Abstract
Background: MicroRNA-374a (miR-374a) has been implicated in several cancers. However, its role in osteosarcoma (OS) remains unclear. Thus the aim of this study was to investigate its expression and role in progression of OS. Methods: Quantitative real-time PCR (qRT-PCR) was performed to detect the expression of miR-374a in OS cell lines and tissues. To further understand its role, we restored expression of miR-374a in MG63 cell line by transfection with miR-374a mimics or inhibitors. Effects of miR-374a on cell proliferation on targets were also determined. Results: In the present study, our results showed that miR-374a was significantly up-regulated in both OS cell lines and OS tissues. Over expression of miR-374a markedly accelerated proliferation of OS cells, while its inhibition significantly suppressed cell proliferation. Moreover, Axin2 was identified to be a functional downstream target of miR-374a, and decreased expression of Axin2 could promote OS cell proliferation. Conclusion: Our study suggested that miR-374a functions as an oncogene in OS, and the miR-374a/Axin2 axis might represent a potential therapeutic target for OS intervention.
Keywords: Osteosarcoma, miR-374a, Axin2, proliferation
Introduction
Osteosarcoma (OS) is the most frequent malignant bone tumor in children and adolescents, comprising 2.4% of all malignancies in pediatric patients [1]. The 5-year survival rate of OS patients has significantly improved over the past decades to approximately 60-70% since the introduction of combinatorial chemotherapy [2]. However, a significant proportion of OS patients still respond poorly to chemotherapy, and they have a risk of local relapse or distant metastasis even after curative resection of the primary tumor and intensive chemotherapy [3]. Therefore, it is important to study the molecular mechanisms of OS and explore new therapeutic agents.
MicroRNAs (miRNAs) are a class of small (19-25 nt), non-coding regulatory RNAs, transcribed from non-protein-coding genes or introns, which mediate translational suppression or cleavage of their target mRNAs by binding to complementary sites in their 3’UTR [4]. They play an essential role in cell cycle regulation, apoptosis and tumorigenesis [5]. Aberrant miRNA expression profiles have been identified in tumors, as compared to normal tissues, thus establishing them as a relatively new and important class of oncogenes and tumor suppressors [6]. For example, Chu et al. found that miR-630 expression level was significantly elevated and association with tumor progression in human gastric cancer [7]. Li et al. showed miR-646 was down regulated in ccRCC and was associated with tumour metastasis through MAPK pathway by targeting NOB1 [8]. Wen et al. revealed that miR-506 expression was downregulated in human cervical cancer and acted as a tumor suppressor by directly targeting the hedgehog pathway transcription factor Gli3 [9].
miRNA profiling has shown that miR-374a is overexpressed in many types of cancers. For example, Xu et al. found that miR-374a expression was up-regulated in human gastric cancer tissues and promoted gastric cancer cell proliferation, migration and invasion by targeting SRCIN1 [10]. Wang et al. found that the expression of miR-374a was increased in gefitinib-resistant lung cancer cells [11]. In contrast, in early-stage non-small cell lung cancer, the expression of miR-374a was decreased and associated with poor patient survival [12]. However, the expression and clinical significance of miR-374a in osteosarcoma are still unknown.
The aim of this study was to understand the role of miR-374a in osteosarcoma progression. MiR-374a expression in OS cell lines and tissues was determined by using qRT-PCR. Furthermore, we introduced miR-374a overexpression and underexpression models to investigate the effect of miR-374a on OS cells growth. Finally, Axis inhibition protein 2 (Axin2) was identified as a direct target of miR-374a.
Materials and methods
OS cancer tissue
A total of 32 pairs of OS tissues and their matched adjacent normal bone were obtained from patients who underwent surgery at The First Affiliated Hospital of Xinxiang Medical University between April 2012 and April 2014 were selected. The diagnosis of osteosarcoma was confirmed pathologically. All the tissue samples were collected, immediately snap frozen in liquid nitrogen, and stored at -80°C until use. The study was approved by the Research Ethics Committee of The First Affiliated Hospital of Xinxiang Medical University. Informed consent was obtained from all patients.
