Abstract
The aim of this study was to determine whether pretreatment status of thymidine phosphorylase (TP), and hypoxia-inducible factor alpha (HIF-1α) could predict pathologic response to neoadjuvant chemoradiation therapy with oxaliplatin and capecitabine (XELOXART) and outcomes for clinical stage II/III rectal cancer patients. A total of 180 patients diagnosed with clinical stage II/III rectal cancer received XELOXART. The status of TP, and HIF-1α were determined in pretreatment biopsies by immunohistochemistry (IHC). Tumor response was assessed in resected regimens using the tumor regression grade system and TNM staging system. 5-year disease free survival (DFS) and 5-year overall survival (OS) were evaluated with the Kaplan-Meier method and were compared by the log-rank test. Over expression of TP and low expression of HIF-1α were associated with pathologic response to XELOXART and better outcomes (DFS and OS) in clinical stage II/III rectal cancer patients (P < 0.05). Our result suggested that pretreatment status of TP and HIF-1α were found to predict pathologic response and outcomes in clinical stage II/III rectal cancer received XELOXART. Additional well-designed, large sample, multicenter, prospective studies are needed to confirm the result of this study.
Keywords: Rectal cancer, response, neoadjuvant chemoradiation therapy, prognosis
Introduction
Rectal cancer is one of the leading causes of cancer-related deaths in the world [1], and in China, the incidence of rectal cancer has increased in recent years [2]. Surgical resection is the cornerstone of curative treatment of rectal cancer. However, the majority of patients who present with advanced tumors require extensive surgery, which may result in severe postoperative complications [3]. The management of distal rectal cancer poses major challenges to the surgeons and oncologists in terms of local tumor control and preservation of the anal sphincter. For these rectal cancer patients, platinum-based neoadjuvant chemoradio therapy (NCRT) is used as the standard first-line preoperative treatment [4], the implementation of NCRT following surgery have reduced the local recurrence risk and increased the sphincter preservation rate [5,6].
To date, a large number of retrospective analyses suggest that the pathological stage of disease after neoadjuvant treatment and/or the tumor regression rate have a significant prognostic impact on disease free survival (DFS) and overall survival (OS) [7-11], especially for the subgroup of patients who achieve a complete pathological response (CPR) has a very limited risk of local or distant recurrence. However, despite these improvements, treatment for rectal cancer is associated with substantial morbidity, and the tumor responses to this regimen cover a wide spectrum, ranging from none to complete [12]. Therefore, an effective tumor biomarker is urgently needed to identify the subgroup of patients who are poor responders to extensive long-course chemo-radiation to avoid unnecessary toxicity.
Thymidine phosphorylase (TP) is an intracellular enzyme that catalyzes the conversion of thymidine and inorganic phosphate into thymine and a-2-deoxy-D-ribose-1-phosphate [13]. TP is upregulated in several tumors such as colorectal, breast and gastric cancer [14-16], and high TP expression is associated with increased angiogenesis, tumor cell metastasis, and poor prognosis [17]. Since Schwartz et al. suggested that a manipulation of intracellular TP levels can affect sensitivity to both 5-FU and 5-FU prodrugs [18], inconsistent results have been published for the predictive role of TP in neoadjuvant therapy patients. Several studies have demonstrated that the pretreatment status of TP could predict tumor responses after neoadjuvant chemoradiation [19-21], but other studies have reported no effects [22,23]. Additionally, whether TP could predict tumor response to neoadjuvant chemoradiation remain unclear. Thus, further studies are needed to clearly define its role as a predictor of therapeutic benefit [24].
Hypoxia-inducible Factor 1-α (HIF-1α) is a key protein regulating cellular response to hypoxia. HIF-1α transcriptionally regulates the expression of target genes and represents a link between oxygen sensors and effectors in the cellular adaptation to hypoxia [25]. HIF-mediated gene expression regulates many critical aspects of tumor biology, including cell survival, metabolic programming, angiogenesis, metastasis, and therapy resistance [26]. Expression of HIF-1α was associated with a pathological response in locally advanced breast cancer patients treated with neoadjuvant chemotherapy [27], and also being associated with response to photodynamic therapy and radiotherapy in early esophageal cancer patients [28]. But only a small number of studies have investigated the possible predictive value of HIF-1α in chemoradiotherapy of rectal cancer [29], and the results have been inconclusive. The role of pretreatment status of HIF1α expression in relation to chemoradiotherapy remains to be explored.
The aim of this study was to explore the predictive role of pathological response to XELOXART regimen and outcomes in clinical stage II/III rectal cancer patients by using TP and HIF-1α.
