Abstract
Preoperative neoadjuvant chemoradiation therapy may be useful in patients with operable rectal cancer, but treatment responses are variable. We examined whether expression levels of circadian clock genes could be used as biomarkers to predict treatment response. We retrospectively analyzed clinical data from 250 patients with rectal cancer, treated with neoadjuvant chemoradiation therapy in a single institute between 2011 and 2013. Gene expression analysis (RT-PCR) was performed in tissue samples from 20 patients showing pathological complete regression (pCR) and 20 showing non-pCR. The genes analyzed included six core clock genes (Clock, Per1, Per2, Cry1, Cry2 and Bmal1) and three downstream target genes (Wee1, Chk2 and c-Myc). Patient responses were analyzed through contrast-enhanced pelvic MRI and endorectal ultrasound, and verified by histological assessment. pCR was defined histologically as an absence of tumor cells. Among the 250 included patients, 70.8% showed regression of tumor size, and 18% showed pCR. Clock, Cry2 and Per2 expressions were significantly higher in the pCR group than in the non-pCR group (P<0.05), whereas Per1, Cry1 and Bmal1 expressions did not differ significantly between groups. Among the downstream genes involved in cell cycle regulation, c-Myc showed significantly higher expression in the pCR group (P<0.05), whereas Wee1 and Chk2 expression did not differ significantly between groups. Circadian genes are potential biomarkers for predicting whether a patient with rectal cancer would benefit from neoadjuvant chemoradiation therapy.
Keywords: Neoadjuvant chemoradiation therapy, circadian clock, rectal cancer, biomarker
Introduction
Colorectal cancer has a high incidence in both developing and developed countries, with recent data from the USA indicating an incidence rate of 50 per 100,000 in males and 37.8 per 100,000 in females [1]. Furthermore, it has been estimated that 2015 will see nearly 40,000 new cases of rectal cancer in the USA alone [1]. Pre-operative neoadjuvant chemoradiation therapy has been recommended for patients with operable rectal cancer, and its use is associated with a low cancer recurrence rate [2-5]. In this regimen, patients receive 2-5 rounds of chemotherapy, accompanied by radiation treatment. There is no current consensus on the choice of chemotherapy drugs and radiation dose/interval, with individual institutions often devising their own empirical protocols [6,7]. Regardless, it has been reported that 10-30% of patients benefit from this treatment, with near complete regression of local cancer. However, approximately 25% of patients do not respond to this therapy, with the remaining patients showing intermediate responses [8,9].
Biomarkers are urgently needed to predict patient responses to neoadjuvant therapy. The treatment itself lasts 4-6 months, during which time patients may experience extensive adverse effects of the chemoradiation therapy, such as hair loss, gastrointestinal syndrome and skin damage; indeed, chemoradiation therapy has been reported to increase toxicity-related mortality [10], highlighting the importance of targeting this treatment to patients who are predicted to benefit. Furthermore, the half-year delay in surgery may pose a significant risk for patients, especially those who respond poorly to the therapy. In addition, the cost of neoadjuvant treatment is a serious consideration for low-income families. Extensive microarray analyses have been conducted to identify biomarkers that can predict patient responses [11-14]. Several candidate genes have been examined, including p53, p21, EGFR, Bcl2/Bax, Ki-67, thymidylate synthase (TS), Hsp27, survivin [15,16], and miRNAs [17]. However, their utilities in predicting patient responses remain to be further verified.
The circadian clock is our biological timer, playing a fundamental role in the orchestration of our daily physiology and behavior. The core molecular oscillator is comprised of six core members encoded by the Clock, Bmal1, Cryptochrome 1 and 2 (Cry1 and Cry2), and Period 1 and 2 (Per1 and Per 2) genes [18,19]. CLOCK and BMAL1 proteins form a heterodimer transcription factor complex, driving the expression of the Cry and Per genes. The PER and CRY proteins in turn heterodimerize and inhibit CLOCK/BMAL1, turning off their own transcription. A diverse array of regulatory mechanisms impinges upon this core mechanism to drive downstream gene expression and coordinate essential physiological processes. In particular, the clock has been shown to regulate various cellular functions that are often deregulated in tumor formation, including cell proliferation, the response to DNA damage, and apoptosis [19,20].