Cell culture
Human OS cell lines (HOS, SaOS2, MG63 and U2OS), and the human osteoblastic cell line (hFOB1.19) were purchased from the American Tissue Culture Collection (ATCC). Human OS cells were cultured in RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin (Invitrogen). hFOB1.19 cells were cultured in Ham’s F12/DMEM (Gibco) supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37°C with 5% CO2 in a humidified chamber.
Overexpression or knockdown of miR-374a
miR-374a mimics, miR-374a inhibitor and scrambled negative control RNA (miR-Ctrl and anti-miR-Ctrl) were synthesized by GenePharma and transfected into the cells to a final oligonucleotide concentration of 10 nmol/l. Transfection was performed using Lipofetamine 2000 Reagent (Invitrogen) following the manufacturer’s protocol. Cells were trypsinized, counted and seeded in plates on the day before transfection to ensure suitable cell confluence. Oligonucleotides and plasmids were used at final concentrations of 10 nmol/L, both in antibiotic-free RPMI 1640 medium (Invitrogen).
Decreased expression of Axin2
The transfection of Axin2 siRNA (si-Axin2) and their negative control siRNA (si-NC) into MG63 cells was performed using lipofectamine 2000 reagent according to the manufacturer’s instructions. The sequences of siRNA and its negative control were as follows: Axin2: 5’-GCAGAGGGACAGGAATCAT-3’, the negative control: 5’-GCAGGGACAAGGTAGACAT-3’.
Luciferase reporter assay
The Axin2 3’UTRs containing predicted miR-374a binding sites were amplified by PCR from human cDNA using primers, and inserted into pMIR-REPORT luciferase reporter vectors (Ambion), to obtain constructs containing wild-type Axin2 3’UTR (Axin2-WT), Axin2-MUT contained sequences with mutations in the first putative binding site of Axin2 3’UTR. Predicted seed regions in these mRNA sequences were created, using primers including mutated sequences. Recombination constructs, pRL-TK (Promega) and miR-374a were co-transfected into MG63 cells using lipofectamine 2000 (Invitrogen). Plasmids of pRL-TK containing Renilla luciferase were used as internal control. Firefly and Renilla luciferase activity were measured using Dual Luciferase Assay (Promega) according to the manufacturer’s instructions, 24 h after transfection. All transfection assays were carried out in triplicate.
Cell proliferation assay
To assess cell proliferation, MG63 cells were plated in 96-well plates overnight and transfected with miR-374a mimic or miR-374a inhibitor. Proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2-5 diphenyltetrazolium bromide (MTT, Sigma) assay at 24, 48, 72, and 96 hours after transfection, and the absorbance of samples was measured with a spectrophotometer reader (Molecular Devices) at 570 nm. All experiments were performed in six replicates and were repeated three times independently.
Cell cycle assay
To assess cell cycle distribution, MG63 cells were plated in 6-well plates and transfected with miR-374a mimic or miR-374a inhibitor. After transfection, the cells were collected by trypsinization, fixed in 70% ice-cold ethanol overnight, then washed with phosphate buffer saline (PBS), and stained with propidium iodide (PI, 50 mg/ml) in PBS supplemented with RNase (50 mg/ml) in the dark at room temperature (RT) for 30 min. Tests were performed in triplicate for each sample, and analyses of cell cycle distribution were performed by flow cytometer in accordance with the manufacturer’s guidelines (BD Bioscience).
Apoptosis assay
The Annexin V-FITC Apoptosis Detection kit (BD Biosciences) and PI was used to assess the apoptotic effect of miR-374a. MG63 cells transfected with miR-374a mimic or miR-374a inhibitor were suspended in RPMI-1640 medium. The cells were re-suspended in 500 μl cold Binding Buffer with 1.25 μl Annexin V-FITC, and incubated for 15 min at RT in the dark. Cells were re-suspended in 500 μl cold Binding Buffer with 10 μl PI, incubated for 4 hours and analyzed by flow cytometry (BD Bioscience).
