Abstract
Cataract is a main cause of blindness worldwide. RAC1 has been reported to have a close relationship with the proliferation and migration of cells. However, the relationship between RAC1 and cataract is not yet clear. The proliferation and migration of lens epithelial cells are key factors in the formation of cataract as well as in the complication of cataract surgery. In this study, the effect of RAC1 overexpression on the proliferation and migration of lens epithelial cells was explored. Results showed that RAC1 overexpression promoted the proliferation of lens epithelial cells and increased the protein level of proliferating cell nuclear antigen. RAC1 overexpression also promoted migration and invasion of lens epithelial cells and had an influence on the epithelial-mesenchymal transition process. These results indicate that RAC1 may become a therapeutic target of cataract and inhibition of RAC1 may become a promising way for the therapy of cataract.
Keywords: RAC1, cataract, proliferation, migration, invasion, epithelial-mesenchymal transition
Introduction
Cataract is one of the main causes of blindness worldwide. Many factors, such as aging, heredity and radiation, can cause the formation of cataract.
Ras-related C3 botulinum toxin substrate 1 (RAC1) is a small GTPase of Rho family. RAC1 has been reported to be implicated in multiple cell events, such as proliferation, differentiation, and migration. RAC1 can regulate cytoskeleton remodeling, cell adhesion, and lamellipodia formation [1,2]. RAC1 was also reported to regulate NF-κB signaling pathway [3] and has a close relationship with inflammation [4,5].
Epithelial-mesenchymal transition (EMT) is a highly conserved cell process in which polarized immotile epithelial cells transform to motile mesenchymal cells. EMT is characterized as the changes of cell morphology, increased cell mobility, and loss of cell adhesion. During EMT, epithelial cell markers, such as E-cadherin, are lost; and mesenchymal cell markers, such as smooth muscle actin-α (α-SMA), are elevatory [6]. EMT is an important process in the development of body. It also plays a critical role in other processes, such as wound healing, fibrosis, and tumor metastasis.
RAC1 is over-expressed in various malignant tumors [7] and is associated with tumor metastasis [8]. However, whether RAC1 has relationship with cataract was still unknown. As the proliferation and migration of lens epithelial cells are key factors in the formation of cataract, we explored the effect of RAC1 on the proliferation, migration and EMT process of lens epithelial cells. Results showed that RAC1 promoted the proliferation, migration and invasion of lens epithelial cells. These results suggest for the first time that RAC1 may have relationship with cataract. It also indicates that inhibition of RAC1 may be a promising way for the treatment of cataract.
Materials and methods
Cell culture
Human lens epithelial cells SRA01/04 were obtained from Bioleaf (Shanghai, China). Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA) and maintained in a humidified atmosphere at 37°C with 5% CO2.
Transfection
Recombinant lentivirus containing RAC1 overexpression plasmid and empty vector was obtained from GenePharma (Shanghai, China). 1×105 cells were seeded in 6-well plates. 24 h later, DMEM medium containing suitable lentivirus was added into each well. 48 h after infection, the medium was changed to fresh DMEM medium. Then DMEM medium containing G418 (400 μg/ml) was added into each well for the filtration of stably transfected cells. The transfection efficiency was measured by quantitative real time PCR (qRT-PCR) and Western blot. Images of cells were captured using a phase contrast microscope.
qRT-PCR
Cells were collected for qRT-PCR. Total RNA was extracted using RNA extraction kit (BioTeke, Beijing, China) according to the manufacturer’s instruction. Then total RNA was reverse transcribed into cDNA using Super M-MLV reverse transcriptase (BioTeke) and oligo (dT)15. The mRNA level of RAC1 was measured by SYBR Green method qRT-PCR using cDNA as templates and primers as follows: forward primer for RAC1, 5’-CCGTGCAAAGTGGTATCCTG-3’; reverse primer for RAC1, 5’-GCTTCTTCTCCTTCAGTTTCTCG-3’; forward primer for β-actin, 5’-CTTAGTTGCGTTACACCCTTTCTTG-3’; reverse primer for β-actin, 5’-CTGTCACCTTCACCGTTC-CAGTTT-3’. The relative mRNA level of RAC1 was calculated using 2-∆∆Ct method.
