, while p62 does the opposite. Therefore, Beclin 1 and p62 may serve as valuable independent prognostic markers in NSCLC and that regulating activities of autophagy may be a new target for NSCLC therapy.
Acknowledgements
The work was supported by the Advanced Project for Returned Personnels Studying Abroad or Doctors of the Second Affiliated Hospital of Soochow University (No. SDFEYBS1102), the Sci-tech Development Planning Project of Suzhou (No. SYS201545), the National Natural Science Foundation of China (81102431) and Natural Science Foundation of Jiangsu Province (BK2011302).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Chen PL, Zhao T, Feng R, Chai J, Tong GX, Wang DB. Patterns and trends with cancer incidence and mortality rates reported by the China National Cancer Registry. Asian Pac J Cancer Prev. 2014;15:6327–32. doi: 10.7314/apjcp.2014.15.15.6327. [DOI] [PubMed] [Google Scholar]
- 3.Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–23. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 4.He C, Levine B. The Beclin 1 interactome. Curr Opin Cell Biol. 2010;22:140–49. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W, Yue C, Deng J, Hu R, Xu J, Feng L, Lan Q, Zhang W, Ji D, Wu J, Liu Q, Liu A. Autophagic protein Beclin 1 serves as an independent positive prognostic biomarker for non-small cell lung cancer. Plos One. 2013;8:e80338. doi: 10.1371/journal.pone.0080338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Zhao X, Subahan NR, Fan L, Gao J, Chen H. The prognostic value of autophagy-related markers beclin-1 and microtubule-associated protein light chain 3B in cancers: a systematic review and meta-analysis. Tumour Biol. 2014;35:7317–26. doi: 10.1007/s13277-014-2060-4. [DOI] [PubMed] [Google Scholar]
- 7.Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–54. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Seibenhener ML, Du Y, Diaz-Meco MT, Moscat J, Wooten MC, Wooten MW. A role for sequestosome 1/p62 in mitochondrial dynamics, import and genome integrity. Biochim Biophys Acta. 2013;1833:452–9. doi: 10.1016/j.bbamcr.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis WD. The 2015 WHO classification of lung tumors. Pathologe. 2014;35(Suppl 2):188. doi: 10.1007/s00292-014-1974-3. [DOI] [PubMed] [Google Scholar]
- 10.Li BX, Li CY, Peng RQ, Wu XJ, Wang HY, Wan DS, Zhu XF, Zhang XS. The expression of beclin 1 is associated with favorable prognosis in stage IIIB colon cancers. Autophagy. 2009;5:303–6. doi: 10.4161/auto.5.3.7491. [DOI] [PubMed] [Google Scholar]
- 11.Park JM, Huang S, Wu TT, Foster NR, Sinicrope FA. Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther. 2013;14:100–7. doi: 10.4161/cbt.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–62. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Chen B, Wu Y, Jin F, Xia Y, Liu X. Genetic and epigenetic silencing of the beclin 1 gene in sporadic breast tumors. BMC Cancer. 2010;10:98. doi: 10.1186/1471-2407-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Q, Chen J, Lv Y, Wang T, Zhang J, Fan J, Wang L. The significance of expression of autophagy-related gene Beclin, Bcl-2, and Bax in breast cancer tissues. Tumour Biol. 2011;32:1163–71. doi: 10.1007/s13277-011-0219-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhou WH, Tang F, Xu J, Wu X, Yang SB, Feng ZY, Ding YG, W-an XB, Guan Z, Li HG, Lin DJ, Shao CK, Liu Q. Low expression of Beclin 1, associated with high Bcl-xL, predicts a malignant ph-enotype and poor prognosis of gastric cancer. Autophagy. 2012;8:389–400. doi: 10.4161/auto.18641. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Chen S, Gou WF, Xiao LJ, Takano Y, Zheng HC. Aberra-nt Beclin 1 expression is closely linked to carcinogenesis, different-iation, progression, and prognosis of ovarian epithelial carcinoma. Tumour Biol. 2014;35:1955–64. doi: 10.1007/s13277-013-1261-6. [DOI] [PubMed] [Google Scholar]
- 17.Wan XB, Fan XJ, Chen MY, Xiang J, Huang PY, Guo L, Wu XY, Xu J, Long ZJ, Zhao Y, Zhou WH, Mai HQ, Liu Q, Hong MH. Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy. 2010;6:395–404. doi: 10.4161/auto.6.3.11303. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, Grishin NV, Peyton M, Minna J, Bhagat G, Levine B. EGFR-mediated Beclin 1 phosphoryla-tion in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–84. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, White M, Rei-chelt J, Levine B. Akt-mediated regulation of autophagy and tumor-igenesis through Beclin 1 phosphorylation. Science. 2012;338:956–9. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koneri K, Goi T, Hirono Y, Katayama K, Yamaguchi A. Beclin 1 g-ene inhibits tumor growth in colon cancer cell lines. Anticancer Res. 2007;27:1453–7. [PubMed] [Google Scholar]
- 21.Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, Ryter SW, Choi AM. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008;4:887–95. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- 22.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis throu-gh elimination of p62. Cell. 2009;137:1062–75. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed A, Ayman A, Deniece J, Wang T, Kovach C, Siddiqui MT, Cohen C. P62/Ubiquitin IHC Expression Correlated with Clinico-pathologic Parameters and Outcome in Gastrointestinal Carcinomas. Front Oncol. 2015;5:70. doi: 10.3389/fonc.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giatromanolaki A, Sivridis E, Mendrinos S, Koutsopoulos AV, Kouk-ourakis MI. Autophagy proteins in prostate cancer: relation with an-aerobic metabolism and Gleason score. Urol Oncol. 2014;32:e11–38. doi: 10.1016/j.urolonc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Moscat J, Diaz-Meco MT. P62 at the crossroads of autophagy, ap-optosis, and cancer. Cell. 2009;137:1001–4. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linares JF, Amanchy R, Greis K, Diaz-Meco MT, Moscat J. Phosp-horylation of p62 bycdk1 controls the timely transit of cells throughmitosis and tumor cell proliferation. Mol Cell Biol. 2011;31:105–17. doi: 10.1128/MCB.00620-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


