Abstract
Objectives: To explore the effects of calcium-sensing receptors (CaSR) on apoptosis in rat hippocampus during hypoxia/reoxygenation (H/R). Methods: After rat hippocampus was isolated, the cultures were subjected to H/R, and meanwhile gadolinium chloride (GdCl3, agonist of CaSR) and NPS 2390 (antagonists of CaSR) were added to reperfusion solution. The number of hippocampal neuron, cell viability and apoptosis rate were determined by inverted microscope, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and flow cytometer (FCM), respectively. Besides, caspase-3, Bax, cytochrome C (Cyt-c), extracellular signal-regulated protein kinase (ERK) 1/2, pERK1/2, P38 and pP38 were analyzed by Western blotting. Results: The hippocampal neuron number and cell viability were significantly decreased during H/R, and were further significantly reduced when co-treatment with CaSR agonist GdCl3. But the effects of GdCl3 were attenuated by NPS-2390. Whereas, apoptosis rate, the expression level of caspase-3, Bax and Cyt-c were all significantly increased under H/R condition, and was further significantly increased by GdCl3, but were reversed by NPS-2390 (P < 0.05). Moreover, there were no significant differences in expression of ERK1/2, P38 and pP38 among different groups. However, the expression of pERK1/2 was significantly increased during H/R, but was significantly reduced by NPS 2390 (P < 0.05). Conclusion: The results suggest that CaSR might play significant roles in the induction of hippocampus apoptosis in rat during H/R through phosphorylation of ERK1/2.
Keywords: Calcium-sensing receptors, hypoxia/reoxygenation, hippocampus, apoptosis, GdCl3, NPS 2390
Introduction
Ischemic cerebrovascular disease (ICVD) is a heterogeneous multifactorial disease, which is harmful to human health [1,2] and significantly contributes to death and long-term disability (such as learning and memory impairment) worldwide [3]. Hippocampus is an important brain structure for learning and memory. Reperfusion after ischemia causes an increase of injury to cell and organ, and apoptosis has been well documented in myocardium after ischemia/reperfusion (I/R) [4,5]. Additionally, previous studies have confirmed that calcium-sensing receptor (CaSR) is involved in the induction of cardiomyocyte apoptosis exposed to hypoxia/reoxygenation (H/R) via diverse signaling pathways, such as endoplasmic reticulum (ER) stress-associated apoptotic pathways, and the sarcoplasmic reticulum (SR)-mitochondrion interface [6-9].
Recently, roles of the CaSR in the central nervous system (CNS) have been studied [10-13]. It has been well demonstrated that CaSR could regulate neuronal cell growth and migration [13]. Besides, CaSR is expressed by almost all areas of brain, practically in postnatal brain development [14,15]. It is abundant in the subfornical organ (SFO) and olfactory bulbs, followed by the hippocampus and hypothalamus [16]. However, little information is available regarding the effects of CaSR on apoptosis in hippocampus during H/R, as well as the underling signaling pathways.
Therefore, the aims of our study were to explore the effects of CaSR on apoptosis in rat hippocampus exposed to H/R, and to determine the possible signaling pathways using a suitable and reliable model of hippocampal H/R.
Materials and methods
Primary hippocampal culture and H/R experiments
Primary cultures were prepared from newborn Wistar rats of either gender (24-hour-old). The animals were purchased from Laboratory Department of Qingdao University Medical College. The animal care and use was in accordance with the Guide for Care and Use of Laboratory Animals published by the China National Institutes of Health. The rats were killed by decapitation under anesthesia (2% pentobarbital sodium, 50 mg/kg intraperitoneally). The hippocampus were collected and submerged in ice-cold Hank’s Buffered Salt Solution (HBSS) for 5 min. Thereafter, the hippocampus were minced with ophthalmic scissors and treated with 0.125% trypsin for 15-30 min at 37°C. Digestion was terminated by adding Dulbecco’s modified Eagle medium (DMEM-F12) supplemented with 10% fetal bovine serum (FBS, Gibco Co. Ltd.). The sample was sequentially filtered through a 74-μm (200 meshes) cell strainer and collected by centrifugation for 10 min at 1,000 rpm. The precipitate was resuspended in neurobasal medium (Invitrogen) supplemented with B-27, 1.0 mM glutamine, 10,000 units/L penicillin, and 10 g/L streptomycin. Then the viable cells were counted with trypan blue staining. Cells were attainted at a final density of 1 × 106 cells per mL. The plates were maintained in a 5% CO2 incubator at 37°C. The medium was substituted for fresh complete DMEM after 24 h and then every three days. To obtain hypoxic conditions, oxygen (O2) was changed with nitrogen (N2). Simultaneously, the medium was switched to a pH 6.8 D-Hanks solution (mM: 5.37 KCl, 0.44 KH2PO4, 136.89 NaCl, 4.166 NaHCO3, 0.338 Na2HPO4, 5 D-glucose) saturated with 95% N2 + 5% CO2. Later, cultures were allowed to a hypoxic incubator that was consisted of 1% O2/5% CO2/94% N2. After treatment with hypoxia, the culture medium was substituted for fresh DMEM supplemented with 20% FBS (20% FBS/DMEM) to initiate reoxygenation.
