Abstract
Gastric cancer is one of the most common causes of digestive tract tumor. Despite of recent advances in surgical techniques and development of adjuvant therapy, the underlying mechanisms of gastric cancer remain poorly understood and relevant insight into novel treatment strategies using gene target remains incomplete. Recently, several studies report that epithelial to mesenchymal transition (EMT) is a crucial process for the invasion and metastasis of epithelial tumors; however, the molecular mechanisms underlying this transition are unknown. As a cis-Golgi matrix protein, GM130 plays an important role in cell cycle progression and transport of protein in the secretory pathway. In this study, we found that GM130 expression has a positive correlation with the pathological differentiation and tumor node metastasis (TNM) stage of gastric cancer. High GM130 expression levels also predict shorter overall survival of gastric cancer patients. RNA interference-mediated knockdown of GM130 expression increased epithelial marker (E-cadherin) and decreased mesenchymal marker (N-cadherin and vimentin) expression in gastric cancer cells, suppressing cell invasion, and tumor formation. Furthermore, we found that GM130 upregulated expression of the key EMT regulator Snail (SNAI1), which mediated EMT activation and cell invasion by GM130. Taken together, our study indicates GM130 may be a promising therapeutic biomarker for gastric cancer.
Keywords: GM130, gastric cancer, EMT, invasion, migration
Introduction
Gastric cancer is the most common type of tumor in humans. Despite of recent advances in surgical techniques and development of chemotherapy/radiotherapy, the mortality of gastric cancer remains high and 5-year survival rate lower than 30% [1,2]. A recently elucidated secretory pathway is attracting considerable interest as a promising anticancer target. To provide insight that will enable the development of new therapeutic strategies, it is crucial to elucidate the molecular mechanisms that promote the migration and invasion properties of gastric cancer. Recent studies have shown that a morphologic conversion, known as epithelial-to-mesenchymal transition (EMT), is associated with the acquisition of malignant characteristics in gastric cancer cells [3,4].
GM130, a cis-Golgi Matrix Protein, is a peripheral membrane protein strongly anchored to the Golgi member which is isolated from the detergent and salt resistant Golgi matrix. Besides maintain of the Golgi structure and stacking of Golgi cisternae, it also is involved in the regulation of ER-to-Golgi transport and glycosylation, cell cycle progression, and the physiology of multicellular organisms. However, emerging evidence has indicated that GM130 has unexpected roles in the control of higher order cell functions such as cell division, polarization and directed cell migration and various disease. It has been reported that GM130 is overexpressed in cervical cancer cell lines [5,6], lung cancer cell lines, and prostate cancer cell lines. Knockdown of GM130 inhibits the lateral fusion of Golgi stacks and disturbs the uniform distribution of Golgi enzymes affecting proper glycosylation of membrane and secretary proteins and also issues in delay of ER-to-Golgi transport or a partial inhibition. In addition, suppression of cells proliferation, invasion, angiogenesis and increase of autophagy have been reported in A549 cells which lack GM130 [7,8].
A number of possible reasons may explain why this novel role for GM130 and in the poor prognosis of oncogenic gastric cancer has not been previously described. In this study, we report that the expression of GM130 was significantly up regulated in gastric cancer tissues compared with their noncancerous counterparts as shown by immunohistochemical staining and RT-PCR. In addition, siRNA-mediated knockdown GM130 expression inhibited cell proliferation and decreased cancer cell migration and invasion in vitro. We show that GM130 decreases the expression of the epithelial markers E-cadherin but increases the expression of mesenchymal markers fibronectin and N-cadherin. The results indicate GM130 induces EMT and cell invasion in gastric cancer cells by promoting Snail expression in gastric cancer.
Materials and methods
Cell lines and tissue samples
Three human gastric cancer cell line MKN-45, SGC-7901 and MKN-28 were purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China). All the cell lines were maintained in RPMI-1640 supplemented with 10% FBS and 1% antibiotics. A total of 84 paraffin-embedded gastric cancer samples of which 42 were with paired adjacent tissues were obtained through the Bayannaoer City Hospital, Inner Mongolia. All gastric cancer patients who had not received any pre-operative chemotherapy, radiotherapy or immunotherapy were diagnosed by at least two experience pathologists independently. This study was approved by the Ethics Committee of The Bayannaoer City Hospital, Inner Mongolia.