Materials and methods
Patients and clinical assessment
This study was approved by the Research Ethics Committee of the Affiliated Cancer Hospital of Guangxi Medical University in China. One hundred-eighty patients were enrolled in this study. These patients were diagnosis as clinical stage II/III rectal cancer patients by pathological examination. The TNM stages were reported according to the American Joint Committee on Cancer (AJCC) [30]. Written informed consent was obtained from all the patients. Fresh cancer specimens were obtained by preoperative gastroscopy and were fixed in 10% formalin and embedded in paraffin, and pathological examination was performed. All the specimens were handled and anonymized according to ethical and legal standards.
Multimodal treatment
The XELOXART regimen was carried on in all patients, according to the NCCN guideline [2].Radiotherapy consisted of 5000 cGy delivered in 25 fractions of 200 cGy five times per week. The iliac lymphatic drainage areas, the surrounding intestines and the tumor bed were considered as target field. 50 mg/m2 oxaliplatin was given on the first day, and 850 mg/m2 capecitabine bid was given for 5 days during the first, second, fourth and fifth weeks of radiotherapy. Within 5-6 weeks after the completion of the XELOXART regimen, low-anterior resection or abdominoperineal resection was scheduled according to the tumor location. Surgery was done by experience surgeon and the principle of TME was strictly followed. Four weeks after surgery, the patients received four more cycles of chemotherapy consisting of 130 mg/m2 oxaliplatin on day 1 and 1000 mg/m2 capecitabine bid on day 1 to day 14. The postoperative regimens were repeated every 3 weeks.
Pathologic assessment
All slides were evaluated by a pathologist who had no access to patient data and clinical status. Pathologic TNM staging was carried out on the surgical specimens to assess for tumor downstaging analysis. We used the tumor regression grade (TRG) system described by Dworak et al. to access tumor response [31]. Grade 0, no regression; grade 1, minor regression, dominant tumor mass with obvious fibrosis in 25% or less of the tumor mass; grade 2, moderate regression, dominantly fibrotic changes with tumor cell fibrosis in 26-50% of the tumor mass; grade 3, good regression, dominant fibrosis outgrowing the tumor mass, more than 50% of tumor regression; and grade 4, total regression, no viable tumor cells, only fibrotic mass. In this study, we considered TRG 0-2 as “poor response”, and TRG 3 and 4 as “good response”.
Immunohistochemical analysis
TP and HIF-1α expression pattern in tissue samples was analyzed by labeled streptavidin-peroxidase (SP) immunohistochemical technique. The process of staining was carried out according to the standard immunohistochemistry (IHC) protocol. Tissue slides were deparaffinized in xylene and rehydrated in graded series of ethanol, followed by heat-induced epitope retrieval in citrate buffer (pH 6.0). Primary antibodies were: TP, anti-TP antibody (mouse monoclonal to TP; Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China); HIF-1α, anti-HIF-1α antibody (rabbit polyclonal to TP; Pharmatechs Co. Ltd, Shanghai, China). The degree of immunostaining was reviewed and scored by two pathologists, taking into account the percentage of positive cells and the staining intensity. Semiquantitative microscopic analyses of protein expression were performed, providing either a negative rating (-) or positive rating (+).
Follow-up
All rectal cancer patients were underwent a systematic follow-up, including physical examinations such as serum level of carcinoembryonic antigen, chest X-ray every 3 months, abdominal and pelvic CT or MRI every 3 months, and colonoscopy within every 6 months after treatment. DFS and OS in these patients were also evaluated.
Statistical analysis
The association between status of TP and HIF-1α and clinicopathologic parameters (age, gender, cT, cN, differentiation, and distance from anal verge et al.) was accessed by using X2-test. To determine the ability of clinic-pathological parameters to predict downstaging and good histo-pathological tumor response to XELOXART, the X2-test was also used. The impact of the TP, and HIF-1α on DFS and OS was determined using the Kaplan-Meier method. All statistical analysis was performed with the Statistical Package for the Social Sciences, version 16.0 (SPSS 16.0), with a P < 0.05 considered to be significant.
Results
The characteristic of the 180 patients were listed in Table 1. Overall, there were 98 male and 82 female involved in this study. Seventy eight of the patients were more than 60 years old, the rest of them were younger than 60 years. Ninety-three patients were clinically assessed as stage II, and 87 patients were clinically assessed as stage III. One hundred and eight tumors were localized in more than 6 cm above the anal verge with rest of which localized in lower 6 cm above the anal verge. Eighty-five of the histological tumors were differentiated, and 95 of which were undifferentiated.
Table 1.