RNA extraction and qRT-PCR analyses
Large and small RNAs from tissue were isolated using the mir-Vana miRNA Isolation Kit (Ambion) according to the manufacturer’s protocol. Total RNAs of the cell lines were extracted using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Five micrograms of the RNA sample was reverse transcribed to cDNA using oligo primers and M-MLV reverse transcriptase (Promega); the cDNA was used for the amplification of Axin2 and GAPDH. Quantitative RT-PCR (qRT-PCR) was performed to detect the relative transcript levels of miR-374a and Axin2. PCR was performed under the following conditions: 94°C for 4 min followed by 40 cycles of 94°C for 1 min, 56°C for 1 min and 72°C for 1 min. The relative expression levels of the gene of interest were calculated using the 2-ΔΔCt method. All primers were synthesized by AuGCT Inc. (Beijing).
Western blotting analysis
Cells were lysed using the mammalian protein extraction reagent RIPA (Beyotime) supplemented with a protease inhibitor cocktail (Roche) and phenylmethylsulfonylfluoride (Roche). Fifty micrograms of protein extracts were separated by 10% SDS-PAGE, transferred to 0.22 mm NC membranes (Sigma) and incubated with specific antibodies. Autoradiograms were quantified by densitometry (Bio-Rad). The Axin2 antibody (1:1000) was purchased from Abcam. Protein was detected with Image Acquisition using ImageQuant™ LAS 4000 (GE Healthcare Life Sciences).
Statistical analysis
Data are expressed as the mean ± SD from at least three separate experiments. Differences between groups were analyzed using Student’s t test or one-way ANOVA analysis. All statistical analyses were performed using SPSS 18.0 software (IBM). P < 0.05 was considered statistically significant.
Results
miR-374a is upregulated in OS cell lines and tissues
We tested the miR-374a levels in OS cell lines (HOS, SaOS2, MG63, U2OS) using qRT-PCR. Our results showed that miR-374a levels were significantly increased in all four OS cells compared to human osteoblastic cell line hFOB1.19 cells (Figure 1A). Furthermore, we examined the expression level of miR-374a in human OS tissues from 32 patients. The expression level of miR-374a was significantly increased in OS tissues versus corresponding non-tumor tissues (Figure 1B). Taken together, these results indicated that miR-374a may act as a tumor oncogene in OS.
Figure 1.
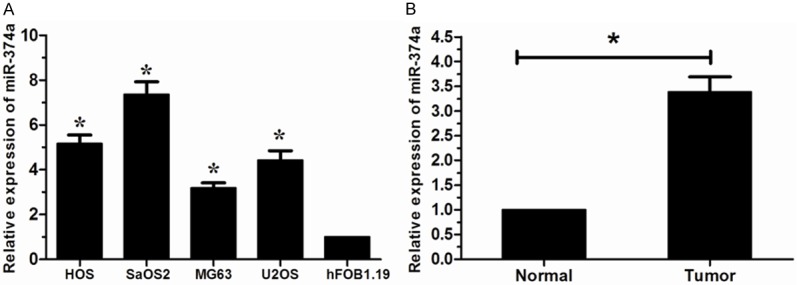
Expression of miR-374a in Os cell lines and tissues. A. miR-374a expression was increased in Os cell lines (HOS, SaOS2, MG63 and U2OS) compared to hFOB1.19 cells determined by using qRT-PCR. B. miR-374a expression was significantly upregulated in OS tissues than in the corresponding non-tumor tissues. *P < 0.05.
