Abstract
The accumulation of cholesterol in macrophages could induce the formation of foam cells and increase the risk of developing atherosclerosis. We wonder if quercetin, one of flavonoids with anti-inflammation functions in different cell types, could elevate the development of foam cells formation in atherosclerosis. We treated foam cells derived from oxLDL induced THP-1 cells with quercetin, and evaluated the foam cells formation, cholesterol content and apoptosis of the cells. We found that quercetin induced the expression of ABCA1 in differentiated THP-1 cells, and increased the cholesterol efflux from THP-1 cell derived foam cells. Eventually, cholesterol level and the formation of foam cell derived from THP-1 cells decreased after quercetin treatment. In addition, quercetin activated PPARγ-LXRα pathway to upregulate ABCA1 expression through increasing protein level of PPARγ and its transcriptional activity. Inhibition of PPARγ activity by siRNA knockdown or the addition of chemical inhibitor, GW9662, abolished quercetin induced ABCA1 expression and cholesterol efflux in THP-1 derived macrophages. Our data demonstrated that quercetin increased cholesterol efflux from macrophages through upregulating the expressions of PPARγ and ABCA1. Taken together, increasing uptake of quercetin or quercetin-rich foods would be an effective way to lower the risk of atherosclerosis.
Keywords: Quercetin, foam cells, atherosclerosis, PPARγ, ABCA1
Introduction
Atherosclerosis was considered as one of chronic metabolic disorders, for it related to both dyslipidemia and low-grade inflammation [1-4]. Macrophages play essential role in atherosclerosis development, as activated in the subendothelium in atherosclerotic lesions after engulfing LDL [1,4]. Circulating monocytes, recruited to the vascular lamina after LDL uptake, would differentiate into macrophages upon infiltration, and with over-loaded oxidized LDL (ox-LDL), these macrophages would transform into foam cells which is a hallmark of atherosclerosis [4]. Inflammatory cytokines and mediators produced by activated macrophages contribute to the inflammatory response in lesion plaques [4]. What’s more, the development of atherosclerotic lesions would be elevated when the macrophage activation or inflammatory mediators were abolished in animals [5-7].
Over-loaded modified LDL through the scavenger receptors would cause foam cell formation and apoptosis [4]. Therefore, the reverse cholesterol transport (RCT) mechanism which mediated cholesterol efflux from foam cells is considered to be very important in macrophage survival. The RCT process in macrophages is mediated by cholesterol transporters, including SR-B1, ATP binding cassette transporter A1 (ABCA1), and ATP binding cassette transporter G1 (ABCG1). ABCA1 is the major transporter that exports cholesterol out of the cells into apolipoprotein A1 (apoA1), forming pre-β-high-density lipoprotein (HDL) [8,9].
High flavonoid intake is positively associated with a decreased incidence of disorders characterized by dyslipidemia, including atherosclerosis, coronary heart diseases, and diabetes [10-13]. Quercetin, one of flavonoid rich in apples and onions [14], is found to have the properties of antioxidant, anti-inflammation, and anti-atherogenesis [15,16]. In consist with these, quercetin was demonstrated to show lots of beneficial effects that reduced dramatically the formation of early stages of atherosclerotic lesion in both humans [15] and animals [11,12,17,18]. The role of quercetin in terms of the uptake of ox-LDL was reported by preventing the activation of the protein kinase C (PKC) and peroxisome proliferator activated receptor γ (PPARγ) signaling pathways, resulting in the decrease of scavenger receptor CD36 and SR-A expression [1,2,4] and the inhibition on the ox-LDL uptaking [19].
However, the roles of quercetin on RCT and the possible mechanism of its action in macrophages still remain unclear. We used the human acute monocyte leukemia cell line (THP-1) as a model to investigate the function of quercetin on cholesterol efflux from foam cells. The results showed that quercetin induced the expression of the ABCA1 and then increased cholesterol efflux through activating PPARγ-LXRα pathway in THP-1 derived foam cells.
Materials and methods
Isolation and oxidation of low density lipoprotein
The native low-density lipoprotein (LDL) was isolated from fresh human blood and transferred into ox-LDL as described previously. In brief, isolated LDL was first incubated with CuSO4 (10°C for 24 hours and then transferred into ethylene diamine tetraacetic acid (EDTA; 200 mM) was added to remove Cu2+ for 24 hours at 4°C. Then, all the product was dialyzed in PBS for 24 hours at 4°C to remove EDTA. The oxidation level of LDL was measured by thiobarbituric acid reaction substances with malondialdehyde as the standard. The ox-LDL was then filtrated and stored at 4°C.