Correlations between clinicopathological parameters and TP status, and HIF-1α status in patients with stage II/III rectal cancer
| Clinicopathological parameters | N | TP status | P-value | HIF-1α status | P-value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Positive (n) | Negative (n) | Positive (n) | Negative (n) | ||||
| Age (years) | |||||||
| > 60 | 78 | 37 | 41 | 0.17 | 40 | 38 | 0.67 |
| ≤ 60 | 102 | 59 | 43 | 49 | 53 | ||
| Gender | |||||||
| Male | 98 | 49 | 49 | 0.33 | 47 | 51 | 0.66 |
| Female | 82 | 47 | 35 | 42 | 40 | ||
| Histology | |||||||
| Differentiated | 85 | 47 | 38 | 0.62 | 45 | 40 | 0.38 |
| Undifferentiated | 95 | 49 | 46 | 44 | 51 | ||
| Distance from anal verge | |||||||
| > 6 cm | 108 | 59 | 49 | 0.67 | 52 | 56 | 0.67 |
| ≤ 6 cm | 72 | 37 | 35 | 37 | 35 | ||
| CT stage | |||||||
| 3 | 101 | 57 | 44 | 0.35 | 46 | 55 | 0.24 |
| 4 | 79 | 39 | 40 | 43 | 36 | ||
| CN stage | |||||||
| Positive | 87 | 43 | 44 | 0.31 | 43 | 44 | 0.15 |
| Negative | 93 | 53 | 40 | 56 | 37 | ||
| AJCC stage | |||||||
| II | 93 | 47 | 46 | 0.44 | 47 | 46 | 0.76 |
| III | 87 | 49 | 38 | 42 | 45 | ||
Note: TP, thymidine phosphorylase; HIF-1α, hypoxia-inducible factor 1-α; AJCC, American Joint Committee on Cancer.
The IHC results revealed that the rectal cancer patients who had positive expression status of TP, and HIF-1α were 53.33% (96/180), and 49.44% (89/180), respectively. The rest of the patients had negative expression status of TP and HIF-1α. The TP, and HIF-1α expression pattern and clinic-pathological factors were listed in Table 1. The expression pattern of TP and HIF-1α were not associated with age, gender, tumor location, histology, distance from anal verge, CT stage, CN stage and American Joint Committee on Cancer (AJCC) stage (P > 0.05).
According to the histopathological TRG system, there were 82 (45.56%) patients with good response (TRG 3-4) and 98 (54.44%) patients with poor response (TRG 0-2). The association between tumor response and clinicopathological parameters was listed in Table 2. The tumor response was not associated with age, gender, tumor location, histology, distance from anal verge, CT stage, CN stage, and AJCC stage (P > 0.05), but was association with pretreament status of TP and HIF-1α (P < 0.05).
Table 2.
Correlations between clinicopathological parameters and tumor response in patients with stage II/III rectal cancer
| Clinicopathological parameters | N | TNM | P-value | TRG | P-value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Downstaging (n) | Non-downstaging (n) | Good response (n) | Poor response (n) | ||||
| Age (years) | |||||||
| > 60 | 78 | 41 | 37 | 0.76 | 34 | 44 | 0.64 |
| ≤ 60 | 102 | 56 | 46 | 48 | 54 | ||
| Gender | |||||||
| Male | 98 | 53 | 45 | 0.96 | 47 | 51 | 0.48 |
| Female | 82 | 44 | 38 | 35 | 47 | ||
| Histology | |||||||
| Differentiated | 85 | 48 | 37 | 0.51 | 37 | 48 | 0.61 |
| Undifferentiated | 95 | 49 | 46 | 45 | 50 | ||
| Distance from anal verge | |||||||
| > 6 cm | 108 | 61 | 47 | 0.39 | 48 | 60 | 0.71 |
| ≤ 6 cm | 72 | 36 | 36 | 34 | 38 | ||
| CT stage | |||||||
| 3 | 101 | 57 | 44 | 0.44 | 49 | 52 | 0.37 |
| 4 | 79 | 40 | 39 | 33 | 46 | ||
| CN stage | |||||||
| Positive | 87 | 47 | 40 | 0.68 | 37 | 50 | 0.43 |
| Negative | 93 | 50 | 43 | 45 | 48 | ||
| AJCC stage | |||||||
| II | 93 | 52 | 41 | 0.57 | 44 | 49 | 0.63 |
| III | 87 | 45 | 42 | 38 | 49 | ||
| TP status | |||||||
| Positive | 96 | 59 | 37 | < 0.05 | 51 | 45 | < 0.05 |
| Negative | 84 | 38 | 46 | 31 | 53 | ||
| HIF-1α status | |||||||
| Positive | 89 | 32 | 67 | < 0.05 | 26 | 63 | < 0.05 |
| Negative | 91 | 65 | 26 | 57 | 24 | ||
Note: TP, thymidine phosphorylase; HIF-1α, hypoxia-inducible factor 1-α; AJCC, American Joint Committee on Cancer; TRG, tumor regression grade.
