Abstract
Loeys-Dietz syndrome (LDS) is an autosomal dominant genetic connective tissue disorder, and most of LDS patients will develop into aortic aneurysm. Unfortunately, there is no known cure, and a high risk of death from aortic aneurysm rupture. However the detailed mechanism is still unknown. In order to explore the mechanism, we firstly used bioinformatics to predict, and then verified with biology methods. Firstly, we found that LncRNA AK056155 was differentially expressed in peripheral blood circulating endothelial cells between normal patients and LDS patients by bioinformatics. Then we further verified that AK056155 was also overexpressed in aortic aneurysm patients by RT-PCR. Moreover, we demonstrated that the expression of AK056155 can be enhanced by TGF-β1 in a concentration or time depended manner in HUVECs by RT-PCR. Furthermore, the expression of AK056155 was reduced with treatment of PI3K inhibitor (LY294002) or AKT inhibitor (GDC-0068) in combination with TGF-β1. These results indicate that AK056155 involved in the development of Loeys-Dietz syndrome through AKT/PI3K signaling pathway, it may provide a promising target gene to prevent LDS develop in to aortic aneurysm.
Keywords: Loeys-Dietz syndrome (LDS), aortic aneurysm, AK056155, TGF-β1, PI3K/AKT
Introduction
Loeys-Dietz syndrome (LDS) is an autosomal dominant genetic connective tissue disorder, and this disorder is marked by aneurysms in the aorta [1]. There are four types of the syndrome, Type 1, Type 2, Type 3, and Type 4 are caused by mutations in TGFBR1, TGFBR2, SMAD3, and TGFB2 respectively. Approximately 75% of LDS patients are type I syndrome [2]. Type 1 LDS is caused by mutations in TGFBR1, which is predicted to result in diminished TGF-β signaling, however, aortic surgical samples from patients show evidence of paradoxically increased TGF-β signaling [3].
The downstream of TGF-β signaling, Smad-independent pathway plays a significant role in tumor initiation and progression. Among these, P13K/Akt signaling pathway is especially outstanding [4]. After P13K/Akt signaling was activated, it contributed to inhibited apoptosis, increased proliferation, enhanced angiogenesis and accelerated migration of tumor cells [5]. For example, Shukla et al. demonstrated that aberrant activation of PI3K/Akt signaling contributed to increased cell invasion and facilitate prostate cancer progression. While the downstream target gene of PI3K/AKT signaling remain unclear.
Long non-coding RNAs (lncRNA) are non-protein coding transcripts longer than 200 nucleotides [6]. There are some many LncRNAs, however only a small proportion has been demonstrated to be biologically relevant. It is known that 118 LncRNAs in human have been functionally annotated. The preponderance of evidences have demonstrated that many transcripts thought to be LncRNAs may, in fact, be translated into proteins [7]. For example, Fu et al. reported that LncRNAPCGEM1 was correlated with increased proliferation and colony formation of prostate cancer cells [8]. MALAT1 (also known as NEAT2) was originally identified as an over expressed LncRNA during metastasis of early-stage non-small cell lung cancer [9]. While whether LncRNAs involved in the development of LDS and aortic aneurysm were still unclear.
In this study, in order to explore the role of LncRNA in the development of LDS, we used bioinformatics to predict and screen out the LncRNAs which differentially expressed between normal and LDS patients. After this we further detected the most differentially expressed LncRNA in aortic aneurysm patients. Moreover we also explored the possible mechanism how the most differentially expressed LncRNA functioned. Our study may provide a promising target for preventing the development of LDS and aortic aneurysm.