miR-374a overexpression promotes cell proliferation
In order to assess the effects of miR-374a on OS cell proliferation, MG63 was transfected with miR-374a mimics. Increased expression of miR-374a was confirmed by qRT-PCR (Figure 2A). We found that over expression of miR-374a significantly increased the proliferation of MG63 cells compared to scrambled negative control (Figure 2B). Then, we analyzed the cell cycle by flow cytometry, we found that ectopic overexpression of miR-374a induced a significant decrease in the percentage of cells in G1/G0 phase and an increase in the percentage of cells in S phase (Figure 2C). Furthermore, we investigated the effect of miR-374a on OS cell apoptosis. Our results showed that overexpression of miR-374a significantly inhibited apoptosis of MG63 cells (Figure 2D). All these results suggested that increased expression of miR-374a promoted the proliferation of MG63 cells.
Figure 2.
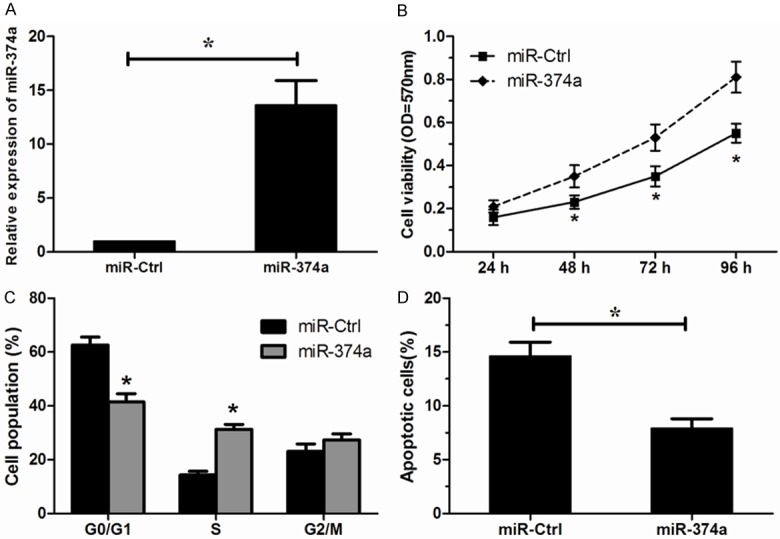
Over expression of miR-374a promotes MG63 cells proliferation. A. Successful overexpression of miR-374a was confirmed by qRT-PCR. B. Effects of miR-374a overexpression on the cell proliferation analyzed by MTT assay. C. Effects of miR-374a overexpression on the cell cycle measured by flow cytometry analysis. D. Effects of miR-374a overexpression on the cell apoptosis measured by apoptosis analysis. *P < 0.05.
Inhibition of miR-374a suppresses cell proliferation
To validate the promotive role of miR-374a in OS cells proliferation, an antisense-based specific inhibitor against miR-374a was applied to inhibit the expression of miR-374a in MG63 cells (Figure 3A). In contrast to miR-374a overexpression, miR-374a inhibition significantly decreased OS cell proliferation (Figure 3B). Additionally, flow cytometry showed a significant increase in the percentage of cells in G1/G0 phase and a decrease in the percentage of cells in S phase by miR-374a inhibitor (Figure 3C). Furthermore, silencing of miR-374a noticeably promoted apoptosis of MG63 cells (Figure 3D). These results demonstrated that decreased expression of miR-374a could inhibit the proliferation of MG63 cells.
Figure 3.