Genetic and epidemiological studies have provided strong evidence for a role of the circadian clock in tumorigenesis [18]. For example, shift work is known to cause a disruption in circadian hormonal rhythm, and is also associated with elevated incidence rates of breast and colorectal cancers. Consistent with the idea that the clock functions as a tumor suppressor, a genetic mutation of the Per2 gene in mice was found to confer exaggerated sensitivity to γ-irradiation-induced lymphoma, likely attributable to defective p53-dependent apoptosis [20]. At the molecular level, core clock genes are also known to regulate the expression and/or function of various downstream genes involved in cell proliferation, the DNA damage response and apoptosis. For example, cell cycle-related genes were among those found to be dysregulated in the liver and muscle of ClockΔ19 mutant mice [21]. Per1 expression has been shown to correlate with DNA damage-induced apoptosis, with lower expression in human tumor samples than in normal tissues [22]. Furthermore, the PER1 protein has been found to interact directly with several checkpoint kinases including Chk2, underscoring a conserved pathway mediating clock control of the DNA damage response [22,23]. Together, these studies highlight a potentially important role of the clock and clock genes in cancer progression. Indeed, the core clock genes have been shown to be associated with tumor formation in skin cancer [24], prostate cancer [25], lung cancer [26], head and neck squamous cell carcinoma [27], endometrial cancer [28], and colorectal cancer [29,30]. These observations raise the interesting possibility that clock genes could serve as prospective biomarkers to predict the response of rectal cancer to neoadjuvant chemoradiation therapy. In the present study, we have examined whether circadian genes could be used to predict patient responses to chemoradiation therapy. We retrospectively analyzed data from patients with rectal cancer, who were treated at the Union Hospital of Fujian Medical University between 2011 and 2013. Among 250 patients with a complete dataset, the response rate to treatment (defined as regression of tumor size or T stage) was 70.8%, and the rate of pathological complete regression (pCR) was 18%. Furthermore, quantitative analysis of the expression levels of core clock genes and their downstream cell cycle genes revealed significant differences in gene expression levels between responders and non-responders, suggesting that clock genes could serve as potential biomarkers to predict patient responses to neoadjuvant chemoradiation therapy.
Patients and methods
Patient population
This was a retrospective analysis of consecutive patients with rectal cancer who were treated by neoadjuvant chemoradiation therapy, between January 2011 and July 2013, at the Department of Radiation Oncology, Affiliated Union Hospital of Fujian Medical University, Fuzhou, Fujian, China. The inclusion criteria were as follows: newly diagnosed and histologically proven T3/4 with N+/- or T1/2 with N+ rectal adenocarcinoma based on the criteria of the 7thedition of the AJCC Cancer Staging Manual [31]; and chemoradiation therapy (see below) administered prior to surgical resection of the tumor. The following exclusion criteria were applied: heart, liver or renal dysfunction; a history of previous malignancy; previous treatment with chemotherapy or radiotherapy; pregnancy or breast-feeding; or the presence of a psychiatric disorder. The study was approved by the Institutional Review Board of The Affiliated Union Hospital of Fujian Medical University. Due to the retrospective nature of this analysis, informed patient consent was deemed not to be required.
Management protocol
All included patients were investigated using standard procedures, including biopsies, electrocardiograms (ECGs), physical examinations, and cancer staging by contrast-enhanced pelvic MRI and endorectal ultrasound. Pre-operatively radiotherapy (total dose of 50.4 Gy, pelvic dose of 45 Gy/25 fractions and 5.4 Gy/28 fractions boosted to the primary tumor) was performed in conjunction with 2 cycles of chemotherapy. After completion of CRT, patients received an additional cycle of chemotherapy. Each chemotherapy cycle included oxaliplatin (Jiangsu Hengrui Medicine Co., Ltd.) 130 mg/m2 on day 1 and capecitabine (La Roche Ltd, Switzerland) 825 mg/m2, twice per day from day 1 to day 14 for a duration of 21 days. Total mesorectal excision (R0 resection) was performed 6-8 weeks after the completion of chemoradiation.
Sample collection
Tumor samples were taken under rectoscope within one week before radiotherapy and after during operation by experienced colorectal surgeons to evaluate patient responses. Tissue samples were fixed in 37% formalin and subjected to standard processing in a pathological laboratory. Samples were used for RNA extraction when parallel specimens contained at least 70% tumor cells.
Histological assessment
Pathological analysis was performed before and after treatment to determine the clinical responsiveness in a blinded fashion from information of the gene expression level results. pCR was defined as the complete absence of all tumor cells. Semi-quantitative evaluation of histological regression was performed according to the Dworak classification [32]. The tissue samples before treatment contained mainly tumor cells (>90%, as assessed by three independent pathologists).