Materials and methods
Materials
M199 medium, fetal bovine serum (FBS), bovine endothelial cell growth supplement, heparin, penicillin/streptomycin, Trizol, OligodT, Super-Script First-Strand cDNA System, Platinum SYBR Green qPCR Super Mix-UDG were purchased from Invitrogen (Grand Island, NY, USA). RIPA lysis buffer was ordered from Beyotime biotechnology (Nantong, China).Protease inhibitor cocktail was obtained from Roche Molecular Biochemicals (Indianapolis, IN, USA). PVDF membranes were ordered from Millipore (Bedford, MA, USA). phospho-AKT, AKT, phospho-PI3K, PI3K and GAPDH were purchased from Cell Signaling Technology (USA). TGF-β1, Na3VO4 and NaF were obtained from Sigma-Aldrich. GDC-0068 was ordered from APExBIO company. LY294002 was ordered from Invitrogen. AK056155 and U6 Primer sequence were commercial synthesis by Funeng Biotechnology (Shanghai, China).
Patients’ information
Thirty serum samples were obtained from LDS patients in China-Japan Union Hospital of Jilin University (Changchun, China) (Table 2). Twenty serum samples were obtained from normal patients as control.
Table 2.
Theaortic aneurysm patients information
| ID | Sex | Age | History of smoking | Diagnosis | Date of Operation |
|---|---|---|---|---|---|
| 1 | Male | 67 | Yes | Abdominal aortic aneurysm | 2013.06.20 |
| 2 | Female | 75 | No | Abdominal aortic aneurysm | 2012.11.15 |
| 3 | Male | 49 | Yes | Abdominal aortic aneurysm | 2013.12.09 |
| 4 | Female | 50 | No | Abdominal aortic aneurysm | 2012.04.18 |
| 5 | Male | 71 | Yes | iliac communis aneurysm | 2008.06.03 |
| 6 | Male | 80 | Yes | Abdominal aortic dissection aneurysm | 2008.05.19 |
| 7 | Male | 35 | No | iliac communis aneurysm | 2008.05.13 |
| 8 | Male | 73 | Yes | Abdominal aortic dissection aneurysm | 2008.04.22 |
| 9 | Male | 65 | Yes | Abdominal aortic dissection aneurysm | 2006.02.06 |
| 10 | Female | 76 | Yes | Abdominal aortic aneurysm | 2011.10.13 |
| 11 | Male | 72 | Yes | Abdominal aortic dissection aneurysm | 2006.04.18 |
| 12 | Male | 61 | Yes | Abdominal aortic aneurysm | 2011.09.01 |
| 13 | Male | 54 | No | Abdominal aortic dissection aneurysm | 2007.05.07 |
| 14 | Male | 62 | Yes | iliac artery aneurysm | 2011.12.13 |
| 15 | Male | 59 | Yes | Abdominal aortic dissection aneurysm | 2005.11.02 |
| 16 | Female | 51 | No | Abdominal aortic dissection aneurysm | 2005.04.29 |
| 17 | Male | 64 | Yes | Abdominal aortic dissection aneurysm | 2005.07.02 |
| 18 | Male | 58 | Yes | Abdominal aortic aneurysm | 2011.06.13 |
| 19 | Male | 62 | Yes | Abdominal aortic dissection aneurysm | 2007.10.17 |
| 20 | Female | 77 | Yes | Abdominal aortic aneurysm | 2011.05.04 |
| 21 | Female | 56 | No | Abdominal aortic dissection aneurysm | 2005.6.21 |
| 22 | Male | 77 | Yes | Abdominal aortic dissection aneurysm | 2006.11.08 |
| 23 | Male | 68 | Yes | Abdominal aortic aneurysm | 2011.03.03 |
| 24 | Male | 73 | Yes | Abdominal aortic aneurysm | 2012.06.21 |
| 25 | Male | 54 | Yes | iliac artery aneurysm | 2010.08.30 |
| 26 | Female | 68 | Yes | Abdominal aortic aneurysm | 2010.06.23 |
| 27 | Male | 76 | Yes | Abdominal aortic aneurysm | 2013.05.27 |
| 28 | Male | 71 | No | Abdominal aortic dissection aneurysm | 2009.04.07 |
| 29 | Male | 84 | Yes | Abdominal aortic dissection aneurysm | 2009.03.03 |
| 30 | Male | 40 | Yes | Abdominal aortic dissection aneurysm | 2008.12.22 |
Cell culture and siRNA transfection
The human umbilical vascular endothelial cells (HUVECs) were obtained from Cambrex (Walkersville, MD, USA). Cells were routinely maintained in M199 medium, which containing 10% FBS, bovine endothelial cell growth supplement (20 ng/mL), heparin (4 u/mL), and 1% of penicillin/streptomycin solution (Invitrogen) at 37°C in a humidified 5% CO2 environment (Thermo). The AK056155-siRNA was designed by GenePharma (Shanghai, China). The X-treme GENE siRNA transfection reagent (Roche, Shanghai, China) was applied to transfected AK056155-siRNA into HUVECs according to the manufacturer’s instruction.