The follow-up end up at May 31, 2015, eight patients were lost follow-up, remaining the 172 patients for evaluating the DFS and OS. In the whole group of these patients, one hundred and twenty-one patients were alive (70.35%), the rest of them were died of disease recurrence and metastasis. The mean disease-free survival (DFS) was 52.8 ± 21.44 months and the mean survival was 57.19 ± 18.00 months. The 5-year DFS of 96 patients with TP positive status was significantly longer than that of 84 patients with TP negative status (P < 0.05) (Figure 1). In the total study population, TP-positive patients had significantly better OS as compared with TP-negative patients (P < 0.05) (Figure 2). Compared with HIF-1α positive expression group, the patients with HIF-1α negative expression had longer statistically significant DFS and OS (P < 0.05, Figures 3 and 4).
Figure 1.
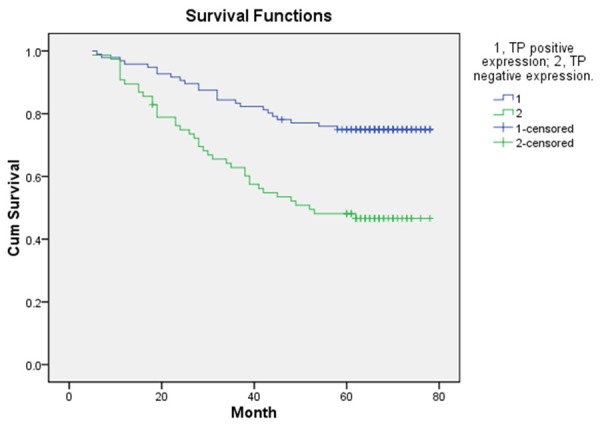
DFS according to positive expression of TP and negative expression of TP.
Figure 2.
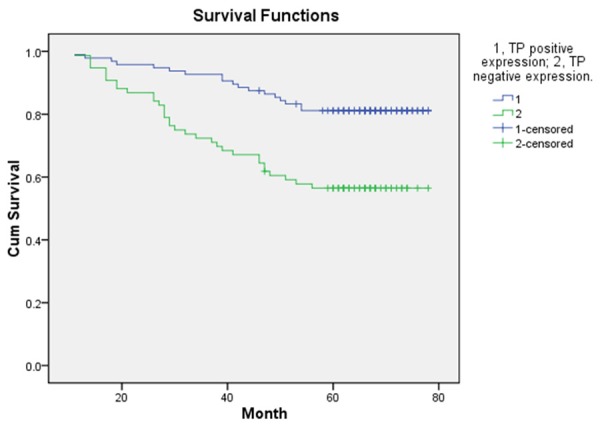
OS according to positive expression of TP and negative expression of TP.
Figure 3.
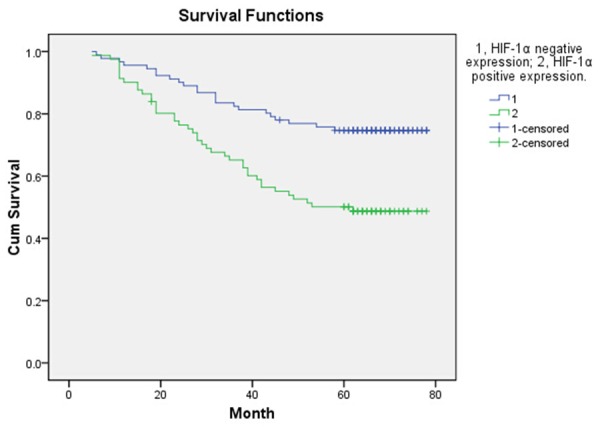
DFS according to positive expression of HIF-1α and negative expression of HIF-1α.
Figure 4.
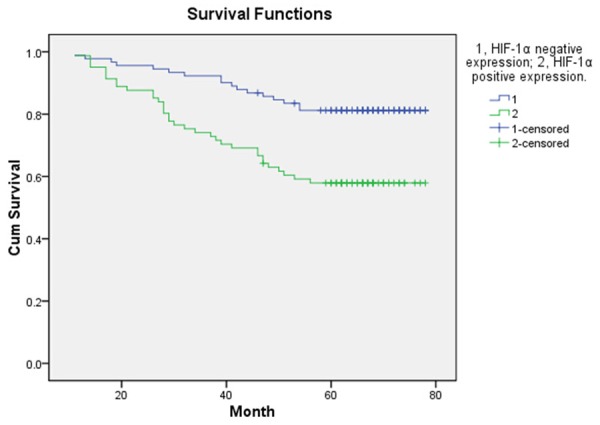
OS according to positive expression of HIF-1α and negative expression of HIF-1α.