RNA extraction and quantitative RT-PCR analysis
Gene expression analysis was carried out on randomly selected tissue samples from 20 patients who showed pCR of rectal cancer after treatment, and 20 patients who showed no response (non-pCR). Gene expression analysis was carried out for six core clock genes (Clock, Cry1, Cry2, Per1, Per2 and Bmal1), three of their downstream target genes (Wee1, Chk2 and c-Myc), and four genes implicated previously as possible biomarkers of patient response to chemoradiation therapy (Hsp27, Survivin, TS and Bcl2). RNA extraction from formalin-fixed and paraffin-embedded (FFPE) pathological samples was performed as described previously [33,34]. Briefly, five paraffin sections, each of 8-µm thickness, were chosen randomly from pre-operative biopsies and used for total RNA extraction. The sections were de-paraffined in xylin (Shangon Biotech, Shanghai, China) and graded ethanol, hydrated in diethylpyrocarbonate (Shangon Biotech, Shanghai, China)-treated water, and scratched off. The tissues were then digested in 0.5 mg/mL proteinase K solution at 55°C for 15 min, and total RNAs extracted with Trizol reagent (Shangon Biotech, Shanghai, China). cDNA synthesis was performed using a Revert-Aid kit (Fermentas, Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. Real-time PCR analysis was performed using a premix from QWBio (Beijing, China) and a LightCycler 480 real-time PCR system (Roche Applied Systems, Penzberg, Germany). Briefly, blocks were heated up to 95°C for 10 min to activate Taq polymerase. The PCR conditions were: 95°C for 15 sec, 60°C for 15 sec, and 72°C for 15 sec for 45 cycles. The primer sequences (Shangon Biotech, Shanghai, China) used are listed in Table 1. Specificity of the PCR reactions was validated by melting point analysis and gel electrophoresis. Expression of the β-actin gene served as the internal control, and the ‘delta-delta CT’ method [35] was used to compare expression levels between different samples.
Table 1.
Primers used in this study
| Gene Name | Primer Sequences |
|---|---|
| CLOCK | GGCACCACCCATAATAGGGTA |
| TGTTGCCCCTTAGTCAGGAAC | |
| CRY1 | ACAGGTGGCGATTTTTGCTTC |
| TCCAAAGGGCTCAGAATCATAC | |
| CRY2 | CGTGTTCCCAAGGCTGTTCA |
| CTCCGTCACTACTTCCACACC | |
| PER1 | TCTGTAAGGATGTGCATCTGGT |
| CAGGCAGTTGATCTGCTGGT | |
| PER2 | AGTTGGCCTGCAAGAACCAG |
| ACTCGCATTTCCTCTTCAGGG | |
| BMAL1 | GCCCATTGAACATCACGAGTAC |
| CCTGAGCCTGGCCTGATAGTAG | |
| WEE1 | AGGGAATTTGATGTGCGACAG |
| TTCAAGCTCATAATCACTGGCT | |
| CHK2 | ATCTGCCTTAGTGGGTATCCA |
| CTGTCGTAAAACGTGCCTTTG | |
| c-MYC | AGCAGCGACTCTGAGGAGGAA |
| ACTCTGACACTGTCCAACTTGA | |
| SURVIVIN | TTCTCAAGGACCACCGCATC |
| CAAGTCTGGCTCGTTCTCAG | |
| TS | TACCTGGGGCAGATCCAACA |
| AGAGGGAATTCATCTCTCAGGC | |
| HSP27 | ACATCTCCCATAACCCCGCC |
| ATCTCGTTGGACTGCGTGGC | |
| BCL2 | AATCCTGTGCTGCTATCCTGC |
| GCGTCCACGTTCTTCATTGTT | |
| β-ACTIN | ACCACCATGTACCCTGGCAT |
| TTGCTGATCCACATCTGCTG |
Statistical analysis
Statistical analyses were performed with SPSS 15.0 (SPSS Inc, Chicago, IL, USA). The gene expression levels in each group are expressed as means ± standard errors. Statistical comparisons between the two groups of patients (pCR vs. non-pCR) were made using the unpaired Student’s t-test. A P value < 0.05 was considered to be statistically significant.
Results
Patient response to chemoradiation therapy
A total of 250 patients (aged 29-74 years, median age 54.2 years) were included in the study (Table 2). In practice, the majority of patients (80%) were treated with a combined platin/5-fluorouracil (5-FU) regimen, although other regimens used included a combination of folic acid/5-FU or 5-FU alone. The total radiation dose was 50.4 Gy, with minor modifications in dose and location [36].
Table 2.