Western blot
Cells were harvested and lysed in RIPA lysis buffer. The concentration of protein was measured using a BCA protein assay kit (Beyotime, Shanghai, China). Then 40 μg of protein was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. After electrophoresis, the separated protein was transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After blockade with 5% skim milk, the membranes were incubated with corresponding primary antibodies against RAC1 (1:200, Santa Cruz, Dallas, TX, USA), vimentin, PCNA (1:500, Bioss, Beijing, China), E-cadherin, fibronectin, N-cadherin, α-SMA (1:400, Boster, Wuhan, China), and β-actin (1:1000, Santa Cruz) at 4°C overnight. After washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000, Beyotime) at 37°C for 45 min. Then the membranes were visualized with enhanced chemiluminescence detection system. The protein signal was analyzed using Gel-Pro-Analyzer software and relative protein level was calculated using β-actin as an internal reference.
MTT assay
Cell viability was determined by MTT assay. Cells were seeded into 96-well plates at a density of 3×103 cells/well in quintuplicate. After the cells were attached to the plates, MTT (Sigma, St. Louis, MO, USA) at a final concentration of 0.2 mg/ml was added into each well at 0h, 24 h, 48 h, 72 h, and 96 h and incubated at 37°C for additional 4 h. Then the supernatant was removed gently, and 200 μl of dimethyl sulfoxide (Sigma) was added into each well. The absorbance at 490 nm was measured with a microplate reader and a growth curve was drawn.
Wound-healing assay
Cell migration capability was determined using wound-healing assay. Cells were seeded into a 6-well plate and grew overnight. When the cell confluency was approximately 90%, 200 µl pipette tips were used to make scratches on the cell surfaces. After washing, cells were cultured in serum-free DMEM medium. At 0 h, 12 h, and 24 h after the scratches were made, images of cells were captured and the relative migration rate was estimated through measuring the gap size and the relative migration rate was calculated.
Transwell assay
Cells were made into cell suspension in serum-free DMEM medium (1×105 cells/ml). 200 μl of cell suspension was added into the upper chamber of the transwell chambers (Corning, Tewksbury, MA, USA) precoated with Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA). And DMEM medium supplemented with 20% FBS was added to the lower chambers. After incubation at 37°C for 24 h, cells upon the micropore membranes were removed with cotton swabs and cells under the micropore membranes were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. Images of cells were captured with a microscope under a 200× magnification.
Imunofluorescence
Cells were made into cell climbing, fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. After blockade with goat serum, the cell climbing was incubated with corresponding primary antibody against E-cadherin (1:200, Boster) at 4°C overnight. After washing, the cell climbing was incubated with the corresponding Cy3 labeled secondary antibody (1:200, Beyotime) at room temperature for 60 min in the dark. Then the cell climbing was incubated with DAPI for nuclear staining. Images of cells were captured with a fluorescence microscope with a 400× magnification.
Statistical analysis
All experiments were repeated three times. The results are presented as mean ± SD. One-way ANOVA and Bonferroni’s Multiple Comparison was used to analyze the statistical significance between different groups. P < 0.05 was considered to be significant.
Results
Expression of RAC1 was increased after transfection with RAC1 overexpression plasmid
To explore the effect of RAC1, a RAC1 overexpression plasmid was constructed and transfected into SRA01/04 cells using lentivirus. The effect of RAC1 overexpression plasmid transfection was detected by qRT-PCR and western blot. Result of qRT-PCR showed that the mRNA level of RAC1 was increased to 3.32±0.34-fold after transfection with RAC1 overexpression plasmid, but no significant difference in RAC1 expression level after transfection with Vector (Figure 1A). The protein level of RAC1 was increased to 3.08±0.45-fold after transfection with RAC1 overexpression plasmid, leaving no significant changes in that of cells transfected with Vector (Figure 1B and 1C). These results demonstrated that the expression of RAC1 was enhanced in cells transfected with RAC1 overexpression plasmid, both in mRNA level and in protein level.
Figure 1.