Discussion
NACR confers a survival benefit to patients with radically resected rectal cancers; however, NACR may not represent the best treatment for all patients, but only for a subgroup of them. Therefore, it would be highly desirable to identify patients responsive to this therapy. In this study, we evaluated the usefulness of TP and HIF-1α expression as molecular biological markers for predicting the effectiveness of definitive NACR in these patients. We thought that expression of TP and HIF-1α, as IHC markers, might contribute to the effective prediction of NACR effects on these rectal cancer patients. Our conclusion is that it is possible to predict a non-responder to preoperative NACR (XELOXART regimen) by the IHC assessment of TP and HIF-1α in biopsy specimens. In fact, positive expression of TP and negative expression of HIF-1α were positively correlated with therapeutic effects.
TP is the enzyme responsible for conversion of FU to its active metabolite fluorodeoxyuridinemonophosphate [32]. TP was also proved to be an angiogenesis-promoting factor with a similar structure to that of platelet-derived endothelial cell growth factor (PD-ECGF) and played an important role in angiogenesis and extracellular matrix remodeling and then can stimulate tumor growth and metastasis [17,33]. Thus, TP may play a dual role in cancer development and responding to fluorouracil based chemotherapy [32]. Meropol et al. revealed that TP gene expression in primary colorectal cancer was associated with response to capecitabineplusirinotecan [34]; Nishimura et al. also showed that high TP levels were associated with a better outcome in patients with radically resected CRC treated with oral fluoropyrimidines [35].Our study was consisted with these reports (Table 2). However, inconsistent results were also presented [36,37]. This discrepancy between these studies may be due to the different in tumor down-staging and the tumor regression grade system in those studies. Other uncontrolled or unmeasured factors in those studies, such as the different in sample size, and the neoadjuvant therapy regimen, might also contributed to this discrepancy result. Additional large scale, prospective studies which include standardized, well-validated assay methodology will be required to determine whether TP expression can be used to predict clinical outcome in patients treated with neoadjuvant therapy.
Solid tumors, such as colorectal cancer, have extensive areas of hypoxia and necrosis, which worsen a patient’s prognosis because necrosis and hypoxia promote a more malignant phenotype, and hypoxic cells are resistant to chemo and radiotherapy [38]. HIF-1α is an important mediator of hypoxia-induced radioresistance, and studies have demonstrated that cell lines with impaired HIF-1α activation ability are more sensitive to radiotherapy compared with cells with intact HIF-1α activation ability [39]. Previous some studies have shown a possible negative predictive and prognostic value of HIF-1α in rectal cancer patients treated with preoperative chemoradiotherapy [40,41], but others has addressed the question of the role of HIF-1α as a predictive marker in this preoperative treatment [42,43]. Our study showed that preoperative HIF-1α low expression was associated with the pathologic response and outcomes in clinical stage II/III rectal cancer patients treated with neoadjuvant chemoradiation therapy. These discrepancy results might also due to the non-uniform in tumor down-staging and tumor regression system in these studies, and other unmeasured factors in those studies, such as the different in sample size, the experimental method. For example, the studies by Lee-Kong et al. and Havelund et al. which did not considered HIF-1α as a predictive marker [40,41], the HIF-1α expression was measured by using IHC, but in others which presented the inconsistent result [42,43], HIF-1α expression was measured by using reverse transcription-polymerase chain reaction. Additionally, the HIF-1α gene polymorphisms may affect the result of its predictive role of treatment response of adjuvant chemotherapy [42,43]. All of these might be the reasons which responsible for these inconsistent results.
In summary, we found that the TP overexpression or HIF-1α low expression can enable us to distinguish the patients who show a robust tumor response to chemoradiotherapy and might obtain a better DFS and OS.
There are a number of limitations to our study that warrant consideration. First, this is a retrospective analysis which was conducted in a single institute with not large enough samples. Second, the TP and HIF-1α expression were evaluated based on protein expression levels, not on genetic levels, which means the results do not reflect molecular changes in cancer cells on a genetic level. Third, some uncontrolled or unmeasured factors, such as non-uniform in tumor down-staging and tumor regression system, different in sample size and neoadjuvant therapy regimen, might potential produce bias. Perchance the inconsistent results between our study and a portion of previous reports might be due to these factors, therefore, additional prospective studies will be required to determine whether TP and HIF-1α expression can be used to predict clinical outcome in patients treated with XELOXART regimen. Such studies must include standardized, well-validated assay methodology, and the sample size must be sufficient to achieve adequate statistical power for specific biomarker end points.