Baseline characteristics of the patients included in the analysis
| N (%) | |
|---|---|
| Gender | |
| Male | 169 (67.6%) |
| Female | 81 (32.4%) |
| Age, years Median (range) | 54.2 (29-74%) |
| Clinical T category | |
| cT2 | 11 (4.4%) |
| cT3 | 106 (42.4%) |
| cT4 | 133 (53.2%) |
| Clinical N category | |
| cN0 | 33 (13.2%) |
| cN+ | 217 (86.8%) |
| Clinical M category | |
| M0 | 227 (90.8%) |
| M1 | 17 (6.8%) |
| Mx | 6 (2.4%) |
| Total | 250 (100%) |
The patient response to neoadjuvant therapy was similar to that described in previous reports [8,9,36], with 70.8% of patients showing down-grading of T stage, and ~30% showing no response (Table 3). pCR (determined by both T-stage and N-stage, with an absence of tumor cells confirmed histologically) was observed in 18% of patients. Tumor progression during treatment was observed in 2.8% of patients (7/250), suggesting uncontrolled cancer. There was no age or gender bias in the patient response to therapy.
Table 3.
Changes in rectal cancer T stage and N stage in patients treated with neoadjuvant chemoradiation therapy
| Change in staging | N (%) |
|---|---|
| T stage | |
| No difference | 66 (26.4%) |
| Down-staging | 177 (70.8%) |
| Up-staging | 7 (2.8%) |
| N stage | |
| No difference | 87 (34.8%) |
| Down-staging | 152 (60.8%) |
| Up-staging | 11 (4.4%) |
| Total | 250 (100%) |
Comparison of the expression levels of core clock genes between the pCR and non-pCR groups
Comparisons of the expression levels of the six core clock genes (Figure 1) revealed that the gene expressions of Clock, Cry2 and Per2 were significantly higher in the pCR group than in the non-pCR group (P = 0.011, 0.014 and 0.003, respectively). In contrast, Per1, Cry1 and Bmal1 did not show significant differences in expression between the two groups (Figure 1).
Figure 1.
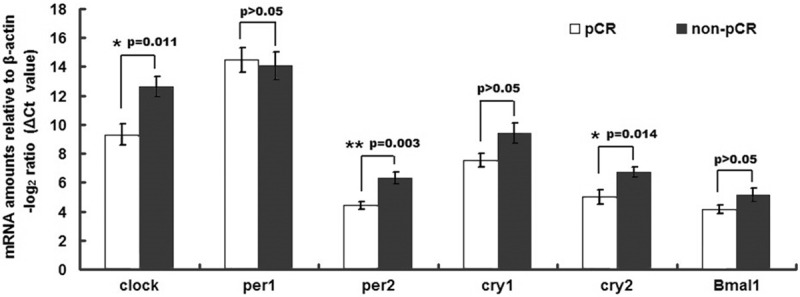
Comparison of core clock gene expression levels in rectal cancer samples. A cohort of 40 patients was assayed for gene expression levels by qRT-PCR, among which 20 showed pCR and 20 showed non-pCR. The data are expressed as delta CT values normalized to β-actin; therefore a higher value represents a lower expression level. The gene expressions of Clock, Cry2 and Per2 were significantly higher in the pCR group than in the non-pCR group, whereas no significant differences were observed for Per1, Cry1 and Bmal1.
Comparison of the expression levels of key genes that are downstream of the core clock genes
We next examined whether the observed differences between the pCR and non-pCR groups in the expressions of Clock, Cry2 and Per2 resulted in the differential expression of key downstream genes. We focused on genes involved in cell cycle regulation and the DNA damage response, because these events are important for the response to chemoradiation therapy. There were no significant differences between the pCR and non-pCR groups in the expressions of Wee1 and Chk2 (Figure 2). Interestingly, the expression of c-Myc was significantly elevated in the pCR group (P = 0.018; Figure 2).
Figure 2.
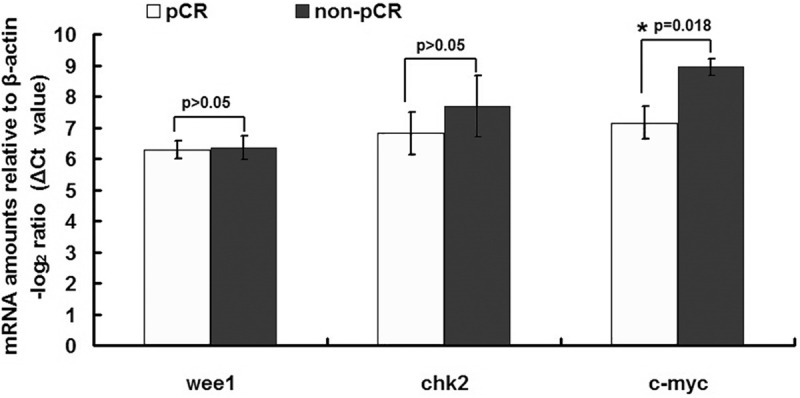
Comparison of key down-stream gene expression levels in rectal cancer samples. A cohort of 40 patients was assayed for gene expression levels by qRT-PCR, among which 20 showed pCR and showed 20 non-pCR. The data are expressed as delta CT values normalized to β-actin; therefore a higher value represents a lower expression level. The gene expression of c-Myc was significantly higher in the pCR group than in the non-pCR group, whereas no significant differences were observed for Wee1 and Chk2.