Experimental protocols
After post-culturing in 20% FBS/ DMEM for 72 h, the cultures were randomly divided into four groups: (1) normal control group, the cultures were continuously maintained in 20% FBS/DMEM for 9 h; (2) model group (H/R group), the cultures were allowed to a hypoxic incubator for 4 h and then reoxygenated for 10 h with 20% FBS/DMEM; (3) gadolinium chloride (GdCl3) group, the cultures received hypoxic treatment for 4 h, and then reoxygenated for 10 h with 20% FBS/DMEM + 300 μM GdCl3 (agonist of CaSR); (4) NPS 2390 group, the cultures received hypoxic treatment for 4 h, and then reoxygenated for 10 h with 20% FBS/DMEM + 10 μM NPS 2390 (antagonists of CaSR). After post-culturing for 24 h, the number of cells in each group was calculated by inverted microscope (Olympus Corporation of the Americas, Inc., Central Valley, PA).
Cell proliferation assay
Cells were seeded in 24-well plate (200 μl per well). Forty μl of 5 mg/ml 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) solution (Sigma, St. Louis, USA) was added to each well, and the 24-well plate was incubated at 37°C for 4 hours. After centrifugation for 10 min at 1,000 rpm, 0.2 ml dimethyl sulfoxide (DMSO) was added to each well. The 24-well plate was incubated at 37°C for 30 min, and then was measured by an microplate reader instrument (SpectraMax M5, Molecular Device, USA) with the absorbance at 570-nm to construct the cell viability. Experiments were carried out 3-5 times.
Flow cytometry (FCM) detection
To assess the apoptosis rate, phosphatidylserine (PS) exposure was analyzed using Annexin V-fluorescein-5-isothiocyanate (Annexin V-FITC) apoptosis detection kit (Sigma, St Louis, MO, USA) according to the manufacturer’s protocol. Briefly, cells (5 × 105) were harvested and rinsed with cold phosphate buffer saline (PBS) for two times. After resuspension with 500 μL 1 × binding buffer, the mixture was incubated with 5 μL Annexin and 5 μL propidium iodide (PI, 10 mg/L) at room temperature in the dark for 5-15 min. Then the cells were read by FCM (Becton Dickinson, San Jose, CA, USA) through FL-1 filter (530 nm) and FL-2 filter (585 nm). The numerical values were determined using CELLQuest 3.0 software (Becton Dickinson, San Jose, CA). Annexin V-positive and PI negative cells were considered as early apoptosis cells, whereas annexin V positive and PI positive were regarded as late apoptotic cells [17].
Western blotting assay detection
Twenty-four hours after culture, cells in each group were collected for protein extraction. The concentration of protein was measured with the Bio-Rad DC protein Assay kit (Bio-Rad, Hercules, CA, USA). Thereafter, 20 µg proteins were resolved with a 10% standard electrophoresis sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gel, and then transferred onto polyvinylidene difluoride (PDVF) membranes (Milipore, Bedford, MA). Membranes were sealed in 5% defatted milk powder for 2 h at room temperature and incubated overnight at 4°C with the following antibodies: anti-CaSR (1:1000, Santa Cruz, CA), anti-caspase-3 (1:1000, Santa Cruz, CA), anti-Bax (1:1000, Santa Cruz, CA), anti-Cyt-c (1:1000, Santa Cruz, CA), anti-extracellular signal-regulated protein kinase (ERK) 1/2 (1:800, Santa Cruz, CA), anti-P38 (1:80, Santa Cruz, CA), anti-p-ERK1/2 (1:1000, Santa Cruz, CA), anti-p-P38 (1:1000, Santa Cruz, CA), and anti-β-actin (1:1000, Sigma) for 2 h at room temperature followed by incubation with a secondary antibody (1:800, Beijing Zhongshan Golden Bridge Biotechnology Co., Beijing, China). Then the samples were performed by enhanced chemiluminescence and densitometric analysis.