In conclusion, our result suggested that pretreatment status of TP and HIF-1α were found to predict pathologic response and outcomes in clinical stage II/III rectal cancer received XELOXART. Additional well-designed, large sample, multicenter, prospective studies are needed to confirm the result of this study.
Acknowledgements
This study was funded by the Guangxi Scientific Research and Technological Development (Foundation body: Standard Treatment for Guangxi Cancer Patients. No. GuiKe Gong 1140003A-35).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Zhao L, Bai C, Shao Y, Guan M, Jia N, Xiao Y, Qiu H, Zhang F, Yang T, Zhong G, Chen S. A phase II study of neoadjuvant chemoradiotherapy with oxaliplatin and capecitabine for rectal cancer. Cancer Lett. 2011;310:134–9. doi: 10.1016/j.canlet.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Laforest A, Bretagnol F, Mouazan AS, Maggiori L, Ferron M, Panis Y. Functional disorders after rectal cancer resection: does a rehabilitation programme improve anal continence and quality of life? Colorectal Dis. 2012;14:1231–7. doi: 10.1111/j.1463-1318.2012.02956.x. [DOI] [PubMed] [Google Scholar]
- 4.Dou X, Wang RB, Meng XJ, Yan HJ, Jiang SM, Zhu KL, Xu XQ, Chen D, Song XR, Mu DB. PDCD4 as a predictor of sensitivity to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Asian Pac J Cancer Prev. 2014;15:825–30. doi: 10.7314/apjcp.2014.15.2.825. [DOI] [PubMed] [Google Scholar]
- 5.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Liu F, Gu W, Lian P, Sheng W, Xu J, Cai G, Shi D, Cai S, Zhang Z. Concomitant boost IMRT-based neoadjuvant chemoradiotherapy for clinical stage II/III rectal adenocarcinoma: results of a phase II study. Radiat Oncol. 2014;9:70. doi: 10.1186/1748-717X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem E, D’Hoore A. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol. 2012;19:2833–41. doi: 10.1245/s10434-012-2327-1. [DOI] [PubMed] [Google Scholar]
- 9.MacGregor TP, Maughan TS, Sharma RA. Pathological grading of regression following neoadjuvant chemoradiation therapy: the clinical need is now. J Clin Pathol. 2012;65:867–71. doi: 10.1136/jclinpath-2012-200958. [DOI] [PubMed] [Google Scholar]
- 10.Schiffmann L, Klautke G, Wedermann N, Gock M, Prall F, Fietkau R, Rau B, Klar E. Prognosis of rectal cancer patients improves with downstaging by intensified neoadjuvant radiochemotherapy-a matched pair analysis. Bmc Cancer. 2013;13:388. doi: 10.1186/1471-2407-13-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdul-Jalil KI, Sheehan KM, Kehoe J, Cummins R, O’Grady A, McNamara DA, Deasy J, Breathnach O, Grogan L, O’Neill BD, Faul C, Parker I, Kay EW, Hennessy BT, Gillen P. The prognostic value of tumour regression grade following neoadjuvant chemoradiation therapy for rectal cancer. Colorectal Dis. 2014;16:O16–25. doi: 10.1111/codi.12439. [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Wang R, Huang Z, Zhang J, Feng R, Xu X, Zhu K, Dou X, Chen D, Yu J. Human epidermal growth factor receptor-2 expression in locally advanced rectal cancer: Association with response to neoadjuvant therapy and prognosis. Cancer Sci. 2014;105:818–24. doi: 10.1111/cas.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liekens S, Bronckaers A, Belleri M, Bugatti A, Sienaert R, Ribatti D, Nico B, Gigante A, Casanova E, Opdenakker G, Pérez-Pérez MJ, Balzarini J, Presta M. The thymidine phosphorylase inhibitor 5’-O-tritylinosine (KIN59) is an antiangiogenic multitarget fibroblast growth factor-2 antagonist. Mol Cancer Ther. 2012;11:817–29. doi: 10.1158/1535-7163.MCT-11-0738. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Sugimoto T, Okabayashi T, Okamoto K, Namikawa T, Tochika N, Moriki T, Araki K. Localization of thymidine phosphorylase in breast cancer tissue. Med Mol Morphol. 2005;38:112–7. doi: 10.1007/s00795-005-0282-7. [DOI] [PubMed] [Google Scholar]