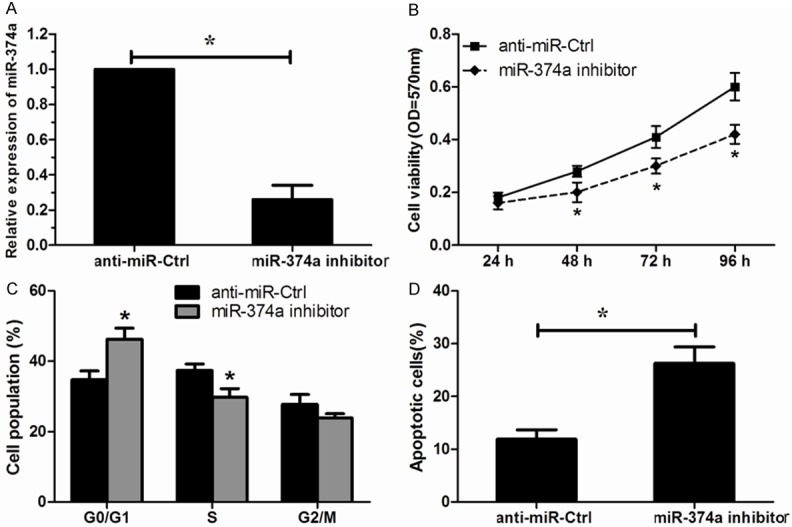
Down regulated expression of miR-374a inhibits MG63 cells proliferation. A. Successful decreased expression of miR-374a was confirmed by qRT-PCR. B. Effects of miR-374a inhibition on the cell proliferation analyzed by MTT assay. C. Effects of miR-374a inhibition on the cell cycle measured by flow cytometry analysis. D. Effects of miR-374a inhibition on the cell apoptosis measured by apoptosis analysis. *P < 0.05.
Axin2 is a direct target gene of miR-374a
To further explore the mechanism by which miR-374a promotes OS cell proliferation, we explored miR-340 targets using the TargetScan bioinformatics algorithm. Our analysis revealed that Axin2 was a potential target of miR-374a based on putative target sequences at position 1321-1327 of the Axin2 3’UTR (Figure 4A). To confirm Axin2 as a direct target of miR-374a, we engineered luciferase reporter constructs containing the wild-type (WT) or mutant (MUT) 3’UTR of the Axin2 gene. Luciferase reporter assay showed that miR-374a significantly decreased the luciferase activity of the Axin2 3’UTR but not that of the mutant in MG63 cells (Figure 4B). Moreover, overexpression of miR-374a in OS cells led to reduced Axin2 protein expression, consistently, inhibition of miR-374a led to an increased expression of Axin2 contents (Figure 4C). Taken together, these data strongly suggest that Axin2 is a direct target of miR-374a in OS.
Figure 4.
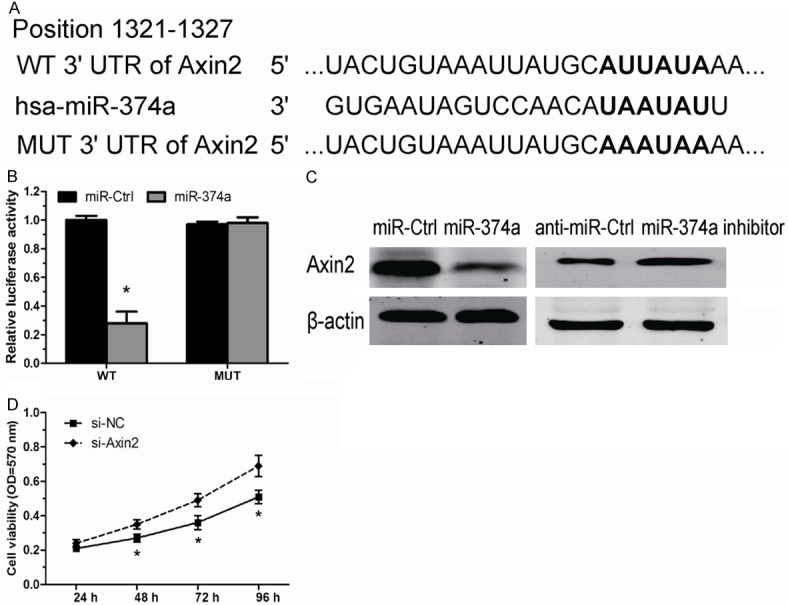
miR-374a negatively regulates Axin2 in OS cell. A. The wild type and mutant complementary sequences of the Axin2 mRNA 3’UTR are shown with the miR-374a sequence. B. luciferase reporter assay showed the inhibitory effect of miR-374a on Axin2 3’UTR luciferase activity in MG63 cells. C. The expression levels of Axin2 protein in MG63 cells transfected with miR-374a mimics and miR-374a inhibitor by Western blot. D. Cell proliferation assay was performed after transfection with si-Axin2 or si-NC. *P < 0.05.