Statistical analysis
All collected data were expressed as mean ± standard deviation (SD). The collected data were firstly tested for normal distribution using one-sample K-S test. Chi-square test or rank-sum test was used for enumeration data. Measurement data were tested by student t-test (for two groups) or analysis of variance (ANOVA, for more than three groups). Comparisons were performed by post-hoc Tukey test. GraphPad Prism software (Version 5, San Diego, CA) was performed to determine the statistical significance. A statistical significance was defined when P < 0.05.
Results
Effects of GdCl3 and NPS 2390 on hippocampal neuron
To explore the effects of CaSR on hippocampal neuron apoptosis evoked by H/R, we used agonist (GdCl3) and antagonist (NPS 2390) of CaSR to demonstrate the role of CaSR in the induction of apoptosis during H/R. Here we set the baseline of cell numbers in control group to be 1. Under H/R condition, the hippocampal neuron numbers and cell viability were significantly decreased compared with the normal control group (P < 0.05). When the hippocampal neuron were exposed to GdCl3, cell numbers and cell viability were further significantly reduced (P < 0.05), but the effects were attenuated by NPS-2390 (P < 0.05) (Figure 1A and 1B). Additionally, FCM results showed that the apoptosis rate of hippocampal neuron was significantly increased when the hippocampal neuron was exposed to H/R condition compared with normal condition. Furthermore, the apoptosis rate was more statistically higher than when exposed to the GdCl3 (P < 0.05), but was significantly lower than when exposed to the NPS-2390 (P < 0.05) (Figure 2). Therefore, these results showed that CaSR was involved in H/R-induced hippocampal neuron apoptosis, but the effects were effectively relieved by NPS 2390.
Figure 1.
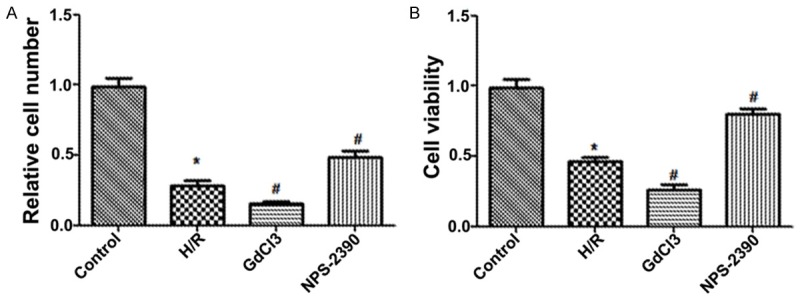
Relative cell number (A) and cell viability (B) after post-culturing for 24 h in each group; H/R, hypoxia/reoxygenation.
Figure 2.