Cell culture
The human THP-1 cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). THP-1 cells were cultured in Roswell Park Memorial Institute medium 1640 (RPMI 1640, Corning) containing 10% fetal bovine serum (Gibico). 100 ng/mL phorbol 12-myristate-13-acetate (PMA, Sigma) were added into the medium to induce the differentiation of THP1 cells into macrophages for 48 hours, and the medium was then replaced with fresh medium with ox-LDL addition (50 mg/mL) to obtain foam cells before use in experiments. And quercetin (Sigma) with indicated concentration or period was added to treat the THP-1 derived foam cell.
siRNA transfection
THP-1 cells were transfected with specific siRNA oligomers directed against Pparγ (80 nM) by the use of Lipofectaminqe 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Scramble siRNA oligomers were used as a negative control. After transfection for 48 h, the cells were exposed to ox-LDL (50 mg/L) for 24 h. The silencing efficiency of target genes was validated by Western blotting.
Western blotting
Total proteins from the treated cells were extracted using RIPA lysis buffer. Equal amounts of protein were separated by SDS-PAGE and probed with various primary antibodies as indicated. Immunoblots were visualized using ECL reagent, and the integrated optical density (IOD) of immunoreactive bands was measured using Image-Pro Plus software and normalized by house-keeping protein (GAPDH).
Quantitative real-time PCR
Total RNA was extracted using Trizol reagent (Invitrogen). 2 g of total RNA was reversely transcripted using MMLV Reverse Transcriptase (Invitrogen). Real-time PCR was performed on a Rotor-Gene Q real-time PCR cycler (Roche, Shanghai, China) using SYBR-green PCR master mix kits. The data were analyzed using the Rotor-Gene Q software (version 1.7, Qiagen), and relative mRNA levels were calculated using the 2--ΔΔCt method and normalized against 18S rRNA. The primers used for real-time PCR were synthesized by Sangon Biotech (Shanghai, China).
Cellular cholesterol efflux experiments
The cells were cultured as described above and then incubated with [3H] cholesterol (0.2 mCi/mL) for 24 hours. After the incubation, the cells were washed with fresh medium for 3 times, and then quercetin was added for indicated time. The cells with PBS washed for 3 times incubated with 0.1% (w/v) BSA dissolved in the RPMI 1640 medium to allow equilibration of [3H] cholesterol in all cellular pools. [3H] cholesterol-labeled cells were cultured in 2 mL of the efflux medium containing 0.1% BSA and 25 mg/mL human plasma apoA-1 in RPMI 1640 medium. The efflux medium was obtained at indicated time and filtered through a 0.45 m filter to remove floating cells. The cell monolayer was extracted by NaOH (0.15 M) to test cellular total [3H] radioactivity. Medium and cell-associated [3H] cholesterol were then measured by liquid scintillation counting. Percentage of cholesterol efflux was calculated using the following equation: [total media counts/(total cellular counts + total media counts)]*100% (Liu et al., 2013).
Statistical analyses
Analysis of variance was conducted to examine whether significant (P, 0.05) main treatment and time effects occurred. Additional post hoc comparisons of treatment means were conducted by using the Dunnett’s t-test (treatments vs. controls) and Bonferroni t-test (selected comparisons) as indicated. Data given represent means standard deviation.
Results
Quercetin induced ABCA1 expression and cholesterol efflux in THP1 derived foam cells
ABCA1 is considered as the key player in RCT for regulating macrophage cholesterol homeostasis. First, the effect of quercetin on ABCA1 expression was tested by real-time quantitative PCR and western blotting assays in THP1 macrophage-derived foam cells. As shown in Figure 1, quercetin induced ABCA1 expression in a dose- and time-dependent manner, at both mRNA and protein levels. With increased ABCA1 expression after quercetin treatment, we detected the effect of quercetin on apoA-1-dependent cholesterol efflux in the THP-1 derived foam cell. The cholesterol to apoA-1 ratio increased dramatically after the addition of quercetin (Figure 1C, 1D). These results indicated that quercetin enhanced apoA-1-dependent cholesterol efflux and induced ABCA1 expression in THP-1 derived foam cells.
Figure 1.