Immunohistochemistry
All tissue sections (4 µm) were routinely dewaxed, rehydrated, and then was subjected to heat with citrate buffer in a microwave oven for antigen retrieval. Endogenous peroxidase activity was blocked for 15 min in 3% hydrogen peroxide in deionized water. The slides were then added blocking normal goat serum and followed by incubation at 4°C overnight with GM130 rabbit polyclonal antibody (1:100). Sections were incubated with biotinylated second antibody and the streptavidin-biotin peroxidase complex (ZSGB-BIO, Beijing, China). In addition, tissue sections were stained with diaminobenzidine (ZSGB-BIO). Ultimately, all the treated sections were lightly counterstained with hematoxylin, dehydrated and mounted. Expression of GM130 was evaluated as the percentage of positive cells in a specimen, and by staining intensity as described previously [9]. The percentage of positive cells was evaluated quantitatively and scored as (0 = 0%, 1 = 1-25%, 2 = 26-50%, 3 = 51-75%, 4 = 76-100%) and the intensity was graded as follows (0 = none, 1 = weak, 2 = moderate, 3 = strong). The final result of GM130 expression was the product of scores of percentage of positively stained cells and the staining intensity (negative, 0-1; weakly positive, 1-2; moderately positive, 2-3; strongly positive, ≥ 3).
Quantitative real-time PCR
Total RNA was extracted from cultured cells using EZNA Total RNA Kit (OMEGA Bio-tek, USA), and cDNA was generated using PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Otsu, Japan). Quantitative real-time PCR was performed using the SYBR Premix ExTaq II (TLiRNaseH Plus) (TaKaRa, Otsu, Japan) with a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA).
Western blot analysis
After measuring the protein concentration of the lysates using the BCA method (Beyotime, China), equal amounts (35 µg) of protein were separated by SDS-PAGE, blotted onto PVDF membranes (Millipore, Billerica, MA). The membranes were blocked with TBST containing 5% skim milk for 1 hour at room temperature, incubated with the corresponding primary antibodies overnight at 4°C and then with secondary antibodies conjugated to horseradish peroxide for 2 h at room temperature. The protein was visualized using enhanced chemiluminescence (GE Healthcare Biosciences).
SiRNA transfection
MKN-45 cell line was transfected with three kinds of siRNAs targeting GM130 (GenePharma, Shanghai, China). The sequences of the siRNAs used to suppress GM130 expression was: sense, 5’-CUGGACUCCAGCUAUGUAATT-3’, and antisense, 5’-UUACAUA GCUGGAGUCCAGTT-3’. The scrambled siRNA as negative control was: sense, 5’-UUCU CCGAACGUGUCACGUTT-3’, and antisense, 5’-ACGUGACACGUUCGGAGAATT-3’. Cell transfection was carried out using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions.
Cell migration and invasion assays
For transwell migration assays, 2×104 cells were seeded in the top chamber with the non-coated membranes (24 well insert, 8.0-mm, BD Bioscience). Cells were seeded in a serum-free medium and then migrated toward complete growth medium. For the invasion assay, cells were serum-starved overnight and 2×104 cells were seeded in a Matrigel-coated chamber and cultured for 48 h. The invaded or migrated cells were fixed with 70% methanol and stained 0.5% crystal violet. Cells invaded or migrated the lower surface of filters were counted in five randomly selected fields. All experiments were carried out in triplicate.
Luciferase reporter assay
The previously reported promoter region for Snail (-1, 558/+92) was amplified and cloned into the pGL4 vector (Promega) [10]. 293T cells were transfected with the pGL4-Snail/promoter together with pQCXIP-GFP-GM130 and pRTK-Luc to normalize transfection efficiency. Forty-eight hours later, the activities of firefly luciferase and Renilla luciferase were measured using the Dual Luciferase Reporter Assay System (Promega). Luciferase activity was measured in triplicate, and 3 independent experiments were carried out. To measure luciferase activity in the absence of GM130 expression, MKN-45 cells were transfected with control or GM130 siRNA together with pRTK-Luc and pGL4-Snail/promoter. Seventy-two hours later, luciferase activities were measured.
Statistical analysis
Statistical analysis for cell invasion, colony formation, and tumor formation in mice was conducted using the Student t test. P < 0.05 was considered statistically significant. The association between GM130 expression and tumor stage was determined by test.