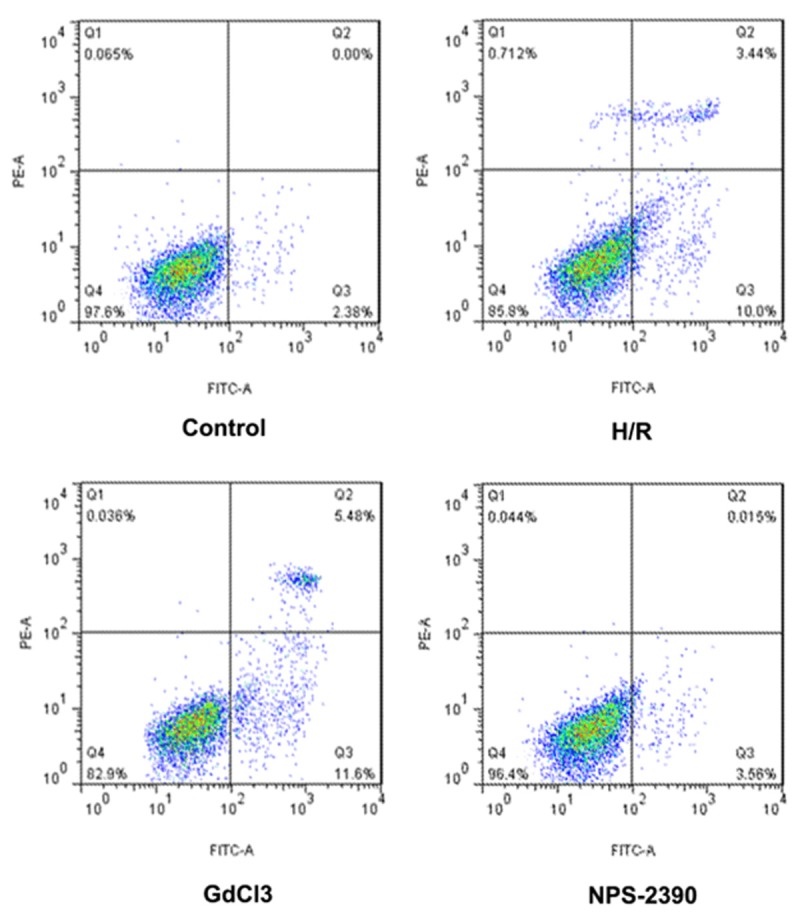
The apoptosis results detected by FCM. FCM, flow cytometry; H/R, hypoxia/reoxygenation.
Expression of caspase-3, Bax and Cyt-c
In order to identify apoptosis-related proteins (caspase-3, Bax and Cyt-c) in hippocampal neuron, western blotting analysis was performed (Figure 3A-D). Here we set the baseline of relative expression level in control group to be 1. The results showed that the expression levels of caspase-3, Bax and Cyt-c were all significantly increased under H/R condition compared with normal control condition (P < 0.05), and were further significantly increased under activation of CaSR (GdCl3 group) compared with under H/R condition (P < 0.05), but were further significantly reduced under inhibition of CaSR (NPS 2390 group) (P < 0.05).
Figure 3.
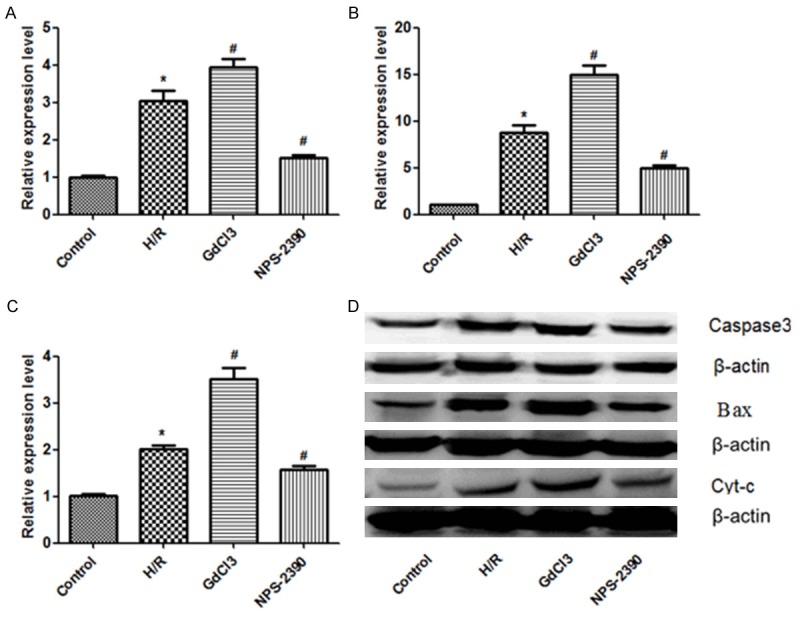
Expression of caspase-3, Bax and Cyt-c detected by western blotting. A. Relative expression level of caspase-3 in each group. B. Relative expression level of Bax in each group. C. Relative expression level of Cyt-c in each group. D. The presence of caspase-3, Bax and Cyt-c confirmed by western blotting. H/R, hypoxia/reoxygenation.
Expression of ERK1/2, pERK1/2, P38 and pP38
To determine whether CaSR induced apoptosis through the ERK signal pathway, the expression levels of ERK1/2 and p38 were analyzed by western blotting. As shown in Figure 4, there were no significant differences in expression levels of ERK1/2, P38 and pP38 among different groups. However, when the cells were exposed to H/R, the expression level of pERK1/2 was significantly upregulated (P < 0.05), but was significantly reduced by NPS 2390 (P < 0.05). The results indicated that the activation of CaSR contributed to the hippocampal neuron apoptosis through phosphorylation of ERK1/2.