Comparison of the expression levels of previously identified putative marker genes
Previous studies have reported that Hsp27, Survivin, TS and Bcl2 could serve as biomarkers for predicting the response to chemoradiation therapy [15,16]. Therefore, we compared the expression levels of these genes between the pCR and non-pCR groups using the same set of samples as above (Figure 3). There was no significant difference between groups in the expressions of Survivin, TS or Bcl2, although the expression of Hsp27 was significantly decreased in the pCR group (P = 0.032; Figure 3).
Figure 3.
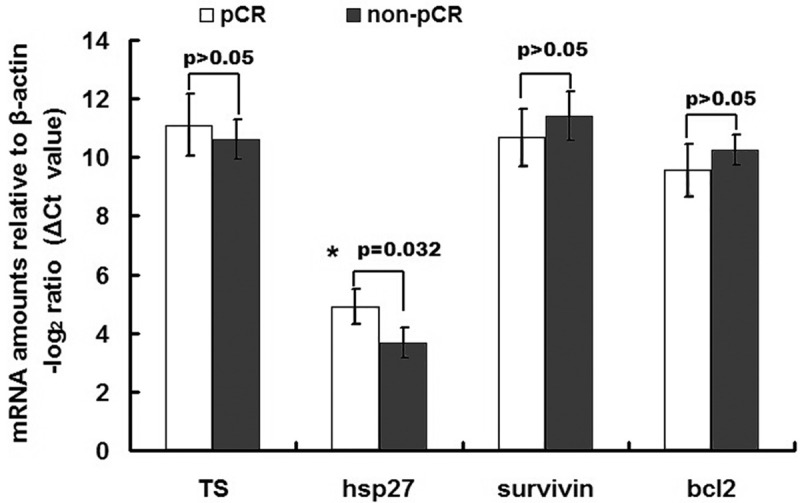
Comparison of the expression levels of some previously identified marker genes in rectal cancer samples. A cohort of 40 patients was assayed for gene expression levels by qRT-PCR, among which 20 showed pCR and 20 showed non-pCR. The data are expressed as delta CT values normalized to β-actin; therefore a higher value represents a lower expression level. There were no significant differences between groups (pCR vs. non-pCR) in the gene expressions of Survivin, Thymidylate synthase (TS) or Bcl2, all of which have been suggested by previous studies to serve as biomarkers for the responsiveness of patients to neoadjuvant chemoradiation therapy. However, the gene expression of Hsp27 was significantly lower in the pCR group than in the non-pCR group.
Discussion
The main findings of the present study were that 18% of patients with rectal cancer treated with neoadjuvant chemoradiation therapy showed pCR, and those patients with pCR showed higher expression levels of three core clock genes (Clock, Cry2 and Per2) and one downstream gene (c-Myc). There were no differences between the pCR and non-pCR groups in the expressions of the other three core clock genes (Per1, Cry1 and Bmal1) or two other downstream genes (Wee1 and Chk2). Interestingly, of four genes (Hsp27, Survivin, TS and Bcl2) previously implicated as potential biomarkers for treatment response, only Hsp27 showed differential expression between the pCR and non-pCR groups. Our novel findings suggest that certain circadian genes show potential as diagnostic biomarkers to prospectively determine whether a rectal cancer patient would benefit from neoadjuvant chemoradiation therapy.
In this study, we found that the expression levels of Per2, Clock and Cry2 were higher in patients that showed a response to chemoradiation therapy. Per2 is a central component of the mammalian clock, displaying the highest sequence similarity with the Drosophila Period gene. Previous reports have indicated that high Per2 expression suppresses tumor growth [37,38]. Thus, it is possible that the pCR group in our study may have stronger circadian activity, which contributes to better outcomes from the neoadjuvant treatment. Per2 mutant mice show abnormal sensitivity to γ-irradiation-induced lymphoma [20], suggesting that Per2 is a tumor suppressor gene. It is also interesting to note that Per1 and Per2 show significant functional redundancy in clock regulation; specifically, only Per2 and Per1 double-knockout mice showed significantly stronger circadian phenotypes, becoming arrhythmic under constant conditions [39]. Therefore, Per2 may share functionality with Per1. The Clock gene encodes a key bHLH-PAS transcription factor that heterodimerizes with BMAL1 to drive downstream target genes via E-box elements. Interestingly, it has been shown that the CLOCK/BMAL1 functional state correlates positively with the responses to two chemotherapeutic drugs, cyclophosphamide [40] and oxaliplatin [41]. Consistent with this, we observed Clock induction in the pCR group, further indicating an important role of this gene in the response to cancer therapeutics.