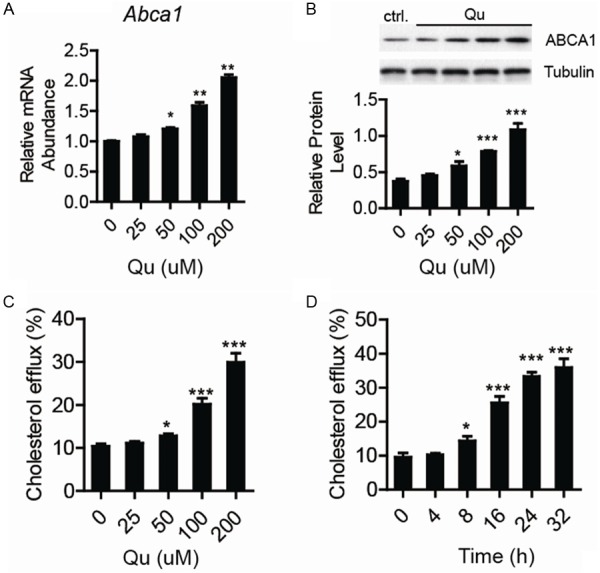
The upregulation of ABCA1 expression and cholesterol efflux in THP1 derived foam cells with quercetin (Qu) treatment. (A) Abca1 mRNA level was tested by Real-time PCR and (B) The protein level tested by western-blotting in THP1 derived macrophages treated by ox-LDL in the presence of quercetin as indicated concentration, and 18S rRNA or Tubulin was used as internal control. Increased cholesterol efflux in THP1 derived foam cells with quercetin treatment of indicated concentration (C) or time (D). Data are presented as the mean ± S.E. of at least four independent experiments. *P<0.05, **P<0.01, ***P<0.001.
Quercetin decreased ox-LDL induced foam cell formation and apoptosis in THP1 cells
With the development of atherosclerosis, the infiltrated macrophages would engulf LDL and then the accumulation of cholesterol in the droplets induces foam cells formation and apoptosis. Macrophages uptake of ox-LDL is the event that triggers the formation of lipid-laden foam cells, which is considered as the important marker of atherosclerosis. The results of oil red O-staining (Figure 2A) and intracellular TC quantitative assay (Figure 2B) showed that lipid content in differentiated THP-1 cells was significantly increased by ox-LDL, which indicated that foam cells were formed. When the cells were co-treated with quercitin, the lipid content decreased dramatically with reduced lipid droplets stained with oil red O-staining (Figure 2A) and lower intracellular TC (Figure 2B). FACS analysis also showed that foam cells induced by ox-LDL decreased after quercetin treatment (Figure 2C). The results demonstrated that quercetin could decrease the accumulation of cholesterol and foam cell formation induced by ox-LDL in macrophages.
Figure 2.
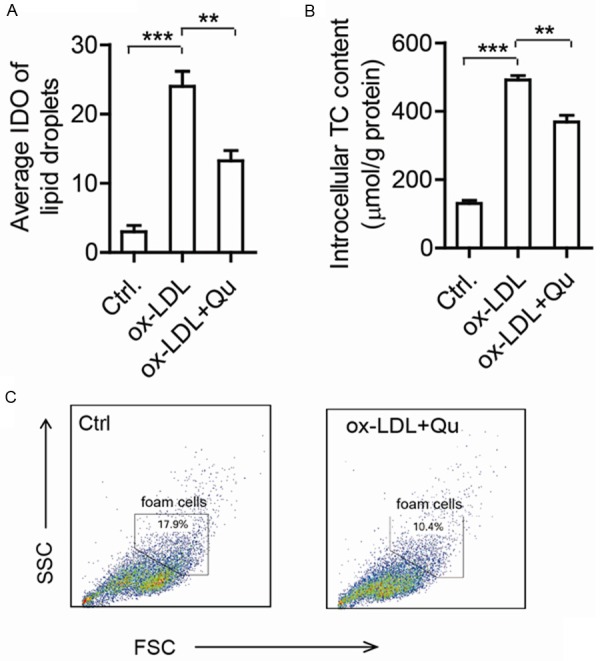
Decreased cholesterol accumulation and foam cell formation in quercetin treated THP1 cells. (A) The average integrated optical density (IOD) of lipid droplets stained with oil red O from differentiated macrophages treated by 50 mg/L ox-LDL in the presence or absence of 200 mM quercetin. (B) The intracellular total cholesterol (TC) content was measured under the same conditions as in (A). (C) Foam cells were identified by FACS assay. Data are presented as the mean ± S.E. of at least four independent experiments. *P<0.05, **P<0.01.