Figure 4.
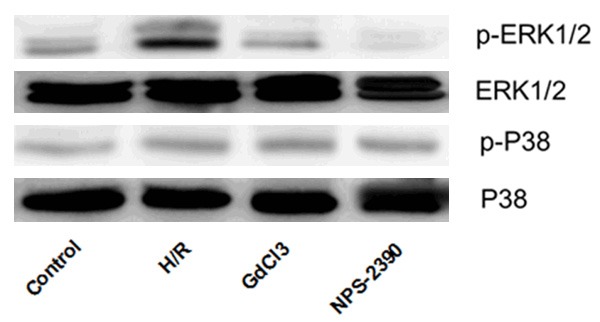
Expression of ERK1/2, pERK1/2, P38 and pP38 detected by western blotting. H/R, hypoxia/reoxygenation.
Discussion
In the present study, we investigated the effects of CaSR on apoptosis in rat hippocampus exposed to H/R. After post-culturing of hippocampus, the cultures were subjected to H/R, H/R + GdCl3, and H/R + NPS 2390. The number of hippocampal neuron, cell viability and apoptosis rate were determined, as well as apoptosis-related proteins (caspase-3, Bax and Cyt-c) and signaling pathway protein (ERK1/2, pERK1/2, P38 and pP38). The results suggested that CaSR might involve in the induction of hippocampus apoptosis in rat during H/R through phosphorylation of ERK1/2.
It has been well acknowledged that extracellular calcium ion (Cao 2+) metabolism plays an important role in cellular proliferation and differentiation. CaSR, a membrane-bound, seven-transmembrane G-protein coupled receptor (GPCR) superfamily, is critical for maintenance of Cao 2+ homeostasis in the body [18]. CaSR has been reported to be involved in the cell differentiation, proliferation, apoptosis, and hormone secretion in numerous cell types [19-22]. In recent years, the role of CaSR in the brain has been investigated, since calcium is an essential regulator for numerous neuronal functions. The induction of apoptosis caused by intracellular calcium overload has been well confirmed during H/R in myocardium; however, there is little investigation about the effect of CaSR on apoptosis during H/R in neuron.
A previous study has revealed that CaSR mRNA and protein are present in hippocampus [23], but the relevance of the CaSR on the function of hippocampus is still unknown. In our study, we performed a suitable and reliable model of hippocampal H/R. In addition, GdCl3 and NPS 2390 were added. The addition of GdCl3 has similar effects on hippocampal neuron as calcium, but these effects were eliminated by NPS2390, a CaSR specific antagonist. After culturing for 24 h, we found that the hippocampal neuron numbers and cell viability were significantly decreased when exposed to H/R compared with the normal group. In addition, cell numbers and viability were further significantly reduced when hippocampal neuron were exposed to GdCl3 and H/R, but were effectively increased when exposed to NPS 2390 and H/R. Whereas, apoptosis rate was significantly increased when exposed to H/R condition, and was further statistically increased by GdCl3, but were attenuated by NPS-2390. In line with previous studies, the same phenomenon was also observed in mesangial cells, cardiac tissue, pulmonary artery, and aortic vascular smooth muscle cells [24-27]. The results indicated that CaSR activation induced hippocampal neuron apoptosis during H/R, while NPS 2390 could effectively inhibit the cell apoptosis. Therefore, we speculated that NPS-2390 might be a potential drug for the treatment of ICVD, especially when exposed to I/R, but the effect must be further verified by animal experiments and clinical trials before introducing it in clinical practice.