RT-PCR
HUVECs were seeded into 12-well plates and incubated for 24 hours, after this 1 ng/ml, 10 ng/ml, 100 ng/ml TGF-β were added respectively, or 10 ng/ml TGF-β treated for 0.5, 3, 12 h respectively, then continued cultured for 24 hours. The cells treated with DMSO as control. Thereafter, total RNA isolation and cDNA synthesis were conducted using TRIzol and OligodT according to the standard procedures. Briefly, first-strand cDNA was reverse-transcribed from 1 μg total RNA using the Super-Script First-Strand cDNA System, and was amplified by Platinum SYBR Green qPCR Super Mix-UDG. A master mix was prepared for each PCR reaction, which included Platinum SYBR Green qPCR Super Mix-UDG, forward primer, reverse primer, and 10 ng of template cDNA. Amplification conditions were as follows: hold at 95°C for 10 minutes, followed by 40 cycles at 15 seconds at 95°C and 1 minute in 60°C. Thermal cycling and fluorescence detection were done using the Step One Plus™ real-time PCR System (Applied Biosystems, USA). The primers used were as follows: 5-AACTCCTAACACATCTCT-3 (Forward) and 5-CTAAGGTAGTCAGTCTCA-3 (Reverse) for AK056155; 5-ATTGGAACGATACAGAGAAGATTA-3 (Forward) and 5-AATATGGAACGCTTCACGAAT-3 (Reverse) For U6.
Western blot
HUVECs were seeded into 12-well plates and incubated for 24 hours, after this 0.1 ng/ml, 1 ng/ml, 10 ng/ml TGF-β were added respectively and continued cultured for 24 hours, the cells treated with DMSO as control. At time point, cells were lysed in 300 μl RIPA lysis buffers (Beyotime biotechnology, Nantong, China) with 1 mM Na3VO4, 25 mM NaF and protease inhibitor cocktail Protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN, USA), and harvested by scraping from the wells. Protein concentrations were determined by spectrophotometry. Twenty-five micrograms of protein was loaded per lane and electrophoresed by 12% SDS-PAGE gel, and followed by transferred to PVDF membranes (Millipore, Bedford, MA, USA) using a wet transblot system. These membranes were then blocked with 5% nonfat dry milk for 1.5 h at room temperature, and incubated with primary antibodies. Rabbit monoclonal anti-human antibodies (phospho-AKT, AKT, phospho-PI3K, PI3K and GAPDH) overnight at 4°C. Secondary antibody, goat anti-rabbit, was incubated at room temperature for 2 h (Boster, Wuhan, China). The detection of specific proteins was carried out with an ECL Western blotting kit according to the recommended procedure.
Bioinformatics
Choice of differentially expressed gene list using volcano plot and heat map analysis. We obtained the microarray date from Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), and the GEO accession number is GSE38961. The date was generated using the genechip Affymetrix Human Genome U133 Plus 2.0 Array (GEO accession number is GPL570), which completely coverage Human Genome U133 Set plus 6500 additional genes for analysis of over 47,000 transcripts.