Quercetin increased the expression of PPARγ and activated PPARγ signaling
The transcriptional cascade in the PPARγ-LXRα pathway plays a critical role in maintaining cellular cholesterol homeostasis in macrophages. Previous studies have shown that the activation of PPARγ upregulates ABCA1 expression and facilitates cellular cholesterol efflux. To investigate whether quercetin could affect the expression of LXRα or PPARγ, we firstly used real-time quantitative PCR and Western blotting analyses. As shown in Figure 3, PPARγ mRNA and protein levels were both increased after the cells were treated by quercetin in a dose-dependent and time-dependent manner, while LXRα protein and mRNA levels did not show any changes. To test if quercetin could induce the activation of PPARγ pathway, we introduced PPRE-luc reporter system to evaluate PPARγ transcriptional activity. As shown in Figure 3C, the luciferase activity increased about 3 folds after 200 μM quercetin treatment. The result demonstrated that quercetin induced PPARγ pathway activation though upregulating the expression level of PPARγ in macrophages.
Figure 3.
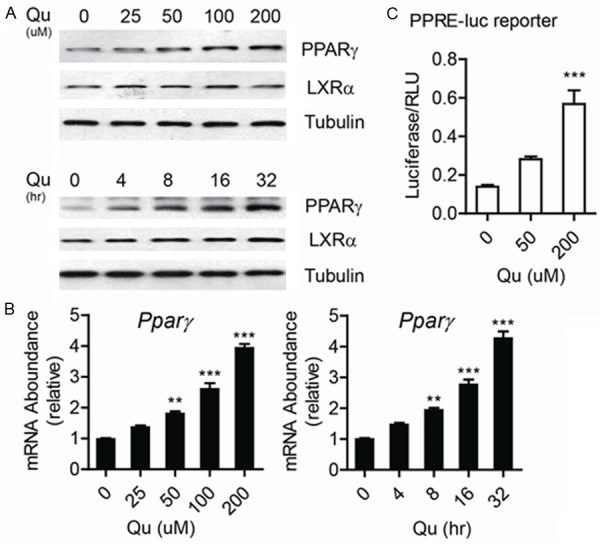
Quercetin increased the expression of PPARγ and activated PPARγ signaling. (A) Pparγ mRNA level was tested by Real-time PCR and (B) The protein level was tested by western-blotting in THP1 derived macrophages treated by ox-LDL in the presence of quercetin as indicated concentration or time, and 18S rRNA or Tubulin was used as internal control. (C) PPARγ reporter (PRE-reporter) assay was done in THP1 derived macrophage with quercetin treatment. Data are presented as the mean ± S.E. of at least four independent experiments. **P<0.01, ***P<0.001.
The inhibition on PPARγ abolished the functions of quercetin on macrophage cholesterol efflux
To confirm the upregulated ABCA1 expression induced by quercetin was PPARγ dependent, we introduced PPARγ antagonist GW9662 and PPARγ specific siRNA to block the activation of PPARγ pathway induced by quercetin. As shown in Figure 4A, the addition of GW9662 dramatically abolished the induction effects of quercetin on ABCA1 expression in macrophages. We then examined the effect of PPARγ siRNA on quercetin-induced change in ABCA1 expression. Upregulation of ABCA1 expression by quercetin was reversed by both PPARγ siRNA. What’s more, with the disruption of PPARγ PPARγ pathway with the addition of GW9662 or PPARγ siRNA, PPARγ transcriptional activity decreased dramatically (Figure 4B). We also examined cholesterol efflux in cells using the liquid scintillation counting assay. Our results showed that treatment with both PPARγ siRNA and quercetin (GW9662 and quercetin) blocked quercetin-mediated upregulation of cholesterol efflux compared with the controls (Figure 4B). All of these indicate that quercetin promotes ABCA1 upregulation through the PPARγ pathway.
Figure 4.