The pCR group also exhibited enhanced expression of Cry2, which, along with its close analog Cry1, is a major transcriptional repressor in the negative arm of the molecular clock. However, a recent study has shown that Cry1 overexpression is associated with tumor progression [42]. It remains to be determined whether these conflicting observations are indicative of functional divergence between Cry1 and Cry2. An alternative possibility is that expression changes in other clock genes, such as Per2 and Clock, modulate the molecular and therapeutic responses to core clock alteration. Given the exquisite feedback mechanisms embedded in the core clock, it is plausible that changes in other clock genes may differentially impact the expression and/or function of the Cry1 and Cry2 genes. Overall, our finding of reduced expression of certain clock genes in tumor samples provides further evidence that the circadian clock serves as a control mechanism for cell growth and death, thus guarding against uncontrolled cell proliferation and cancer progression.
The present study did not detect any differences between the two groups in the expressions of Wee1 and Chk2. Wee1 is a negative cell cycle regulator that contributes to tumor progression and is an important downstream target of the clock machinery [43,44]. Chk2 is a DNA damage checkpoint kinase that plays a conserved role in clock regulation of the DNA damage response [22,23]. Interestingly, we did observe higher expression levels of c-Myc in the pCR group. c-Myc is a clock-controlled gene that displays rhythmic expression over the circadian cycle [20], and is an important oncogene mutated or activated in many cancers. c-Myc has a variety of cellular functions, with downstream transcriptional target genes involved in mitosis, metabolism and apoptosis [45-47]. On the one hand, our observation was somewhat unexpected, because c-Myc is an important oncogene that promotes cell cycle progression and tumor formation. On the other, c-Myc has also been reported to promote apoptosis by enhancing cytochrome-c release. It has been suggested that c-Myc sensitizes cells to various pro-apoptotic stimuli including genotoxic stress [48,49]. Thus, c-Myc may have a dual role as a “death priming” and “growth promoting” factor. Our observation that elevated tumor expression of c-Myc confers responsiveness to chemoradiation therapy is consistent with its “death priming” function, highlighting the complex role of this important clock-controlled gene in cancer pathobiology. Regardless of the exact mechanism by which a higher c-Myc expression level confers responsiveness to chemoradiation therapy, this observation is consistent with a role of the clock machinery in cancer therapy.
Our findings that Survivin, TS and Bcl2 expression did not differ significantly between the pCR and non-pCR groups are not consistent with previous reports [15,16]. However, this apparent discrepancy could involve a number of factors, including patient sample size, patient ethnicity, treatment regimen and institutional differences. Thus, additional research is needed to identify universal biomarkers that would be useful in a clinical setting.
Our study has several limitations. All included patients were from a single institution, thus our results may not be generalizable to other populations. Gene expression analysis was undertaken in only a small sample of the patients, and may not reflect the gene expression changes of the entire patient cohort. In addition, correlation analysis was not undertaken to investigate the relationship between gene expression levels and response to chemoradiation therapy.
In conclusion, circadian genes are potential diagnostic biomarkers that could be used to prospectively determine whether a rectal cancer patient would benefit from neoadjuvant chemoradiation therapy.