Statistic analysis
All experiments were repeated at least three times. SPASS 16.0 was used for statistical analysis. All data were expressed as mean ± standard error. Differences between the groups were analyzed using one-way analysis of variance (ANOVA), and differences were considered statistically significant when P<0.05.
Results
LncRNA AK056155 significantly overexpressed in peripheral blood circulating endothelial cells of LDS patients
Firstly, we used bioinformatics to analyze the differentially expressed LncRNA genes of peripheral blood circulating endothelial cells between normal patients and LDS patients (Figure 1A and 1B). After removed the redundant and unannoated sequences, with FDR <1%, 5 gene was found to be significantly down-regulated and 5 genes to be significantly up-regulated in Notch 1-transfer group compared to pcDNA3 vector group (Table 1). Among these up-regulated LncRNA, AK056155 was significant higher than others (P<0.01). Suggesting LncRNA AK056155 may participate in the development of LDS.
Figure 1.
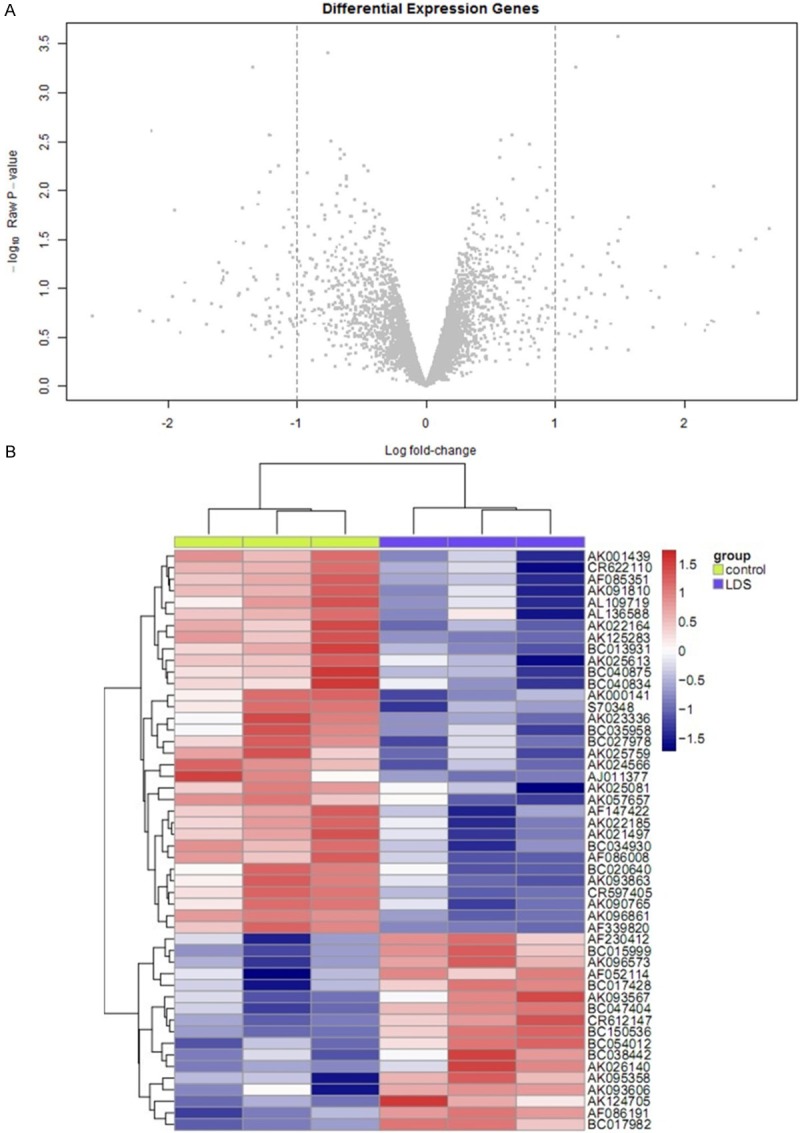
A, B. The volcano plot and heat map screen differentially expressed LncRNA genes.