Moreover, in order to explore the relationship between CaSR activation and apoptosis pathway, we analyzed the expression of apoptosis related protein (caspase-3, Bax and Cyt-c) and signal protein (ERK 1/2, pERK1/2, P38 and pP38) by western blotting. Caspase-3 is a key effector molecule in apoptosis, which could be activated by the release of Cyt-c via Cyt-c/Apaf-1/caspase-9 [28]. Bax is a regulator of the programmed cell death pathway, and belongs to Bcl-2 family protein, which can initiate the release of Cyt-c from mitochondria both in vitro and in vivo [29]. The ERK and p38 play significant roles in cell proliferation and apoptosis [30,31]. Our study demonstrated that caspase-3, Bax and Cyt-c were all increased when exposed to H/R, and was further significantly increased by GdCl3 group, but the effect was alleviated by NPS 2390 group. Besides, we found that the expression level of ERK1/2, P38 and pP38 were equivalent among different groups. When the cells were exposed to H/R, the pERK1/2 expression was significantly upregulated, but was significantly reduced by NPS 2390. The results indicated that the apoptosis of hippocampal neuron induced by CaSR activation might be through phosphorylation of ERK1/2.
In conclusion, our results suggest that CaSR might be involved in the induction of hippocampus apoptosis in rat during H/R through phosphorylation of ERK1/2.
Disclosure of conflict of interest
None.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL. Stroke epidemiology in the developing world. Lancet. 2005;365:2160–2161. doi: 10.1016/S0140-6736(05)66755-4. [DOI] [PubMed] [Google Scholar]
- 3.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- 4.Scarabelli TM, Stephanou A, Pasini E, Comini L, Raddino R, Knight RA, Latchman DS. Different signaling pathways induce apoptosis in endothelial cells and cardiac myocytes during ischemia/reperfusion injury. Circ Res. 2002;90:745–748. doi: 10.1161/01.res.0000015224.07870.9a. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb RA, Engler RL. Apoptosis in myocardial ischemia-reperfusion. Ann N Y Acad Sci. 1999;874:412–426. doi: 10.1111/j.1749-6632.1999.tb09255.x. [DOI] [PubMed] [Google Scholar]
- 6.Lu F, Tian Z, Zhang W, Zhao Y, Bai S, Ren H, Chen H, Yu X, Wang J, Wang L, Li H, Pan Z, Tian Y, Yang B, Wang R, Xu C. Calcium-sensing receptors induce apoptosis in rat cardiomyocytes via the endo (sarco) plasmic reticulum pathway during hypoxia/reoxygenation. Basic Clin Pharmacol Toxicol. 2010;106:396–405. doi: 10.1111/j.1742-7843.2009.00502.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu FH, Tian Z, Zhang WH, Zhao YJ, Li HL, Ren H, Zheng HS, Liu C, Hu GX, Tian Y, Yang BF, Wang R, Xu CQ. Calcium-sensing receptors regulate cardiomyocyte Ca2+ signaling via the sarcoplasmic reticulum-mitochondrion interface during hypoxia/reoxygenation. J Biomed Sci. 2010;17:50. doi: 10.1186/1423-0127-17-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong S, Teng Z, Lu FH, Zhao YJ, Li H, Ren H, Chen H, Pan ZW, Lv YJ, Yang BF, Tian Y, Xu CQ, Zhang WH. Post-conditioning protects cardiomyocytes from apoptosis via PKC(epsilon)-interacting with calcium-sensing receptors to inhibit endo (sarco) plasmic reticulum-mitochondria crosstalk. Mol Cell Biochem. 2010;341:195–206. doi: 10.1007/s11010-010-0450-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang WC, Zhang WH, Wu B, Zhao YJ, Li QF, Xu CQ. [The role of calcium-sensing receptor on ischemia/reperfusion-induced rat cardiomyocyte apoptosis] . Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:740–744. [PubMed] [Google Scholar]
- 10.Bandyopadhyay S, Tfelt-Hansen J, Chattopadhyay N. Diverse roles of extracellular calcium-sensing receptor in the central nervous system. J Neurosci Res. 2010;88:2073–2082. doi: 10.1002/jnr.22391. [DOI] [PubMed] [Google Scholar]