Acknowledgements
This work is supported by The National Natural Science Foundation of China (NSFC), grants 31071285 and 31371472 (to ZY). ZY is a Minjiang scholar at Fuzhou University. BX is supported by the Fujian Provincial Health Department Innovation Fund (2011-CX-8). ZC is supported by the Welch Foundation (AU-1731) and NIH/NIA (R01AG045828).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ, Wolmark N. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J. Clin. Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Galanis E, Alberts SR, O’Connell MJ. New adjuvant therapy for colon cancer: justified hope or commercial hype. Surg Oncol Clin N Am. 2000;9:813–823. discussion 825-816. [PubMed] [Google Scholar]
- 5.Macdonald JS. Adjuvant therapy of colon cancer. CA Cancer J Clin. 1999;49:202–219. doi: 10.3322/canjclin.49.4.202. [DOI] [PubMed] [Google Scholar]
- 6.Edwards MS, Chadda SD, Zhao Z, Barber BL, Sykes DP. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis. 2012;14:e31–47. doi: 10.1111/j.1463-1318.2011.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minsky BD. Adjuvant therapy for rectal cancer--the transatlantic view. Colorectal Dis. 2003;5:416–422. doi: 10.1046/j.1463-1318.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- 8.Ciccocioppo A, Stephens JH, Hewett PJ, Rieger NA. Complete pathologic response after preoperative rectal cancer chemoradiotherapy. ANZ J Surg. 2009;79:481–484. doi: 10.1111/j.1445-2197.2009.04950.x. [DOI] [PubMed] [Google Scholar]
- 9.Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG, Wagman R, Saltz LB, Wong WD. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131–135. doi: 10.1016/s1072-7515(01)01159-0. discussion 135-136. [DOI] [PubMed] [Google Scholar]
- 10.Fiorica F, Cartei F, Licata A, Enea M, Ursino S, Colosimo C, Camma C. Can chemotherapy concomitantly delivered with radiotherapy improve survival of patients with resectable rectal cancer? A meta-analysis of literature data. Cancer Treat Rev. 2010;36:539–549. doi: 10.1016/j.ctrv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Nishioka M, Shimada M, Kurita N, Iwata T, Morimoto S, Yoshikawa K, Higashijima J, Miyatani T. Gene expression profile can predict pathological response to preoperative chemoradiotherapy in rectal cancer. Cancer Genomics Proteomics. 2011;8:87–92. [PubMed] [Google Scholar]
- 12.Brettingham-Moore KH, Duong CP, Greenawalt DM, Heriot AG, Ellul J, Dow CA, Murray WK, Hicks RJ, Tjandra J, Chao M, Bui A, Joon DL, Thomas RJ, Phillips WA. Pretreatment transcriptional profiling for predicting response to neoadjuvant chemoradiotherapy in rectal adenocarcinoma. Clin Cancer Res. 2011;17:3039–3047. doi: 10.1158/1078-0432.CCR-10-2915. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB 3rd, Hamilton SR. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimkus C, Friederichs J, Boulesteix AL, Theisen J, Mages J, Becker K, Nekarda H, Rosenberg R, Janssen KP, Siewert JR. Microarray-based prediction of tumor response to neoadjuvant radiochemotherapy of patients with locally advanced rectal cancer. Clin Gastroenterol Hepatol. 2008;6:53–61. doi: 10.1016/j.cgh.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673–688. doi: 10.1016/j.ijrobp.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Chen MB, Wu XY, Yu R, Li C, Wang LQ, Shen W, Lu PH. P53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment: a meta-analysis in rectal cancer. PLoS One. 2012;7:e45388. doi: 10.1371/journal.pone.0045388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della Vittoria Scarpati G, Falcetta F, Carlomagno C, Ubezio P, Marchini S, De Stefano A, Singh VK, D’Incalci M, De Placido S, Pepe S. A specific miRNA signature correlates with complete pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2012;83:1113–1119. doi: 10.1016/j.ijrobp.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- 20.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 21.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, McKnight SL. A conserved DNA damage response pathway responsible for coupling the cell division cycle to the circadian and metabolic cycles. Cell Cycle. 2007;6:2906–2912. doi: 10.4161/cc.6.23.5041. [DOI] [PubMed] [Google Scholar]
- 24.Lengyel Z, Lovig C, Kommedal S, Keszthelyi R, Szekeres G, Battyani Z, Csernus V, Nagy AD. Altered expression patterns of clock gene mRNAs and clock proteins in human skin tumors. Tumour Biol. 2013;34:811–819. doi: 10.1007/s13277-012-0611-0. [DOI] [PubMed] [Google Scholar]
- 25.Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, Koeffler HP. A role for the clock gene per1 in prostate cancer. Cancer Res. 2009;69:7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gery S, Komatsu N, Kawamata N, Miller CW, Desmond J, Virk RK, Marchevsky A, McKenna R, Taguchi H, Koeffler HP. Epigenetic silencing of the candidate tumor suppressor gene Per1 in non-small cell lung cancer. Clin Cancer Res. 2007;13:1399–1404. doi: 10.1158/1078-0432.CCR-06-1730. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CM, Lin SF, Lu CT, Lin PM, Yang MY. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol. 2012;33:149–155. doi: 10.1007/s13277-011-0258-2. [DOI] [PubMed] [Google Scholar]