Table 1.
Differentially expressed LncRNA genes of peripheral blood circulating endothelial cells between normal patients and LDS patients
| logFC | P Value | The mean expression (Control) | The mean expression (LDS) | |
|---|---|---|---|---|
| AK056155 | 2.661901 | 0.024678 | 4.845335622 | 7.507236686 |
| AK123427 | 2.569561 | 0.178763 | 4.183462117 | 6.753022725 |
| uc003qev.1 | 2.557344 | 0.031157 | 4.916085531 | 7.473429658 |
| AK130720 | 2.437549 | 0.040699 | 2.596636016 | 5.034185079 |
| AK130067 | 2.382366 | 0.060831 | 2.932106337 | 5.314472487 |
| AK024680 | -1.99304 | 0.210594 | 9.779094335 | 7.786053435 |
| AF086187 | -2.10882 | 0.218108 | 10.53881079 | 8.429992527 |
| AK000141 | -2.12346 | 0.002462 | 6.48063853 | 4.357179043 |
| AK001884 | -2.21636 | 0.169681 | 8.711327327 | 6.494969882 |
| BC040870 | -2.57869 | 0.194742 | 9.916714571 | 7.338027259 |
LncRNA AK056155 significantly overexpressed in aortic aneurysm patients
Most of LDS patients may develop into aortic aneurysm. So we also detected the expression of LncRNA AK056155 in aortic aneurysm patients, normal patients as control. The patients’ information was shown in Table 2. We found there was a significant difference in AK056155 expression between normal and aortic aneurysm patients (P<0.01) (Figure 2). The expression of LncRNA AK056155is significant high in both LDS and aortic aneurysm patients, which suggests AK056155 may become an effective target gene to prevent LDS develop into aortic aneurysm, but how the AK056155 functions still unclear.
Figure 2.
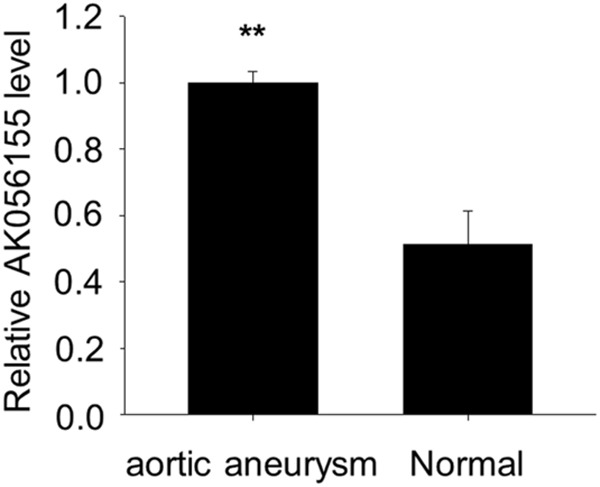
The expression of LncRNA AK056155 in normal and aortic aneurysm patients.
TGF-β1 increased the expression of LncRNA AK056155 in HUVECs
Type I LDS is caused by mutations in TGFBR1.So after we screened out the differentially expressed LncRNA AK056155, we further explored the relationship between LncRNA AK056155 and TGF-β1. We used different concentration of TGF-β1 to treat the HUVECs for 24 h. As shown in Figure 3A, the expression of LncRNA AK056155 was enhanced in a concentration depended manner, it was dramatically higher in 10 ng/ml and 100 ng/ml TGF-β1 treated group than DMSO treated group (P<0.05). Moreover, the expression of LncRNA AK056155 also could be increased in a time depended manner when the HUVECs treated with 10 ng/ml TGF-β1. The expression of LncRNA AK056155 reached the maximum after HUVECs treated with TGF-β1 for 3 h Figure 3B. These results revealed TGF-β1 can increase the expression of LncRNA AK056155 in HUVECs.