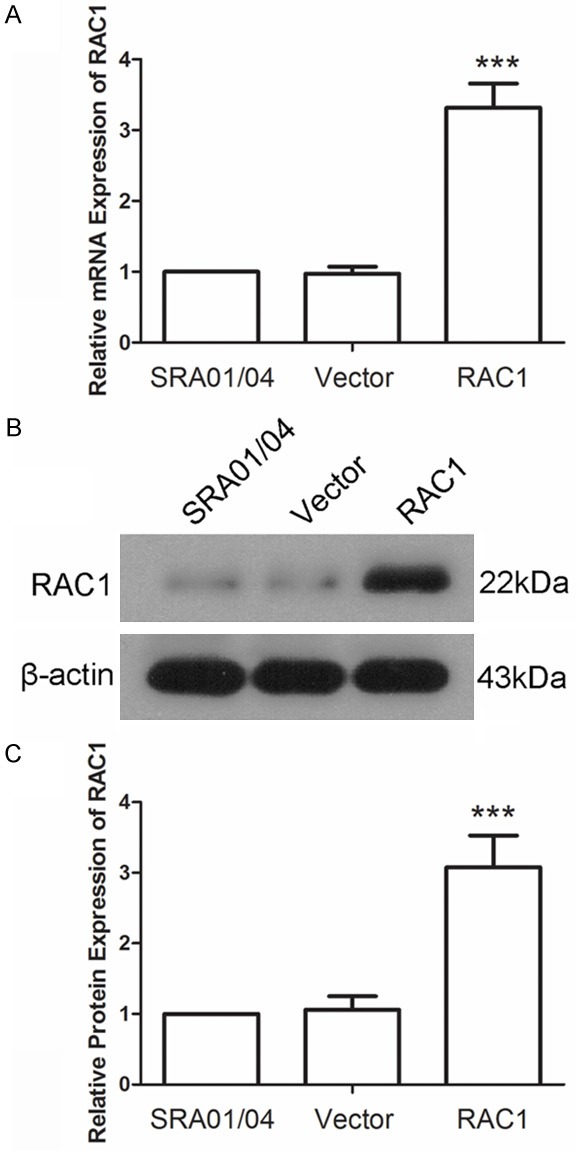
RAC1 level was increased after transfection with RAC1 overexpression plasmid. A. The mRNA level of RAC1 was detected by quantitative real time PCR. The relative mRNA level was calculated using 2-∆∆Ct method. B, C. The protein level of RAC1 was detected by western blot using β-actin as an internal reference. All experiments were repeated three times and the results are presented as mean ± SD. ***P < 0.001.
RAC1 overexpression promoted the proliferation of lens epithelial cells
To explore the effect of RAC1 overexpression on the proliferation of lens epithelial cells, a MTT assay was carried out. As shown in Figure 2A, cells transfected with RAC1 overexpression plasmid showed a quick growth compared with that of cells transfected with Vector. Then PCNA, which is a marker of proliferation, was also detected by western blot. Result showed that the protein level of PCNA was increased to 2.37±0.38-fold by RAC1 overexpression compared with Vector (Figure 2B and 2C). These results suggested that RAC1 overexpression promoted the proliferation of lens epithelial cells.
Figure 2.
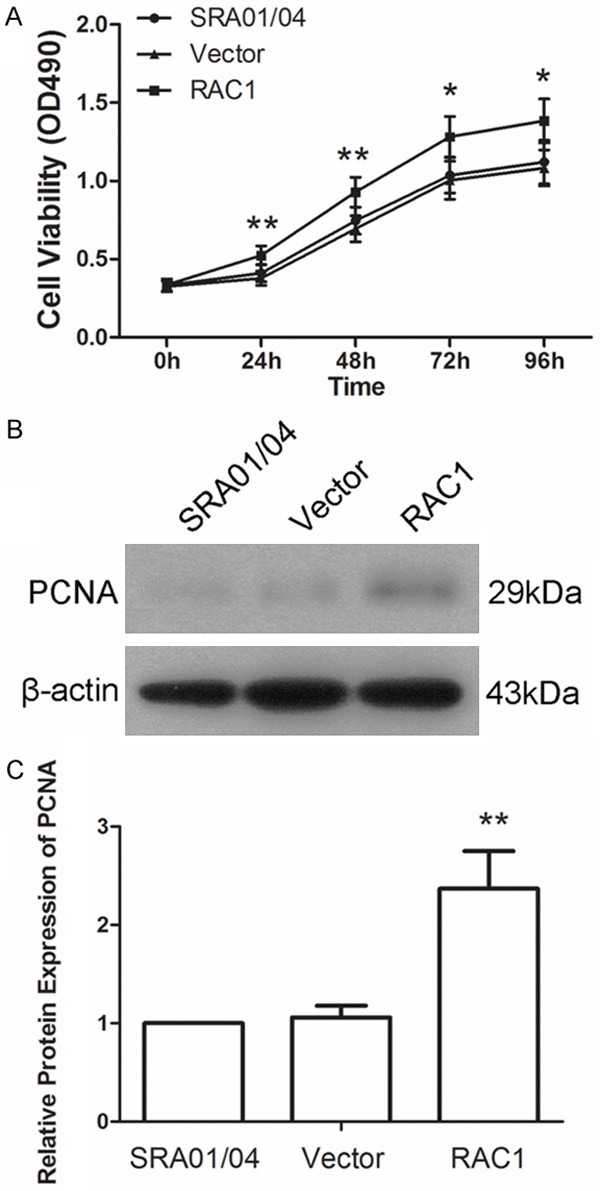
Overexpression of RAC1 accelerated the proliferation of lens epithelial cells. A. Cell viability was determined by MTT assay. B, C. Protein level of proliferating cell nuclear antigen (PCNA) was detected using western blot. The relative protein level of PCNA was calculated using β-actin as internal reference. Each experiment was repeated three times. The results are presented as mean ± SD. *P < 0.05, **P < 0.01.