Results
Upregulation of GM130 expression in human gastric cancer
To determine the clinicopathological significance of GM130, GM130 expression was evaluated by immunohistochemistry in 84 pairs of human gastric and adjacent non-cancerous counterparts. We found that strong expression of was observed in some cancer tissues, and the expression was correlated with tumor stage (Figure 1A). Increased GM130 expression was also found to correlate with shorter overall survival (P < 0.001) of patients (Figure 1B). Of the 84 samples, we observed that GM130 expression was more frequently in carcinomas (88.1%) than adjacent non-cancerous tissues cells (52.4%). To explore the role of GM130 in human gastric cancer progression, we first evaluated GM130 expression levels in various gastric cancer cell lines. Interestingly, we found that GM130 expression was related with the malignant characteristics of gastric cancer cells. MKN-45 was more invasive than other gastric cancer cell lines and was able to grow under anchorage-independent conditions. These findings show that GM130 is overexpressed in primary human gastric cancer and metastatic gastric cancer cells.
Figure 1.
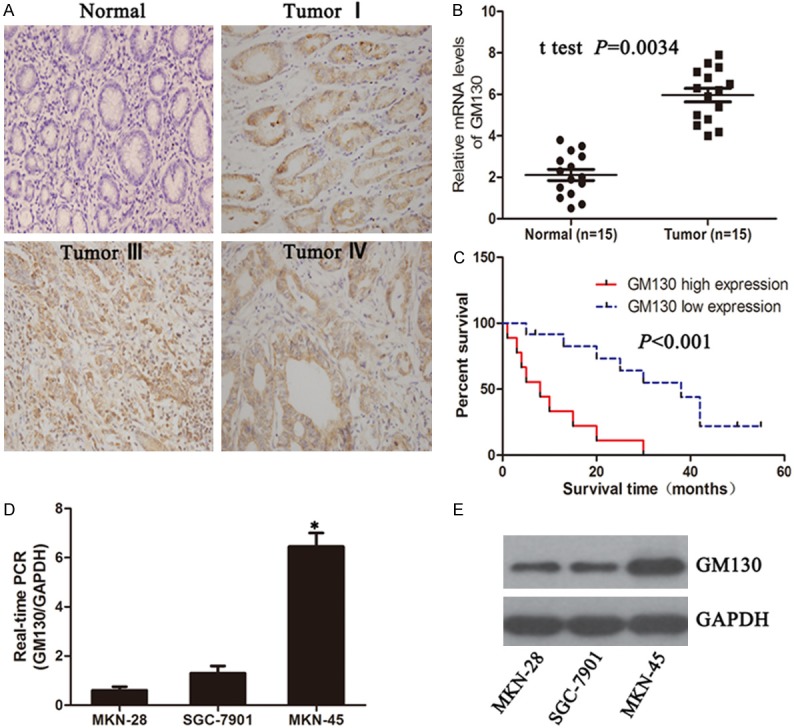
Expression of GM130 in human gastric cancer patient specimens and cell lines. (A) Expression of GM130 in normal gastric tissue and different stage gastric cancer (400×). (B) Over-expression of GM130 at mRNA level in gastric cancer tissues. Expression of GM130 in normal and gastric cancer tissue was analyzed by qRT-PCR (C) Kaplan-Meier plots of GM130 expression in 20 cases of gastric cancer patients. Overall survival rate was performed by log-rank test. P < 0.05 indicate significant differences between two groups. (D, E) Expression of GM130 in breast cancer cell lines (P < 0.001).
Downregulation of GM130 reduces migration and invasion of MKN-45 cells
To determine the effect of GM130 on cell migration and invasion. Migration was assessed using a short-term transwell assay (Figure 2A and 2B). And then, we assessed cell invasiveness, we used Matrigel-coated Boyden chambers. GM130 knockdown reduced cell migration and invasion of MKN-45 cells (Figure 2C and 2D).
Figure 2.