Figure 3.
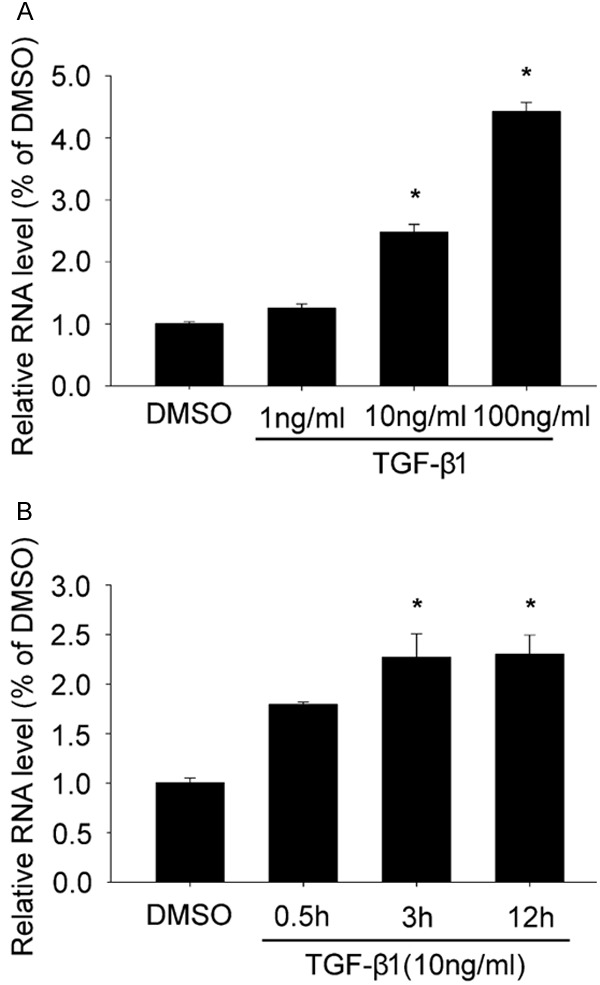
TGF-β1 increased the expression of LncRNA AK056155 in HUVECs. A. The expression of LncRNA AK056155 in HUVECs treated with DMSO, 1 ng/ml, 10 ng/ml and 100 ng/ml TGF-β1 respectively. B. The expression of LncRNA AK056155 in HUVECs treated with 10 ng/ml TGF-β1 for 0.5, 3, 12 h respectively, DMSO treated group as control. *P<0.05.
AKT/PI3K signaling involved in the process of TGF-β1 regulating AK056155 expression
As Figure 4A showed, the phosphorylation-level of PI3K and AKT were gradually enhanced with the increased concentration of TGF-β1, we hypothesis that AKT/PI3K signaling pathway may involve in the process of TGF-β1 regulating AK056155 expression. In order to further verify this hypothesis, we used PI3K inhibitor (LY294002) or AKT inhibitor (GDC-0068) in combination with TGF-β1 to co-treat the HUVECs. As shown in Figure 4B, the relative expression of LncRNA AK056155 in TGF-β1 treated group was obvious higher than DMSO treated group. Interestingly, when used AKT inhibitor (GDC-0068) to treat the HUVECs, the expression of LncRNA AK056155 was reduced (no matter whether TGF-β1 was added) compared to TGF-β1 alone treated group (P<0.05). Similarly, when used PI3K inhibitor (LY294002) to treat the HUVECs, the expression of LncRNA AK056155 was significant decreased (P<0.01). These results indicated that AKT/PI3K signaling involved in the process of TGF-β1 regulating AK056155 expression.
Figure 4.