- 28.Yeh KT, Yang MY, Liu TC, Chen JC, Chan WL, Lin SF, Chang JG. Abnormal expression of period 1 (PER1) in endometrial carcinoma. J Pathol. 2005;206:111–120. doi: 10.1002/path.1756. [DOI] [PubMed] [Google Scholar]
- 29.Alhopuro P, Bjorklund M, Sammalkorpi H, Turunen M, Tuupanen S, Bistrom M, Niittymaki I, Lehtonen HJ, Kivioja T, Launonen V, Saharinen J, Nousiainen K, Hautaniemi S, Nuorva K, Mecklin JP, Jarvinen H, Orntoft T, Arango D, Lehtonen R, Karhu A, Taipale J, Aaltonen LA. Mutations in the circadian gene CLOCK in colorectal cancer. Mol Cancer Res. 2010;8:952–960. doi: 10.1158/1541-7786.MCR-10-0086. [DOI] [PubMed] [Google Scholar]
- 30.Sotak M, Polidarova L, Ergang P, Sumova A, Pacha J. An association between clock genes and clock-controlled cell cycle genes in murine colorectal tumors. Int J Cancer. 2013;132:1032–1041. doi: 10.1002/ijc.27760. [DOI] [PubMed] [Google Scholar]
- 31.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. New York: Springer; 2010. [Google Scholar]
- 32.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 33.Bohmann K, Hennig G, Rogel U, Poremba C, Mueller BM, Fritz P, Stoerkel S, Schaefer KL. RNA extraction from archival formalin-fixed paraffin-embedded tissue: a comparison of manual, semiautomated, and fully automated purification methods. Clin Chem. 2009;55:1719–1727. doi: 10.1373/clinchem.2008.122572. [DOI] [PubMed] [Google Scholar]
- 34.Antonov J, Goldstein DR, Oberli A, Baltzer A, Pirotta M, Fleischmann A, Altermatt HJ, Jaggi R. Reliable gene expression measurements from degraded RNA by quantitative real-time PCR depend on short amplicons and a proper normalization. Lab Invest. 2005;85:1040–1050. doi: 10.1038/labinvest.3700303. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Xu BH, Chi P, Guo JH, Guan GX, Tang TL, Yang YH, Chen MQ, Song JY, Feng CY. Pilot study of intense neoadjuvant chemoradiotherapy for locally advanced rectal cancer: retrospective review of a phase II study. Tumori. 2014;100:149–157. doi: 10.1177/030089161410000206. [DOI] [PubMed] [Google Scholar]
- 37.Oda A, Katayose Y, Yabuuchi S, Yamamoto K, Mizuma M, Shirasou S, Onogawa T, Ohtsuka H, Yoshida H, Hayashi H, Rikiyama T, Kim H, Choe Y, Kim K, Son H, Motoi F, Egawa S, Unno M. Clock gene mouse period2 overexpression inhibits growth of human pancreatic cancer cells and has synergistic effect with cisplatin. Anticancer Res. 2009;29:1201–1209. [PubMed] [Google Scholar]
- 38.Miyazaki K, Wakabayashi M, Hara Y, Ishida N. Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes Cells. 2010;15:351–358. doi: 10.1111/j.1365-2443.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- 39.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 40.Antoch MP, Kondratov RV, Takahashi JS. Circadian clock genes as modulators of sensitivity to genotoxic stress. Cell Cycle. 2005;4:901–907. doi: 10.4161/cc.4.7.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng ZL, Luo HY, Yang J, Wu WJ, Chen DL, Huang P, Xu RH. Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin Cancer Res. 2014;20:1042–1052. doi: 10.1158/1078-0432.CCR-13-0171. [DOI] [PubMed] [Google Scholar]
- 42.Yu H, Meng X, Wu J, Pan C, Ying X, Zhou Y, Liu R, Huang W. Cryptochrome 1 overexpression correlates with tumor progression and poor prognosis in patients with colorectal cancer. PLoS One. 2013;8:e61679. doi: 10.1371/journal.pone.0061679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obi-Ioka Y, Ushijima K, Kusama M, Ishikawa-Kobayashi E, Fujimura A. Involvement of Wee1 in the circadian rhythm-dependent intestinal damage induced by docetaxel. J Pharmacol Exp Ther. 2013;347:242–248. doi: 10.1124/jpet.113.203299. [DOI] [PubMed] [Google Scholar]
- 44.Kelleher FC, Rao A, Maguire A. Circadian molecular clocks and cancer. Cancer Lett. 2014;342:9–18. doi: 10.1016/j.canlet.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 45.Gohil K, Brooks GA. Exercise tames the wild side of the Myc network: a hypothesis. Am J Physiol Endocrinol Metab. 2012;303:E18–30. doi: 10.1152/ajpendo.00027.2012. [DOI] [PubMed] [Google Scholar]
- 46.Wolfer A, Ramaswamy S. MYC and metastasis. Cancer Res. 2011;71:2034–2037. doi: 10.1158/0008-5472.CAN-10-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- 48.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 49.Larsson LG, Henriksson MA. The Yin and Yang functions of the Myc oncoprotein in cancer development and as targets for therapy. Exp Cell Res. 2010;316:1429–1437. doi: 10.1016/j.yexcr.2010.03.025. [DOI] [PubMed] [Google Scholar]