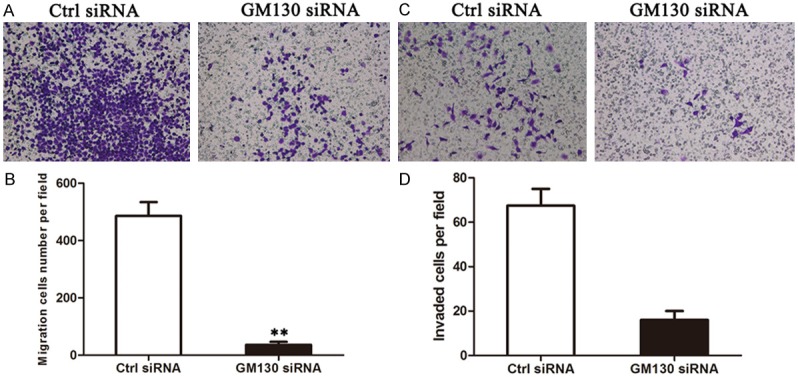
Depletion of GM130 reduces migration and invasion of MKN-45 cells. A, C. Cells were transfected with siRNA and then subjected to the in vitro migration and invasion assay 72 hours later. Representative images of invading cells are shown. B, D. The graph indicates the average number of migrated and invaded cells per field. Three independent experiments were carried out, and the data are shown as the mean with SD (P < 0.05).
GM130 regulates EMT in gastric cancer cells
Epithelial-to-mesenchymal transition is associated with malignant properties, such as invasion and anchorage-independent growth. We hypothesized that the upregulation of GM130 may promote EMT in gastric cancer cells. High metastatic gastric cancer cell line (MKN-45) demonstrated elevated GM130 expression, were spindle shaped and exhibited reduced cell contact. GM130 knockdown resulted in a dramatic shift from spindle shaped to a cobblestone-like morphology. To test this hypothesis, the expression levels of epithelial and mesenchymal markers were examined. By western blot and immunofluorescence, we found that depletion GM130 expression caused a increase in epithelial marker (E-cadherin), but an decrease in mesenchymal markers (N-cadherin and vimentin) in MKN-45 cells (Figure 3A). These results indicate that GM130 has an important role in EMT regulation in gastric cancer cells.
Figure 3.
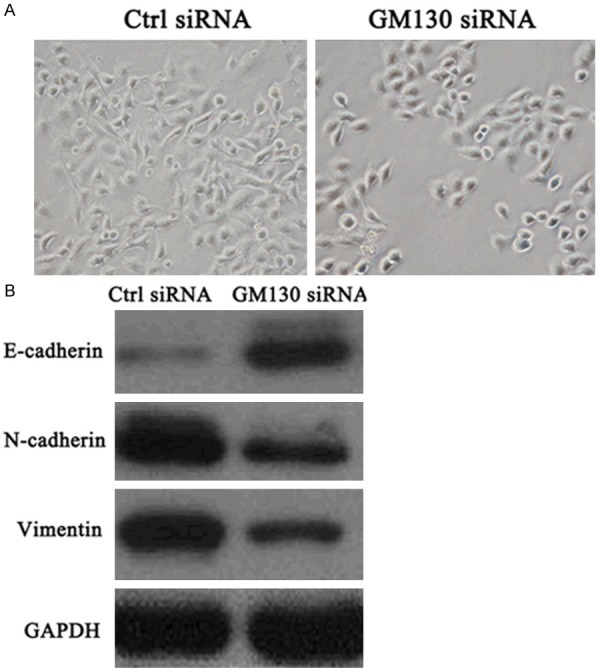
GM130 induces EMT of gastric cancer cells. A. Phase contrast microscopic images show that GM130 knockdown (GM130 siRNA) induced epithelial cell morphology in MKN-45 cell lines. B. GM130 depletion upregulated E-cadherin expression and downregulated N-cadherin and vimentin expression in MKN-45 cells.
GM130 directly regulates Snail expression
Aberrant expression of EMT transcription factors contributes to the appearance of an invasive phenotype by suppressing E-cadherin and inducing EMT in a wide variety of human cancers [11,12]. We aimed to identify transcription factors whose expression is regulated by GM130. RNA was extracted from MKN-45 with GM130 siRNA, and the mRNA and protein expression of EMT-related transcription factors were examined by qRT-PCR and immunoblot. Among the transcription factors examined, Snail expression was decreased by the knockdown of GM130 in MKN-45 cells (Figure 4A and 4B).
Figure 4.