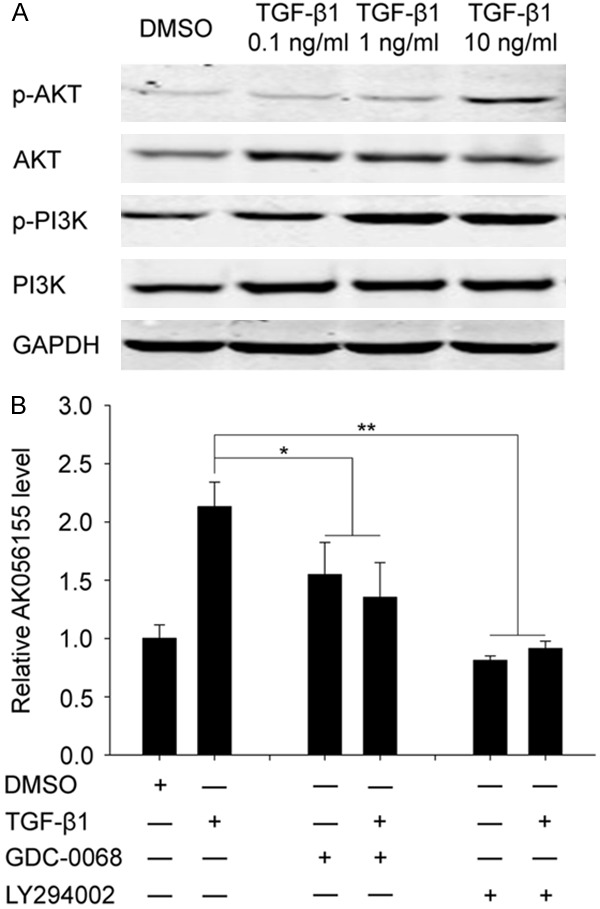
AKT/PI3K signaling involved in the process of TGF-β1 regulating LncRNAAK056155 expression. A. The expression of AKT and PI3K in HUVECs treated with DMSO, 0.1 ng/ml, 1 ng/ml and 10 ng/ml TGF-β1 respectively. B. The expression of LncRNA AK056155 in HUVECs treated with DMSO, TGF-β1, GDC-0068 with or without TGF-β1, LY294002 with or without TGF-β1 respectively. *P<0.05, **P<0.01.
Discussion
Loeys-Dietz syndrome (LDS) is an autosomal dominant aortic aneurysm syndrome and first identified by Loeys and Dietz in 2005 [10]. Unfortunately, there is no known cure for LDS, and a high risk of death from aortic aneurysm rupture is existed. In order to find out effective therapeutic targets, the mechanism of LDS development should be clear first. In this study, we illustrated that LncRNA AK056155 as the downstream of PI3K/AKT signaling involved in development of LD. So it may become a therapeutic target gene for LDS.
One characterization of LDS is abnormal TGF-β signaling. Other’s studies have shown that TGF-β signaling performs important role in tumor growth, development and metastasis [11]. The overexpression of TGF-β1 is connected with breast cancer, colorectal cancer, esophagus cancer, gastric cancer, liver cancer and lung cancer [12,13]. For example, Jong JS et al. reported 67% of invasive breast cancer patients with over expressed TGF-β1 [14]. Moreover, TGF-β1 was able to promote the development of tumor during the late stages. Wikstr et al. showed the over expressed TGF-β1 was associated with angiogenesis, metastasis of prostatic cancer, and poor prognosis of patients, also the survival rate dropped significantly [15]. TGF-β1 performs its biological effects mainly through Smad-dependence or Smad-independence signaling, PI3K/AKT is an important Smad-independence signaling and widely exists in pathogenetic process of many tumors.
It has been identified that PI3K/AKT signaling pathway is involved in many biological process, including proliferation, apoptosis, angiogenesis, tumorigenesis, tumor growth [16,17]. For example, Wu et al. demonstrated that TGF-β1 could promote epithelial-mesenchymal transition through PI3K/AKT pathway [18]. Also Andreas et al. concluded that the promoted tumor progression and metastasis that induced by TGF-β1 was related to PI3K/AKT pathway in human chondrosarcoma cells [19]. Similarly, in this study, we found that TGF-β1 was able to enhance the expression of PI3K and AKT, our results was consistent with other studies.