RAC1 overexpression promoted the migration and invasion of lens epithelial cells
Wound-healing assay was performed to detect the impact of RAC1 overexpression on the migration capability of lens epithelial cells. As shown in Figure 3A, cells transfected with RAC1 overexpression plasmid showed a rapid migration at 12 h and 24 h compared with Vector. Transwell assay was then performed to detect the effect of RAC1 overexpression on the invasion of lens epithelial cells. Results of transwell assay showed that there were 107.4±11.7 cells passing through the micropore membrane in the RAC1 overexpression group, significantly more than that of Vector (72.6±7.92) (Figure 3B, P < 0.001). These results indicated that RAC1 overexpression promoted the migration and invasion of lens epithelial cells.
Figure 3.
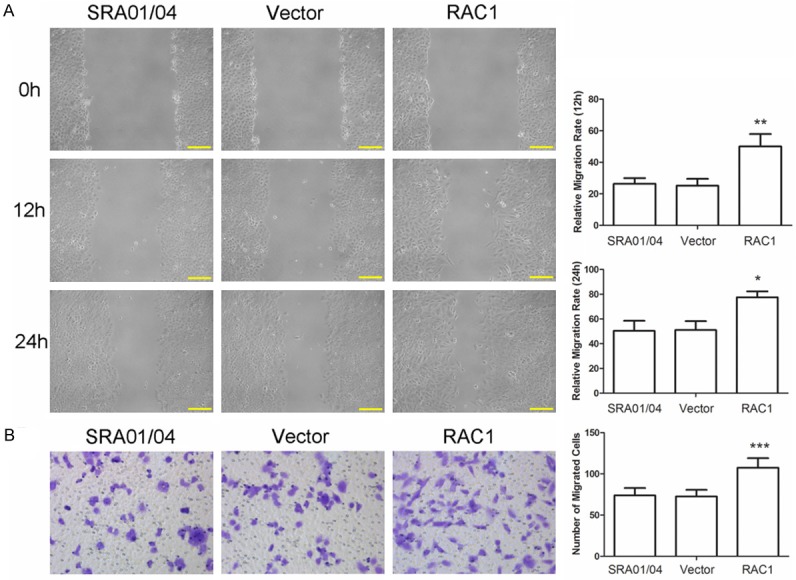
RAC1 overexpression promoted the migration and invasion of lens epithelial cells. A. Wound-healing assay was performed to evaluate the migration capability of cells. The relative migration rate at 12 h and 24 h was calculated. Scale bar = 100 μm. B. Transwell assay was carried out to evaluate the invasion capability of cells. The number of cells passing through the micropore membrane was calculated. Each experiment was repeated three times and the results are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
RAC1 overexpression promoted EMT of lens epithelial cells
EMT is a key process in the metastasis of cells and the effect of RAC1 overexpression on EMT of lens epithelial cells was examined in this study. The morphological analysis of cells was first carried out. Cells transfected with Vector showed round or diamond appearance, but cells transfected with RAC1 overexpression plasmid showed long spindle appearance (Figure 4A). Then markers of EMT were detected by western blot. Results of western blot showed that after transfection with RAC1 overexpression plasmid, the protein level of E-cadherin was decreased to 61±8%, and the protein levels of N-cadherin, vimentin, fibronectin and α-SMA was increased to 3±0.3-fold, 2.02±0.23-fold, 2.2±0.34-fold, and 1.66±0.23-fold respectively (Figure 4B and 4C). The expression and distribution of E-cadherin was also detected by imunofluorescence. As shown in Figure 4D, E-cadherin (red fluorescence) was distributed on the cell membranes, with a strong fluorescence. However, in the RAC1 overexpression group, the fluorescence of E-cadherin distributed on the cell membranes was weakened and the scope was reduced, which indicated a decrease in function of E-cadherin. These results demonstrated that RAC1 overexpression promoted the EMT process of lens epithelial cells.