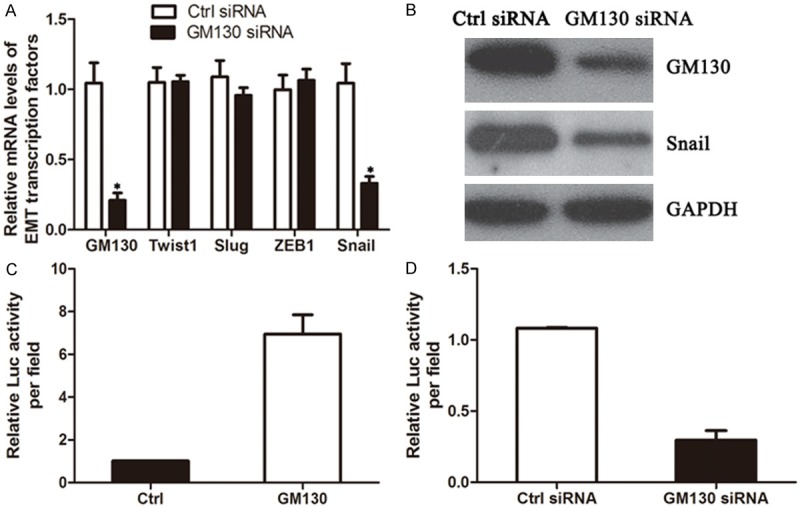
Snail expression is regulated by GM130. A. Seventy-two hours after transfection with siRNA, total RNA was extracted to examine changes in the mRNA expression levels of the indicated genes. B. The expression of Snail in siRNA-transfected cells was examined by immunoblot. C. 293T cells were cotransfected with the GM130 expression vector together with the pGL4-Snail/promoter and pRK-Luc expression vectors. Three independent experiments were carried out (P < 0.001). D. MKN-45 cells were transfected with GM130 siRNAs together with the pGL4-Snail/promoter and pRK-Luc vectors. After 72 hours, luciferase activity was measured. Three independent experiments were carried out, and the relative luciferase activity is indicated (P < 0.001).
We next conducted luciferase assays to determine whether Snail promoter activity was regulated by GM130 expression. 293T cells were transiently cotransfected with full-length GM130, and a reporter construct in which the human Snail promoter region was cloned upstream of firefly luciferase (pGL4-Snail/promoter). Exogenous expression of GM130 increased Snail promoter activity approximately 6-fold (Figure 4C). In addition, the transfection of GM130 siRNA into MKN-45 cells decreased promoter activity 60% to 80% compared with that of luciferase siRNA (Figure 4D). These results demonstrate that GM130 induces the transcription of the Snail gene.
Discussion
Gastric cancer is a very common disease worldwide and the second most frequent cause of cancer death. During gastric cancer carcinogenesis, genomic damage accumulate, affecting cellular functions such as self-sufficiency in growth signals, escaping antigrowth signals, apoptosis resistance, Sustained replicative potential, angiogenesis induction, and invasive or metastatic potential [13-15]. In addition, a cag pathogenicity island methylator phenotype (CIMP) has been a third pattern of genomic instability [16-18]. However, the exact mechanism which implicated in carcinogenesis and progression has not been fully elucidated.
Recently, novel pathway involving the ER, Golgi apparatus, and lysosomes are being recognized as potential targets for therapeutic interventions. GM130 plays an important role in the maintenance of Golgi structure and transport of proteins and lipids in the secretary pathway. Recent reports support the view that the downregulation of GM130 inhibits the protein transportation between ER and Golgi and induces autophagy. While, autophagy contributes to tumor suppression and defects of autophagy are associated with tumorgenesis [18-20]. In this study, we first examined the expression of GM130 in human gastric cancer tissues and cell lines to explore the molecular mechanisms of gastric cancer tumorgenesis, and then to reveal new predictive markers. Our results showed that GM130 protein was primarily expressed in the cytoplasm of gastric cancer cells and GM130 expression was evidently increased in gastric cancer specimens compared with adjacent normal tissues. Furthermore, also immunohistochemistry results demonstrated that the overexpression of GM130 was associated with differentiation grade and TNM stage in gastric cancer. Recent evidence supports aerosol delivery of GTP-SPE/shGM130 can suppress tumor progression to lung adenocarcinoma. In addition, in support of a role for GM130 in tumor progression, knockdown of GM130 in a Caco-2 cells lead to a loss of E-cadherin expression, a phenotype that is often considered to be linked to tumor progression and a loss of epithelial identity and is in line with the notion that loss of polarity is a typical phenomenon in epithelial-mesenchymal transition [5,6,21,22]. We also observed that GM130 expression, at both the mRNA and protein levels, in the human gastric cancer lines MKN-28, SGC-7901, MKN-45. Our results are consistent with GM130 might be involved in tumorgenesis and the progression of cancer.