The performance of PI3K/AKT signaling need activated the downstream gene. In this study, we found that the expression of AK056155 can be shortened when blocked PI3K/AKT signaling with PI3K or AKT special inhibitor. Thus we thought AK056155 functioned as the downstream of PI3K/AKT signaling. AK056155 is a 2500 bp mRNA, the role of AK056155 in tumor development was rarely reported. In this study, we firstly found AK056155 was overexpressed in both LDS and aortic aneurysm patients. Moreover, in vitro we found TGF-β1 was able to increase the expression of AK056155. So we thought AK056155 participated in development of LDS and aortic aneurysm.
In summary, according to the aforementioned, we concluded that the increased TGF-β1 signaling in LDS patients activated PI3K/AKT signaling, and then activated LncRNA AK056155. The new founded signaling suggests AK056155 may become a new target for preventing the development of LDS and aortic aneurysm.
Disclosure of conflict of interest
None.
References
- 1.Williams JA, Loeys BL, Nwakanma LU, Dietz HC, Spevak PJ, Patel ND, François K, DeBacker J, Gott VL, Vricella LA, Cameron DE. Early surgical experience with Loeys-Dietz: A new syndrome of aggressive thoracic aortic aneurysm disease. Ann Thorac Surg. 2007;83:S757–S63. doi: 10.1016/j.athoracsur.2006.10.091. [DOI] [PubMed] [Google Scholar]
- 2.Loeys BL, Dietz HC. Loeys-dietz syndrome. 2013 [Google Scholar]
- 3.Gallo EM, Loch DC, Habashi JP, Calderon JF, Chen Y, Bedja D, van Erp C, Gerber EE, Parker SJ, Sauls K, Judge DP, Cooke SK, Lindsay ME, Rouf R, Myers L, ap Rhys CM, Kent KC, Norris RA, Huso DL, Dietz HC. Angiotensin II-dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124:448–60. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–67. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 6.Perkel JM. Visiting “noncodarnia”. Biotechniques. 2013;54:301, 303–4. doi: 10.2144/000114037. [DOI] [PubMed] [Google Scholar]
- 7.Smith JE, Alvarez-Dominguez JR, Kline N, Huynh NJ, Geisler S, Hu W, Coller J, Baker KE. Translation of small open reading frames within unannotated RNA transcripts in Saccharomyces cerevisiae. Cell Rep. 2014;7:1858–66. doi: 10.1016/j.celrep.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu X, Ravindranath L, Tran N, Petrovics G, Srivastava S. Regulation of apoptosis by a prostate-specific and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell Biol. 2006;25:135–41. doi: 10.1089/dna.2006.25.135. [DOI] [PubMed] [Google Scholar]
- 9.Gutschner T, Hämmerle M, Eißmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, Zörnig M, MacLeod AR, Spector DL, Diederichs S. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–9. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–81. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 11.Levy L, Hill CS. Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006;17:41–58. doi: 10.1016/j.cytogfr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–20. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 13.Bierie B, Moses HL. TGF-β and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 14.de Jong JS, van Diest PJ, van der Valk P, Baak J. Expression of growth factors, growth-inhibiting factors, and their receptors in invasive breast cancer. II: Correlations with proliferation and angiogenesis. J Pathol. 1998;184:53–7. doi: 10.1002/(SICI)1096-9896(199801)184:1<53::AID-PATH6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Wikström P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor β1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37:19–29. doi: 10.1002/(sici)1097-0045(19980915)37:1<19::aid-pros4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 17.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, Huang XF, Chen ZN, Bian H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-β signaling and is transcriptionally regulated by Slug. Oncogene. 2011;30:4410–27. doi: 10.1038/onc.2011.149. [DOI] [PubMed] [Google Scholar]
- 19.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–70. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]