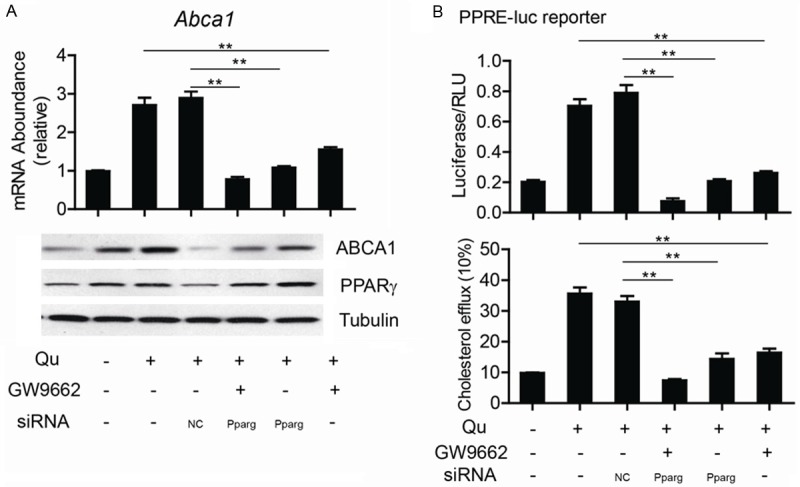
The inhibition on PPARγ abolished the functions of quercetin on macrophage cholesterol efflux. Abca1 mRNA and ABCA1 and PPARγ protein levels (A), PRE reporter assay (B) and cholesterol efflux (C) were tested in quercetin treated macrophages with or without the addition of GW9662 or PPARγ specific siRNA as indicated. Data are presented as the mean ± S.E. of at least three independent experiments. **P<0.01, ***P<0.001.
Discussion
Our study here supports the hypothesis that quercetin could promote macrophage RCT process via upregulating the cholesterol transporter ABCA1 and transcriptional activator PPARγ expression. This effect of quercetin results in decreased foam cell formation to alleviate atherogenesis. The presence of sustained macrophage activation in the subendothelium of atherosclerotic lesions suggested that macrophages may play an essential role during the initiation and progression of atherosclerosis [1,4].
Quercetin-driven ABCA1 expression is likely to be mediated by the increased transcriptional activities of PPARγ, as demonstrated by the upregulated expression of these transcriptional factors after quercetin treatment. PPARγ expression induced by quercetin occurred somehow earlier than that of ABCA1. In accordance with our results in human macrophages, a previous study showed that rutin, glycosylated quercetin, induced the expression of PPARγ in lung cells [20]. However, the effects of quercetin on PPARγ expression may be different among cells and tissues, as shown that quercetin lowered PPARγ transcript levels and exerted no effects on PPARγ transcriptional activity in rat adipose tissue [21]. Although it has not been examined whether quercetin acts as agonist or antagonist, quercetin should have no measurable effects as PPARγ ligand as well. Taken account of all of these, quercetin may regulate ABCA1 gene expression through having some effects on its related transcription factors.
ABCA1 is considered as a major regulator of reverse cholesterol transport. Quercetin modulation effects on ABCA1 expression may lower the deposition of cholesterol in macrophages. As characterized in atherosclerosis, dysregulated cholesterol metabolism and chronic inflammation could be improved after quercetin treatment, resulting in the reduction of foam cell formation which is a critical feature in the initial stage of atherosclerosis [22]. In addition of the quercetin effects that we observed here, it has been previously shown that quercetin could have health benefits in anti-atheroslerosis by suppressing the expression of scavenger receptors such as SR-A and CD36 in macrophages and blocking free radical-mediated oxidative modification of LDL [11,12,23,24]. Corroborating these previous reports, our study provides convincing evidences to reveal beneficial roles of quercetin in improving atherosclerosis.
Although most of the experiments were in vitro, this study provides a rationale to investigate anti-atherogenic effects of quercetin in vivo. Further researches are necessary by using hyperlipidemic animal models and humans.
Acknowledgements
This work was supported by grants from the key scientific research projects of Colleges and universities in Henan Province (No. 15A320019) and the science and technology projects in Henan province (No. 142102310109 and 122102310088). We are grateful to Prof. Zongfang Liu and Prof. Lihua Zhang for helpful discussions and technical assistance. We thank Prof. Jian Liguo for helpful comments.
Disclosure of conflict of interest
None.