Figure 4.
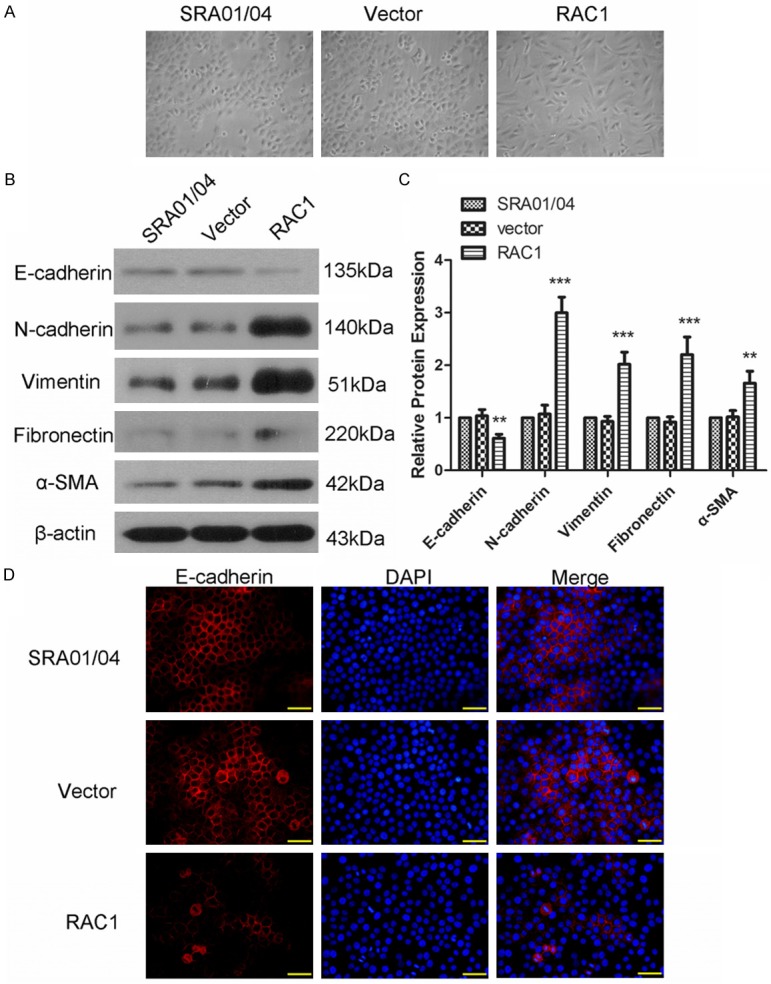
RAC1 overexpression promoted epithelial-mesenchymal transition (EMT). A. The cellular morphology of cells in each group. B, C. Protein level of E-cadherin, N-cadherin, vimentin, fibronectin and smooth muscle actin-α (α-SMA) was detected using western blot. The relative protein level was calculated using β-actin as internal reference. D. The expression and distribution of E-cadherin was detected by immunofluorescence. Scale bar = 50 μm. All experiments were repeated three times. The results are presented as mean ± SD. **P < 0.01, ***P < 0.001.
Discussion
In the present study, we explored the effect of RAC1 overexpression on cataract. Results of our study showed that overexpression of RAC1 can promote the proliferation and migration of lens epithelial cells and impact the EMT process, thus RAC1 has a relationship with cataract.
As a small GTPase, RAC1 plays an essential role in the assembly and activation of NADPH oxidase which can trigger the generation of reactive oxygen species (ROS) [3,9,10]. ROS is a critical production of oxidative stress and is associated with the proliferation and apoptosis of different types of cells. Overexpression of RAC1 was found to increase the cell viability of lens epithelial cells, upregulate the protein level of PCNA, and accelerate the proliferation of lens epithelial cells in the present study. RAC1 also acts as an oncogene in tumor cells, promotes the proliferation and migration of tumor cells [11].