- 15.Ruckhaberle E, Karn T, Engels K, Turley H, Hanker L, Muller V, Schmidt M, Ahr A, Gaetje R, Holtrich U, Kaufmann M, Rody A. Prognostic impact of thymidine phosphorylase expression in breast cancer--comparison of microarray and immunohistochemical data. Eur J Cancer. 2010;46:549–57. doi: 10.1016/j.ejca.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Huang X, Chen Y, Jin X, Li Q, Yi TN. Prognostic value of TP/PD-ECGF and thrombocytosis in gastric carcinoma. Eur J Surg Oncol. 2012;38:568–73. doi: 10.1016/j.ejso.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Bronckaers A, Gago F, Balzarini J, Liekens S. The dual role of thymidine phosphorylase in cancer development and chemotherapy. Med Res Rev. 2009;29:903–53. doi: 10.1002/med.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz EL, Baptiste N, Wadler S, Makower D. Thymidine phosphorylase mediates the sensitivity of human colon carcinoma cells to 5-fluorouracil. J Biol Chem. 1995;270:19073–7. doi: 10.1074/jbc.270.32.19073. [DOI] [PubMed] [Google Scholar]
- 19.Jakob C, Liersch T, Meyer W, Baretton GB, Hausler P, Schwabe W, Becker H, Aust DE. Immunohistochemical analysis of thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase in rectal cancer (cUICC II/III): correlation with histopathologic tumor regression after 5-fluorouracil-based long-term neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2005;29:1304–9. doi: 10.1097/01.pas.0000170346.55304.88. [DOI] [PubMed] [Google Scholar]
- 20.Huh JW, Lee JH, Kim HR. Pretreatment expression of 13 molecular markers as a predictor of tumor responses after neoadjuvant chemoradiation in rectal cancer. Ann Surg. 2014;259:508–15. doi: 10.1097/SLA.0b013e31829b3916. [DOI] [PubMed] [Google Scholar]
- 21.Jakob C, Liersch T, Meyer W, Baretton GB, Schwabe W, Hausler P, Kulle B, Becker H, Aust DE. Prognostic value of histologic tumor regression, thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase in rectal cancer UICC Stage II/III after neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2006;30:1169–74. doi: 10.1097/01.pas.0000213302.13435.6e. [DOI] [PubMed] [Google Scholar]
- 22.Tateishi Y, Tatemoto Y, Ohno S, Morishita K, Ueta E, Yamamoto T. Combined evaluation of dihydropyrimidine dehydrogenase and thymidine phosphorylate mRNA levels in tumor predicts the histopathological effect of 5-fluorouracil-based chemoradiotherapy. Cancer Lett. 2009;274:187–93. doi: 10.1016/j.canlet.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Hahnvajanawong C, Chaiyagool J, Seubwai W, Bhudhisawasdi V, Namwat N, Khuntikeo N, Sripa B, Pugkhem A, Tassaneeyakul W. Orotate phosphoribosyl transferase mRNA expression and the response of cholangiocarcinoma to 5-fluorouracil. World J Gastroenterol. 2012;18:3955–61. doi: 10.3748/wjg.v18.i30.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciaparrone M, Quirino M, Schinzari G, Zannoni G, Corsi DC, Vecchio FM, Cassano A, La Torre G, Barone C. Predictive role of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase expression in colorectal cancer patients receiving adjuvant 5-fluorouracil. Oncology. 2006;70:366–77. doi: 10.1159/000098110. [DOI] [PubMed] [Google Scholar]
- 25.Semenza GL. Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res. 2001;49:614–7. doi: 10.1203/00006450-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Sadri N, Zhang PJ. Hypoxia-inducible factors: mediators of cancer progression; prognostic and therapeutic targets in soft tissue sarcomas. Cancers (Basel) 2013;5:320–33. doi: 10.3390/cancers5020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiezzi DG, Clagnan WS, Mandarano LR, de Sousa CB, Marana HR, Tiezzi MG, de Andrade JM. Expression of aldehyde dehydrogenase after neoadjuvant chemotherapy is associated with expression of hypoxia-inducible factors 1 and 2 alpha and predicts prognosis in locally advanced breast cancer. Clinics (Sao Paulo) 2013;68:592–8. doi: 10.6061/clinics/2013(05)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koukourakis MI, Giatromanolaki A, Skarlatos J, Corti L, Blandamura S, Piazza M, Gatter KC, Harris AL. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001;61:1830–2. [PubMed] [Google Scholar]