References
- 1.Detmers PA, Hernandez M, Mudgett J, Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock J, Weikel R, Bergstrom JD, Shevell DE, Hermanowski-Vosatka A, Sparrow CP, Chao YS, Rader DJ, Wright SD, Puré E. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J Immunol. 2000;165:3430–3435. doi: 10.4049/jimmunol.165.6.3430. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz G, Kaminski WE, Porsch-Ozcurumez M, Klucken J, Orso E, Bodzioch M, Buchler C, Drobnik W. ATP-binding cassette transporter A1 (ABCA1) in macrophages: a dual function in inflammation and lipid metabolism? Pathobiology. 1999;67:236–240. doi: 10.1159/000028100. [DOI] [PubMed] [Google Scholar]
- 3.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 4.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 5.Detmers PA, Patel S, Hernandez M, Montenegro J, Lisnock JM, Pikounis B, Steiner M, Kim D, Sparrow C, Chao YS, Wright SD. A target for cholesterol absorption inhibitors in the enterocyte brush border membrane. Biochim Biophys Acta. 2000;1486:243–252. doi: 10.1016/s1388-1981(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 6.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 7.Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation. 2001;103:3099–3104. doi: 10.1161/01.cir.103.25.3099. [DOI] [PubMed] [Google Scholar]
- 8.Oram JF, Vaughan AM. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr Opin Lipidol. 2000;11:253–260. doi: 10.1097/00041433-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 10.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 11.Hayek T, Fuhrman B, Vaya J, Rosenblat M, Belinky P, Coleman R, Elis A, Aviram M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler Thromb Vasc Biol. 1997;17:2744–2752. doi: 10.1161/01.atv.17.11.2744. [DOI] [PubMed] [Google Scholar]
- 12.Juzwiak S, Wojcicki J, Mokrzycki K, Marchlewicz M, Bialecka M, Wenda-Rozewicka L, Gawronska-Szklarz B, Drozdzik M. Effect of quercetin on experimental hyperlipidemia and atherosclerosis in rabbits. Pharmacol Rep. 2005;57:604–609. [PubMed] [Google Scholar]
- 13.Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011;5:107–111. doi: 10.4162/nrp.2011.5.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer J, Brown D, Liu RH. Uptake of quercetin and quercetin 3-glucoside from whole onion and apple peel extracts by Caco-2 cell monolayers. J Agric Food Chem. 2004;52:7172–7179. doi: 10.1021/jf030733d. [DOI] [PubMed] [Google Scholar]
- 15.Ishizawa K, Yoshizumi M, Kawai Y, Terao J, Kihira Y, Ikeda Y, Tomita S, Minakuchi K, Tsuchiya K, Tamaki T. Pharmacology in health food: metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J Pharmacol Sci. 2011;115:466–470. doi: 10.1254/jphs.10r38fm. [DOI] [PubMed] [Google Scholar]
- 16.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Enkhmaa B, Shiwaku K, Katsube T, Kitajima K, Anuurad E, Yamasaki M, Yamane Y. Mulberry (Morus alba L. ) leaves and their major flavonol quercetin 3-(6-malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor-deficient mice. J Nutr. 2005;135:729–734. doi: 10.1093/jn/135.4.729. [DOI] [PubMed] [Google Scholar]
- 18.Motoyama K, Koyama H, Moriwaki M, Emura K, Okuyama S, Sato E, Inoue M, Shioi A, Nishizawa Y. Atheroprotective and plaque-stabilizing effects of enzymatically modified isoquercitrin in atherogenic apoE-deficient mice. Nutrition. 2009;25:421–427. doi: 10.1016/j.nut.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Choi JS, Bae JY, Kim DS, Li J, Kim JL, Lee YJ, Kang YH. Dietary compound quercitrin dampens VEGF induction and PPARgamma activation in oxidized LDL-exposed murine macrophages: association with scavenger receptor CD36. J Agric Food Chem. 2010;58:1333–1341. doi: 10.1021/jf9032443. [DOI] [PubMed] [Google Scholar]
- 20.Yeh SL, Yeh CL, Chan ST, Chuang CH. Plasma rich in quercetin metabolites induces G2/M arrest by upregulating PPAR-gamma expression in human A549 lung cancer cells. Planta Med. 2011;77:992–998. doi: 10.1055/s-0030-1250735. [DOI] [PubMed] [Google Scholar]
- 21.Wein S, Behm N, Petersen RK, Kristiansen K, Wolffram S. Quercetin enhances adiponectin secretion by a PPAR-gamma independent mechanism. Eur J Pharm Sci. 2010;41:16–22. doi: 10.1016/j.ejps.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 23.Auger C, Teissedre PL, Gerain P, Lequeux N, Bornet A, Serisier S, Besancon P, Caporiccio B, Cristol JP, Rouanet JM. Dietary wine phenolics catechin, quercetin, and resveratrol efficiently protect hypercholesterolemic hamsters against aortic fatty streak accumulation. J Agric Food Chem. 2005;53:2015–2021. doi: 10.1021/jf048177q. [DOI] [PubMed] [Google Scholar]
- 24.Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K, Terao J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]