Migration and invasion of lens epithelial cells have a close relationship with the formation of cataract. We found that overexpression of RAC1 promoted the migration and invasion of lens epithelial cells. RAC1 plays an important role on actin dynamics and cytoskeleton regulation [12]. RAC1 has been previously reported to be involved in cell migration induced by cytokines [13-15], promote cell mesenchymal mode movement [16,17] and deletion of RAC1 was reported to inhibit cell migration and wound healing of epidermal keratinocytes [18]. Metal matrix proteinases (MMPs) play a critical role in the process of cell metastasis. RAC1 can also regulate the protein level of MMP-3 and MMP-9 [19,20] and mediate Twist1-induced cancer cell migration [21]. Yao et al also showed that RAC1 was essential for lung metastasis of hematogenous [8].
EMT is a critical process that impacts the migration and invasion capability of cells. RAC1 regulates actin cytoskeleton dynamics [22] and promotes cell lamellipodia formation [16]. Previous studies showed that RAC1 induced EMT in transformed keratinocytes [19] and podocytes [23]. During the process of EMT, epithelial cells lose their compact organization and change to motile and invasive spindle mesenchymal cells. In our study, we also discovered a similar mesenchymal change in morphology of cells in RAC1 group, which indicated the regulation of RAC1 on EMT. E-cadherin is a marker of epithelial cells and is very important to the tight junction between cells. N-cadherin, vimentin, fibronectin, and α-SMA are important markers of mesenchymal cells. Overexpression of RAC1 leaded to a decrease in E-cadherin level, especially on the membranes, and increases in levels of N-cadherin, vimentin, fibronectin, and α-SMA in the present study, which enhanced our hypothesis that RAC1 regulated the EMT process.
In summary, we found that overexpression of RAC1 promoted the proliferation, migration, and invasion of lens epithelial cells, and regulated the EMT process. Proliferation and migration of lens epithelial cells are associated with the formation of cataract. Our results indicate for the first time that there is a possible relationship between RAC1 and cataract, and that inhibition of RAC1 may be a potential method for the treatment of cataract. Main means of cataract therapy is surgical intervention and artificial intraocular lens implantation. However, in cataract surgery, after the entire lens is removed, lens epithelial cells usually undergo EMT process and move to the surface of posterior capsular, which usually causes posterior capsule opacification [24]. Inhibition of RAC1 may also have a positive effect on this complication and may be used as an adjuvant therapy of cataract surgery. Moreover, Oxidative stress is usually observed in surgery. Zhou et al demonstrated that inhibition of RAC1 could attenuate oxidative stress [25], which will be beneficial to cataract surgery. Overall, inhibition of RAC1 could become a promising way in the treatment of cataract.
Disclosure of conflict of interest
None.
References
- 1.Leng R, Liao G, Wang H, Kuang J, Tang L. Rac1 expression in epithelial ovarian cancer: effect on cell EMT and clinical outcome. Med Oncol. 2015;32:329. doi: 10.1007/s12032-014-0329-5. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 3.Myant KB, Cammareri P, McGhee EJ, Ridgway RA, Huels DJ, Cordero JB, Schwitalla S, Kalna G, Ogg EL, Athineos D, Timpson P, Vidal M, Murray GI, Greten FR, Anderson KI, Sansom OJ. ROS production and NF-kappaB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwaiz R, Hasan Z, Rahman M, Zhang S, Palani K, Syk I, Jeppsson B, Thorlacius H. Rac1 signaling regulates sepsis-induced pathologic inflammation in the lung via attenuation of Mac-1 expression and CXC chemokine formation. J Surg Res. 2013;183:798–807. doi: 10.1016/j.jss.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 5.Henson PM, Bratton DL. Allergy: airway epithelial Rac1 suppresses allergic inflammation. Curr Biol. 2013;23:R104–106. doi: 10.1016/j.cub.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Crnogorac-Jurcevic T, Efthimiou E, Capelli P, Blaveri E, Baron A, Terris B, Jones M, Tyson K, Bassi C, Scarpa A, Lemoine NR. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20:7437–7446. doi: 10.1038/sj.onc.1204935. [DOI] [PubMed] [Google Scholar]