- 29.Havelund BM, Spindler KL, Ploen J, Andersen RF, Jakobsen A. Single nucleotide polymorphisms in the HIF-1alpha gene and chemoradiotherapy of locally advanced rectal cancer. Oncol Lett. 2012;4:1056–60. doi: 10.3892/ol.2012.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–9. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 31.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 32.Huang L, Chen F, Chen Y, Yang X, Xu S, Ge S, Fu S, Chao T, Yu Q, Liao X, Hu G, Zhang P, Yuan X. Thymidine phosphorylase gene variant, platelet counts and survival in gastrointestinal cancer patients treated by fluoropyrimidines. Sci Rep. 2014;4:5697. doi: 10.1038/srep05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyadera K, Sumizawa T, Haraguchi M, Yoshida H, Konstanty W, Yamada Y, Akiyama S. Role of thymidine phosphorylase activity in the angiogenic effect of platelet derived endothelial cell growth factor/thymidine phosphorylase. Cancer Res. 1995;55:1687–90. [PubMed] [Google Scholar]
- 34.Meropol NJ, Gold PJ, Diasio RB, Andria M, Dhami M, Godfrey T, Kovatich AJ, Lund KA, Mitchell E, Schwarting R. Thymidine phosphorylase expression is associated with response to capecitabine plus irinotecan in patients with metastatic colorectal cancer. J. Clin. Oncol. 2006;24:4069–77. doi: 10.1200/JCO.2005.05.2084. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura G, Terada I, Kobayashi T, Ninomiya I, Kitagawa H, Fushida S, Fujimura T, Kayahara M, Shimizu K, Ohta T, Miwa K. Thymidine phosphorylase and dihydropyrimidine dehydrogenase levels in primary colorectal cancer show a relationship to clinical effects of 5’-deoxy-5-fluorouridine as adjuvant chemotherapy. Oncol Rep. 2002;9:479–82. [PubMed] [Google Scholar]
- 36.Ciaparrone M, Quirino M, Schinzari G, Zannoni G, Corsi DC, Vecchio FM, Cassano A, La Torre G, Barone C. Predictive Role of Thymidylate Synthase, Dihydropyrimidine Dehydrogenase and Thymidine Phosphorylase Expression in Colorectal Cancer Patients Receiving Adjuvant 5-Fluorouracil. Oncology. 2006;70:366–77. doi: 10.1159/000098110. [DOI] [PubMed] [Google Scholar]
- 37.Huh JW, Lee JH, Kim HR. Pretreatment Expression of 13 Molecular Markers as a Predictor of Tumor Responses After Neoadjuvant Chemoradiation in Rectal Cancer. Ann Surg. 2014;259:508–15. doi: 10.1097/SLA.0b013e31829b3916. [DOI] [PubMed] [Google Scholar]
- 38.Kaynar MY, Sanus GZ, Hnimoglu H, Kacira T, Kemerdere R, Atukeren P, Gumustas K, Canbaz B, Tanriverdi T. Expression of hypoxia inducible factor-1alpha in tumors of patients with glioblastoma multiforme and transitional meningioma. J Clin Neurosci. 2008;15:1036–42. doi: 10.1016/j.jocn.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 39.Ravizza R, Molteni R, Gariboldi MB, Marras E, Perletti G, Monti E. Effect of HIF-1 modulation on the response of two-and three-dimensional cultures of human colon cancer cells to 5-fluorouracil. Eur J Cancer. 2009;45:890–8. doi: 10.1016/j.ejca.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 40.Lee-Kong SA, Ruby JA, Chessin DB, Pucciarelli S, Shia J, Riedel ER, Nitti D, Guillem JG. Hypoxia-related proteins in patients with rectal cancer undergoing neoadjuvant combined modality therapy. Dis Colon Rectum. 2012;55:990–5. doi: 10.1097/DCR.0b013e31825bd80c. [DOI] [PubMed] [Google Scholar]
- 41.Havelund BM, Sorensen FB, Lindebjerg J, Spindler KL, Jakobsen A. Pretreatment HIF-1alpha and GLUT-1 expressions do not correlate with outcome after preoperative chemoradiotherapy in rectal cancer. Anticancer Res. 2011;31:1559–65. [PubMed] [Google Scholar]
- 42.Toiyama Y, Inoue Y, Saigusa S, Okugawa Y, Yokoe T, Tanaka K, Miki C, Kusunoki M. Gene expression profiles of epidermal growth factor receptor, vascular endothelial growth factor and hypoxia-inducible factor-1 with special reference to local responsiveness to neoadjuvant chemoradiotherapy and disease recurrence after rectal cancer surgery. Clin Oncol (R Coll Radiol) 2010;22:272–80. doi: 10.1016/j.clon.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Wang P, Zhou XC, Bao GQ, Lyu ZM, Liu XN, Wan SG, He XL, Huang QC. Genetic variations in the HIF1A gene modulate response to adjuvant chemotherapy after surgery in patients with colorectal cancer. Asian Pac J Cancer Prev. 2014;15:4637–42. doi: 10.7314/apjcp.2014.15.11.4637. [DOI] [PubMed] [Google Scholar]