To further explore the effects of GM130 on proliferation, invasion and migration in carcinoma cell lines. We transfected the GM130 sequence-specific siRNA with Lipofectamine 2000 into the MKN-45 cells, which had high invasive and metastatic potential and expressed high levels of GM130 proteins. We found that the cell proliferation and growth of GM130-siRNA cells was markedly inhibited and the levels of invasion and migration capacity was significantly lower in comparison with the cells in the negative control group, indicating that downregulation of GM130 in MKN-45 gastric cancer lines suppressed proliferation and inhibited migration and invasion. In addition, we also found that downregulation of GM130 decreased the protein level of MMPs. It has been shown that small interfering RNA against GOLGA2/GM130 results in inhibition of cell proliferation, angiogenesis and invasion in A549 cells.
Recent studies reported that EMT-associated transcription factors confer cancer cells with malignant characteristics, such as invasion, metastasis, and resistance to chemotherapy. Interestingly, silencing GM130 can induce E-cadherin downregulation in Caco-2 cells. The loss of E-cadherin expression not only disrupts cell- cell adhesion but also activates multiple pathways that induce cellular migration, invasion, and metastasis. Divergent findings have been made concerning the role of GM130 in cancer, which seems to depend on the type of cancer investigated. For instance, in lung cancer, the downregulation of GM130 induces autophagy and suppresses angiogenesis, invasion. GM130 expression is elevated in lung cancer but downregulated in colorectal and breast cancer [6,21,22]. Future experiments are necessary to clarify the proposed mechanism to the effect of GM130 on tumor cells. These studies are currently under way. And then, this is the first report show that variation in GM130 level participate in tumorigenesis and the progression of gastric cancer, as well as we have identified GM130 as a novel regulator of EMT and cell invasion. In addition, the induction of Snail expression was shown to be critical for the GM130-mediated promotion of EMT and cell invasion. Thus, GM130 is a critical regulator of cancer progression and may be an important target for human gastric cancer treatment.
Disclosure of conflict of interest
None.
References
- 1.Mao Z, Zhou J, Luan J, Sheng W, Shen X, Dong X. Tamoxifen reduces P-gp-mediated multidrug resistance via inhibiting the PI3K/Akt signaling pathway in ER-negative human gastric cancer cells. Biomed Pharmacother. 2014;68:179–183. doi: 10.1016/j.biopha.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Deng H, Huang XH, Fan J, Wang L, Xia Q, Yang X, Wang Z, Liu L. A variant of estrogen receptor-alpha, ER-alpha36 is expressed in human gastric cancer and is highly correlated with lymph node metastasis. Oncol Rep. 2010;24:171–176. [PMC free article] [PubMed] [Google Scholar]
- 3.Baschieri F, Confalonieri S, Bertalot G, Di Fiore PP, Dietmaier W, Leist M, Crespo P, Macara IG, Farhan H. Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis. Nat Commun. 2014;5:4839. doi: 10.1038/ncomms5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou W, Chang J, Wang X, Savelieff MG, Zhao Y, Ke S, Ye B. GM130 is required for compartmental organization of dendritic golgi outposts. Curr Biol. 2014;24:1227–1233. doi: 10.1016/j.cub.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang SH, Hong SH, Jiang HL, Minai-Tehrani A, Yu KN, Lee JH, Kim JE, Shin JY, Kang B, Park S, Han K, Chae C, Cho MH. GOLGA2/GM130, cis-Golgi matrix protein, is a novel target of anticancer gene therapy. Mol Ther. 2012;20:2052–63. doi: 10.1038/mt.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy E, Bruyère J, Flamant P, Bigou S, Ausseil J, Vitry S, Heard JM. GM130 gain-of-function induces cell pathology in a model of lysosomal storage disease. Hum Mol Genet. 2012;21:1481–95. doi: 10.1093/hmg/ddr584. [DOI] [PubMed] [Google Scholar]