- 8.Yao H, Shi W, Wu J, Xu C, Wang J, Shao Y, Wu X, Zhang Z. Endothelial Rac1 is essential for hematogenous metastasis to the lung. Oncotarget. 2015;6:17501–17513. doi: 10.18632/oncotarget.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrizzo A, Forte M, Lembo M, Formisano L, Puca AA, Vecchione C. Rac-1 as a new therapeutic target in cerebro- and cardio-vascular diseases. Curr Drug Targets. 2014;15:1231–1246. doi: 10.2174/1389450115666141027110156. [DOI] [PubMed] [Google Scholar]
- 10.Tobar N, Caceres M, Santibanez JF, Smith PC, Martinez J. RAC1 activity and intracellular ROS modulate the migratory potential of MCF-7 cells through a NADPH oxidase and NF-kappaB-dependent mechanism. Cancer Lett. 2008;267:125–132. doi: 10.1016/j.canlet.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Espinha G, Osaki JH, Magalhaes YT, Forti FL. Rac1 GTPase-deficient HeLa cells present reduced DNA repair, proliferation, and survival under UV or gamma irradiation. Mol Cell Biochem. 2015;404:281–297. doi: 10.1007/s11010-015-2388-0. [DOI] [PubMed] [Google Scholar]
- 12.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Eller-Borges R, Batista WL, da Costa PE, Tokikawa R, Curcio MF, Strumillo ST, Sartori A, Moraes MS, de Oliveira GA, Taha MO, Fonseca FV, Stern A, Monteiro HP. Ras, Rac1, and phosphatidylinositol-3-kinase (PI3K) signaling in nitric oxide induced endothelial cell migration. Nitric Oxide. 2015;47:40–51. doi: 10.1016/j.niox.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Lai Y, Liu XH, Zeng Y, Zhang Y, Shen Y, Liu Y. Interleukin-8 induces the endothelial cell migration through the Rac 1/RhoA-p38MAPK pathway. Eur Rev Med Pharmacol Sci. 2012;16:630–638. [PubMed] [Google Scholar]
- 16.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki D, Kurisu S, Takenawa T. Involvement of Rac and Rho signaling in cancer cell motility in 3D substrates. Oncogene. 2009;28:1570–1583. doi: 10.1038/onc.2009.2. [DOI] [PubMed] [Google Scholar]
- 18.Tscharntke M, Pofahl R, Chrostek-Grashoff A, Smyth N, Niessen C, Niemann C, Hartwig B, Herzog V, Klein HW, Krieg T, Brakebusch C, Haase I. Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J Cell Sci. 2007;120:1480–1490. doi: 10.1242/jcs.03426. [DOI] [PubMed] [Google Scholar]
- 19.Santibanez JF, Kocic J, Fabra A, Cano A, Quintanilla M. Rac1 modulates TGF-beta1-mediated epithelial cell plasticity and MMP9 production in transformed keratinocytes. FEBS Lett. 2010;584:2305–2310. doi: 10.1016/j.febslet.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 20.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang WH, Lan HY, Huang CH, Tai SK, Tzeng CH, Kao SY, Wu KJ, Hung MC, Yang MH. RAC1 activation mediates Twist1-induced cancer cell migration. Nat Cell Biol. 2012;14:366–374. doi: 10.1038/ncb2455. [DOI] [PubMed] [Google Scholar]
- 22.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 23.Lv Z, Hu M, Zhen J, Lin J, Wang Q, Wang R. Rac1/PAK1 signaling promotes epithelial-mesenchymal transition of podocytes in vitro via triggering beta-catenin transcriptional activity under high glucose conditions. Int J Biochem Cell Biol. 2013;45:255–264. doi: 10.1016/j.biocel.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Jing J, Hu J, Li T, Sun Y, Guan H. RNA Interference Targeting Snail Inhibits the Transforming Growth Factor beta 2-Induced Epithelial-Mesenchymal Transition in Human Lens Epithelial Cells. J Ophthalmol. 2013;2013:869101. doi: 10.1155/2013/869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, Yu D, Ning S, Zhang H, Jiang L, He L, Li M, Sun M. Augmented Rac1 Expression and Activity are Associated with Oxidative Stress and Decline of beta Cell Function in Obesity. Cell Physiol Biochem. 2015;35:2135–2148. doi: 10.1159/000374019. [DOI] [PubMed] [Google Scholar]