- 11.Kawamoto EM, Vivar C, Camandola S. Physiology and pathology of calcium signaling in the brain. Front Pharmacol. 2012;3:61. doi: 10.3389/fphar.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SS, Spitzer NC. Calcium signaling in neuronal development. Cold Spring Harb Perspect Biol. 2011;3:a004259. doi: 10.1101/cshperspect.a004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruat M, Traiffort E. Roles of the calcium sensing receptor in the central nervous system. Best Pract Res Clin Endocrinol Metab. 2013;27:429–442. doi: 10.1016/j.beem.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Ruat M, Molliver ME, Snowman AM, Snyder SH. Calcium sensing receptor: molecular cloning in rat and localization to nerve terminals. Proc Natl Acad Sci U S A. 1995;92:3161–3165. doi: 10.1073/pnas.92.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yano S, Brown EM, Chattopadhyay N. Calcium-sensing receptor in the brain. Cell Calcium. 2004;35:257–264. doi: 10.1016/j.ceca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Mudo G, Trovato-Salinaro A, Barresi V, Belluardo N, Condorelli DF. Identification of calcium sensing receptor (CaSR) mRNA-expressing cells in normal and injured rat brain. Brain Res. 2009;1298:24–36. doi: 10.1016/j.brainres.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 17.Zeng KW, Wang XM, Ko H, Yang HO. Neuroprotective effect of modified Wu-Zi-Yan-Zong granule, a traditional Chinese herbal medicine, on CoCl2-induced PC12 cells. J Ethnopharmacol. 2010;130:13–18. doi: 10.1016/j.jep.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Brown EM. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract Res Clin Endocrinol Metab. 2013;27:333–343. doi: 10.1016/j.beem.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Tu CL, Bikle DD. Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function. Best Pract Res Clin Endocrinol Metab. 2013;27:415–427. doi: 10.1016/j.beem.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diez-Fraile A, Lammens T, Benoit Y, D’Herde KG. The calcium-sensing receptor as a regulator of cellular fate in normal and pathological conditions. Curr Mol Med. 2013;13:282–295. doi: 10.2174/156652413804810763. [DOI] [PubMed] [Google Scholar]
- 21.Riccardi D, Kemp PJ. The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol. 2012;74:271–297. doi: 10.1146/annurev-physiol-020911-153318. [DOI] [PubMed] [Google Scholar]
- 22.Brennan SC, Davies TS, Schepelmann M, Riccardi D. Emerging roles of the extracellular calcium-sensing receptor in nutrient sensing: control of taste modulation and intestinal hormone secretion. Br J Nutr. 2014;111(Suppl 1):S16–22. doi: 10.1017/S0007114513002250. [DOI] [PubMed] [Google Scholar]
- 23.Chattopadhyay N, Legradi G, Bai M, Kifor O, Ye C, Vassilev PM, Brown EM, Lechan RM. Calcium-sensing receptor in the rat hippocampus: a developmental study. Brain Res Dev Brain Res. 1997;100:13–21. doi: 10.1016/s0165-3806(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 24.Kwak JO, Kwak J, Kim HW, Oh KJ, Kim YT, Jung SM, Cha SH. The extracellular calcium sensing receptor is expressed in mouse mesangial cells and modulates cell proliferation. Exp Mol Med. 2005;37:457–465. doi: 10.1038/emm.2005.56. [DOI] [PubMed] [Google Scholar]
- 25.Smajilovic S, Hansen JL, Christoffersen TE, Lewin E, Sheikh SP, Terwilliger EF, Brown EM, Haunso S, Tfelt-Hansen J. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;348:1215–1223. doi: 10.1016/j.bbrc.2006.07.192. [DOI] [PubMed] [Google Scholar]
- 26.Li GW, Xing WJ, Bai SZ, Hao JH, Guo J, Li HZ, Li HX, Zhang WH, Yang BF, Wu LY, Wang R, Yang GD, Xu CQ. The calcium-sensing receptor mediates hypoxia-induced proliferation of rat pulmonary artery smooth muscle cells through MEK1/ERK1,2 and PI3K pathways. Basic Clin Pharmacol Toxicol. 2011;108:185–193. doi: 10.1111/j.1742-7843.2010.00639.x. [DOI] [PubMed] [Google Scholar]
- 27.Qi H, Cao Y, Huang W, Liu Y, Wang Y, Li L, Liu L, Ji Z, Sun H. Crucial role of calcium-sensing receptor activation in cardiac injury of diabetic rats. PLoS One. 2013;8:e65147. doi: 10.1371/journal.pone.0065147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamilselvan J, Jayaraman G, Sivarajan K, Panneerselvam C. Age-dependent upregulation of p53 and cytochrome c release and susceptibility to apoptosis in skeletal muscle fiber of aged rats: role of carnitine and lipoic acid. Free Radic Biol Med. 2007;43:1656–1669. doi: 10.1016/j.freeradbiomed.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 30.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]