- 7.Yuan H, Kajiyama H, Ito S, Yoshikawa N, Hyodo T, Asano E, Hasegawa H, Maeda M, Shibata K, Hamaguchi M, Kikkawa F, Senga T. ALX1 induces snail expression to promote epithelial-to-mesenchymal transition and invasion of ovarian cancer cells. Cancer Res. 2013;73:1581–1590. doi: 10.1158/0008-5472.CAN-12-2377. [DOI] [PubMed] [Google Scholar]
- 8.Lyons JG, Lobo E, Martorana AM, Myerscough MR. Clonal diversity in carcinomas: its implications for tumour progression and the contribution made to it by epithelial-mesenchymal transitions. Clin Exp Metastasis. 2008;25:665–77. doi: 10.1007/s10585-007-9134-2. [DOI] [PubMed] [Google Scholar]
- 9.Nieto MA. Epithelial-Mesenchymal Transitions in development and disease: old views and new perspectives. Int J Dev Biol. 2009;53:1541–1547. doi: 10.1387/ijdb.072410mn. [DOI] [PubMed] [Google Scholar]
- 10.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Du L, Yang X, Qu A, Zhang X, Zhou C, Wang C. Aberrant CCR4 Expression Is Involved in Tumor Invasion of Lymph Node-Negative Human Gastric Cancer. PLoS One. 2015;10:e0120059. doi: 10.1371/journal.pone.0120059. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Liu Y, Ling Y, Qi Q, Zhu M, Wan M, Zhang Y, Zhang C. Trastuzumab increases the sensitivity of HER2-amplified human gastric cancer cells to oxaliplatin and cisplatin by affecting the expression of telomere-associated proteins. Oncol Lett. 2015;9:999–1005. doi: 10.3892/ol.2014.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han X, Chen Y, Yao N, Liu H, Wang Z. MicroRNA let-7b suppresses human gastric cancer malignancy by targeting ING1. Cancer Gene Ther. 2015;22:122–9. doi: 10.1038/cgt.2014.75. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Duan J, Jiang Y, Wang L, Huang N, Lin L, Liao Y, Liao W. Metastasis-associated in colon cancer-1 upregulates vascular endothelial growth factor-C/D to promote lymphangiogenesis in human gastric cancer. Cancer Lett. 2015;357:242–253. doi: 10.1016/j.canlet.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Zhang H, Wang J, Yang Y, Wu Q. RNA interference-mediated silencing of G protein-coupled receptor 137 inhibits human gastric cancer cell growth. Mol Med Rep. 2015;11:2578–2584. doi: 10.3892/mmr.2014.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T, Liu X, Yi S, Zhang J, Ge J, Liu Z. EphB4 is overexpressed in gliomas and promotes the growth of glioma cells. Tumour Biol. 2013;34:379–385. doi: 10.1007/s13277-012-0560-7. [DOI] [PubMed] [Google Scholar]
- 17.Yuan CX, Zhou ZW, Yang YX, He ZX, Zhang X, Wang D, Yang T, Wang NJ, Zhao RJ, Zhou SF. Inhibition of mitotic Aurora kinase A by alisertib induces apoptosis and autophagy of human gastric cancer AGS and NCI-N78 cells. Drug Des Devel Ther. 2015;9:487–508. doi: 10.2147/DDDT.S74127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang G, Wang Z, Qian F, Zhao C, Sun C. Silencing of the ABCC4 gene by RNA interference reverses multidrug resistance in human gastric cancer. Oncol Rep. 2015;33:1147–1154. doi: 10.3892/or.2014.3702. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura N. Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions. J Pharmacol Sci. 2010;112:255–264. doi: 10.1254/jphs.09r03cr. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Pan Y, Zhou Y. Depletion of ALX1 causes inhibition of migration and induction of apoptosis in human osteosarcoma. Tumour Biol. 2015;36:5965–70. doi: 10.1007/s13277-015-3271-z. [DOI] [PubMed] [Google Scholar]
- 21.Sundaramoorthy S, Goh JB, Rafee S, Murata-Hori M. Mitotic Golgi vesiculation involves mechanisms independent of Ser25 phosphorylation of GM130. Cell Cycle. 2010;9:3100–3105. doi: 10.4161/cc.9.15.12522. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z, Sun X, Zou Z, Sun L, Zhang T, Guo S, Wen Y, Liu L, Wang Y Qin J, Li L, Gong W, Bao S. PRMT5 regulates Golgi apparatus structure through methylation of the golgin GM130. Cell Res. 2010;20:1023–1033. doi: 10.1038/cr.2010.56. [DOI] [PubMed] [Google Scholar]