Axin2 knockdown increases MG63 cells proliferation
Axin2 is a negative regulator of the canonical Wnt/β-catenin signaling pathway, and acts as a tumor suppressor protein in a number of human cancers [13]. However, it is less involved the role of Axin2 in OS. We further investigated whether decreased Axin2 by miR-374a plays a tumor suppressor role in OS, we transfected si-Axin2 into MG63 cells. Our data showed decreased expression of Axin2 significantly promoted OS cell proliferation (Figure 4D), similar to those induced by miR-374a. Taken together, these findings indicated that Axin2 is a functionally important target of miR-374a that is involved in the proliferation of OS cells.
Discussion
The present study was set forth to evaluate the role of miR-374a in the regulation of proliferation of OS cells and possible mechanisms. We found that the miR-374a expression was significantly increased in OS cell lines and tissues. In OS cells with miR-374a overexpression, the proliferation ability was significantly increased. In contrast, in cells with miR-374a underexpression, the proliferation ability was significantly decreased. The overexpression and underexpression of miR-374a exhibited a decrease and increase effect on the transcription activity and expression of Axin2, respectively, which acted as a tumor suppressor in OS. Thus, our results identify a new role of miR-374a in OS.
miRNAs have been shown to be a significant gene modulator during OS progression [14]. In the present study, we used qRT-PCR to identify the expression of miR-374a in OS. The biological functions of miR-374a are quite different according to tumor types. For example, Wang et al. found that miR-374a were up-regulated in colonic cancer tissues compared with the para-cancerous controls [15]. Zhang et al. showed that miR-374a was significantly up-regulated in bladder urothelial carcinoma samples compared with corresponding normal counterparts [16]. However, Võsa et al. indicated that miR-374a was down regulation in early-stage non-small cell lung cancer and associated with poor patient survival [12]. miR-374a was markedly increased in primary breast cancer, and over-expression of miR-374a directly promoted epithelial-mesenchymal transition and metastasis both in vitro and in vivo [17], which showed a directly effect that miR-374a promoted breast cancer tumorigenesis. Consistent with these studies, our study indicated that miR-374a play an oncogene role on the proliferation of OS cells. Thus, these results, together with the high expression in OS tissue, suggesting the important role of miR-374a in OS progression.
miRNAs exert their function by interacting with the 3’UTR of the target mRNA. miR-374a was reported to directly target and suppressed multiple negative regulators of the Wnt/catenin signaling cascade [17]. As a member of β-catenin/T-cell factor-dependent genes, Axin2 has complementary sequences of miR-374a core region, which indicated the possible interaction between miR374a and Axin2. Luciferase reporter assay showed that miR-374a significantly decreased the luciferase activity of the Axin2 3’UTR in MG63 cells. Furthermore, Western blot showed that the Axin2 protein level was decreased by miR-374a, but increased by miR-374a inhibition. Taken together, these results suggested that Axin2 was a directed target of miR-374a in OS.
Wnt/β-catenin signaling is important to the development of a broad range of human cancers [18]. However, the investigation of Wnt pathway components in OS is less involved. In the present study, we knockdowned Axin2 expression using Axin2 specific siRNA. Downregulated expression of Axin2 significantly increased the proliferation of MG63 cells, indicating the suppressive role of Axin2 in OS. Thus, Axin2 suppression by miR-374a may underline the mechanism by which miR-374a promotes OS growth.
In summary, the present study showed that miR-374a was dramatically increased in OS cell lines and tissues. Moreover, decreased expression of miR-374a in MG63 OS cells suppressed cell proliferation in vitro by inducing cell cycle arrest in G0/G1 phase and promoting cell apoptosis through the targeting of Axin2. These results provide a strategy for targeting the miR-347a/Axin2 interaction in a novel therapeutic application to treat OS patients.
Disclosure of conflict of interest
None.
References
- 1.Picci P. Osteosarcoma (osteogenic sarcoma) Orphanet J Rare Dis. 2007;2:6. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: Where do we go from here? Paediatr Drugs. 2008;10:315–327. doi: 10.2165/00148581-200810050-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ottaviani G, Jaffe N. Pediatric and Adolescent Osteosarcoma. Springer; 2010. The epidemiology of osteosarcoma; pp. 3–13. [Google Scholar]
- 4.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Chu D, Zhao Z, Li Y, Li J, Zheng J, Wang W, Zhao Q, Ji G. Increased MicroRNA-630 Expression in Gastric Cancer Is Associated with Poor Overall Survival. PLoS One. 2014;9:e90526. doi: 10.1371/journal.pone.0090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Liu M, Feng Y, Xu YF, Huang YF, Che JP, Wang GC, Yao XD, Zheng JH. Downregulated miR-646 in clear cell renal carcinoma correlated with tumour metastasis by targeting the nin one binding protein (NOB1) Br J Cancer. 2014;111:1188–1200. doi: 10.1038/bjc.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen S, Lin Y, Yu Y, Cao S, Zhang R, Yang X, Li J, Zhang Y, Wang Y, Ma M. miR-506 acts as a tumor suppressor by directly targeting the hedgehog pathway transcription factor Gli3 in human cervical cancer. Oncogene. 2015;34:717–25. doi: 10.1038/onc.2014.9. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Wang W, Su N, Zhu X, Yao J, Gao W, Hu Z, Sun Y. miR-374a promotes cell proliferation, migration and invasion by targeting SRCIN1 in gastric cancer. FEBS Lett. 2015;589:407–413. doi: 10.1016/j.febslet.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Xia H, Zhuang Z, Miao L, Chen X, Cai H. Axl-altered microRNAs regulate tumorigenicity and gefitinib resistance in lung cancer. Cell Death Dis. 2014;5:e1227. doi: 10.1038/cddis.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Võsa U, Vooder T, Kolde R, Fischer K, Välk K, Tõnisson N, Roosipuu R, Vilo J, Metspalu A, Annilo T. Identification of miR-374a as a prognostic marker for survival in patients with early-stage nonsmall cell lung cancer. Genes Chromosomes Cancer. 2011;50:812–822. doi: 10.1002/gcc.20902. [DOI] [PubMed] [Google Scholar]
- 13.Ma C, Liu C, Huang P, Kaku H, Chen J, Guo K, Ueki H, Sakai A, Nasu Y, Kumon H. Significant association between the Axin2 rs2240308 single nucleotide polymorphism and the incidence of prostate cancer. Oncol Lett. 2014;8:789–794. doi: 10.3892/ol.2014.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones KB, Salah Z, Del Mare S, Galasso M, Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, Volinia S, Stein GS, Croce CM, Lian JB, Aqeilan RI. miRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 2012;72:1865–1877. doi: 10.1158/0008-5472.CAN-11-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen XM, Gao HJ. Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. J Dig Dis. 2010;11:50–54. doi: 10.1111/j.1751-2980.2009.00413.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZC, Huang Y, Wang XJ, Wang M, Ma LL. [Expression of circulating microRNAs in patients with bladder urothelial carcinoma] . Beijing Da Xue Xue Bao. 2013;45:532–536. [PubMed] [Google Scholar]
- 17.Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan J, Wu J, Li M. MicroRNA-374a activates Wnt/beta-catenin signaling to promote breast cancer metastasis. J Clin Invest. 2013;123:566–579. doi: 10.1172/JCI65871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]


