Abstract
Background: There is increasing evidence demonstrating the role of human trophoblast cell surface antigen 2 (TROP2) in cancer development and progression. However, their prognostic value in Epstein-Barr virus (EBV) associated nasopharyngeal carcinoma (NPC) remains to be elucidated. Method: The prognostic significances of TROP2 and Ki-67 were determined by immunohistochemistry in 58 NPC samples. TROP2 mRNA expression level and biological functions were evaluated. The presence of EBV was assessed using in situ hybridization. Analyses were conducted on the association between each of these variables as well as clinical outcome. Results: TROP2 was exhibited over expression in 64% of NPC samples and significantly associated with highly proliferative tumor cells (P = 0.05) and lymph node metastases (P = 0.03). Overexpression of TROP2 significantly correlated with worse overall survival (P = 0.026) and poor disease-free survival (P = 0.021). By univariate analysis, high expression of TROP2 significantly correlated with patients with distant metastases, Ki-67 and EBV infection. Multivariate analysis further revealed that TROP2 along with Ki-67 and distant metastasis are independent prognostic predictors for NPC patients. Conclusion: Our findings have demonstrated that overexpression of TROP2 appears to be an independent predictor for poor clinical outcome in NPC. The strong correlation of overexpression of TROP2 with Ki-67 and distant metastases indicates a potentially therapeutic strategies targeting TROP2 for NPC patients.
Keywords: Nasopharyngeal carcinoma, TROP2, EBV, prognostic predictor, clinical outcome
Introduction
Head and neck squamous cell carcinoma is the sixth most common malignant tumor worldwide [1], among of which, Nasopharyngeal carcinoma (NPC) represents a unique epithelial malignancy, with distinct biological and epidemiologic features from other cancers of the head and neck region. More than 80% of nasopharyngeal carcinomas harbor Epstein-Barr virus (EBV) and majority of these patients distribute in Southeast Asia regions [2]. Ionizing radiation therapy is the major curative treatment modality for early disease. Despite progress in combination with chemotherapy for more advanced stages, 30-40% of patients will still develop distant metastases within 4 years [2], underlining the importance to better understand the molecular basis of tumor characteristics and to search for new tumor mediators that contribute to disease progression and developing novel therapeutic strategies.
There is increasing evidence supporting the role for TROP2 (human trophoblast cell surface antigen 2) in tumor development and progression. TROP2 is a cell surface transmembrane glycoprotein receptor, which is also known as Tumor-Associated Calcium Signal Transducer 2 (TACSTD2). It belongs to a family with at least two closely related genes (TACSTD1-EPCAM, and TACSTD2-TROP2) [3]. Both of them share sequence homology with both thyroglobulin type I and interleukin-2 receptors [4]. TROP2 was first discovered in human trophoblast cells, and subsequently it has been found significantly different expression between cancer cells and normal epithelial cells. The higher expression of TROP2 was found in the majority of human epithelial carcinomas, including gastric [5], colorectal [6], cervical [7], oral cavity [8] and laryngeal carcinomas [9]. In recent years, overexpression of TROP2 has been recognized to closely associate with cancer progression and poor prognosis [7,8,10] and it has emerged as an attractive cancer therapy target.
Although the function of TROP2 is not well understood, early studies have shown that TROP2 not only acts as a calcium signal transducer but also plays a role in regulating the growth of carcinoma cells [11]. To date, the role of TROP2 in NPC remains unclear, we therefore investigated TROP2 expression and its correlation with clinicopathologic features in nasopharyngeal carcinoma samples.
Material and methods
Patient characteristics
This study was conducted according to the regulations of the local Medical Ethics Committee. A total of 58 nasopharyngeal carcinoma punch or cone formalin-fixed and paraffin-embedded biopsy samples were retrieved during the period of 2001-2011. The clinical, demographic and tumor-related characteristics of the patients have been described in Table 1. The majority of patients were male (42 or 72.4%), median age was 45 years. 77.6% patients had lymph node metastasis at the diagnosis and 67% patients had locally advanced disease with stage III and IV. All 58 patients shared an average follow-up time of 96 months. During the follow-up period, 26 patients were dead (44.9%) and all of the patients were not treated to either radiotherapy or chemotherapy prior to the biopsy. All studied tumour specimens were assessed on hematoxylin-and-eosin-stained slides using standard diagnostic criteria. This study was approval from the Second Hospital of Jilin University and all patients have consented for the study.
Table 1.
Characteristics of Patients
| Characteristic | N = 58 | (%) |
|---|---|---|
| Sex | ||
| Male | 42 | (72.4) |
| Female | 16 | (27.6) |
| Age (years) | ||
| Range | 24-7 | |
| Median | 45 | |
| Tumor stage | ||
| T1-2 | 19 | (32.8) |
| T3-4 | 39 | (67.2) |
| N0 | 13 | (22.4) |
| N1-3 | 45 | (77.6) |
| M1 | 12 | (20.7) |
| Lymph node metastasis | ||
| No | 21 | (36.2) |
| Yes | 37 | (63.8) |
| Distant metastasis | ||
| No | 46 | (79.3) |
| Yes | 12 | (20.7) |
| TROP2 membrane expression | ||
| Low (0-1.5) | 21 | (36.2) |
| High (2-3) | 37 | (63.8) |
| Ki-67 | ||
| ≤ 10% | 23 | (39.7) |
| > 10% | 35 | (60.3) |
| EBER | ||
| 0-1 | 17 | (29.3) |
| 2-3 | 41 | (70.7) |
Immunohistochemistry
The expression of TROP2 was determined by immunohistochemistry using a purified goat polyclonal antibody (AF650, R&D Systems, Inc., Minneapolis, MN, USA), which identifies TROP2 extracellular domain as described previously [12]. Ki-67 expression was evaluated by using the monoclonal antibody (Dako Cytomation). Briefly, 4-µm sections were deparaffinised and rehydrated, antigens were retrieved by using citrate acid buffer in a microwave oven. Endogenous peroxidase was treated with 3% hydrogen peroxide and non-specific binding was blocked with normal goat serum. A 1:50 dilution of goat anti-TROP2 or an anti-Ki-67 (1:100) antibody was then applied on the sections overnight at 4°C. In combination with the Universal LSABTM2 detective system (Dako Cytomation), the positive signal was detected by 3,3’-diaminobenzidine in chromogenic solution (DAB) with haematoxylin as a counterstain. Sections without primary antibodies were used as negative controls.
Immuno-activity evaluation
TROP2 expression was evaluated by two individuals using light microscopy with a double-blind method. Positive TROP2 expression was assessed by calculating the tumor cell membrane staining proportion and intensity, as described previously [5,10]. The proportion of positive cells was scored as: 0 = none; 1 < 10%; 2 = 10-50%; 3 ≥ 50%. Staining intensity was defined as: 0 = no staining; 1 = weak; 2 = moderate; 3 = strong. The overall score was a combination of staining proportion and intensity. Low = score 0-1.5; high = score 2-3. Immunostaining of Ki-67 was evaluated by scanning the entire section at low power (×100) and then counting at least 500 tumor cells in the five most densely staining fields at high power (×400). The percentage of positively staining tumor nuclei was calculated. Ki-67 positive was considered when > 10% of tumor cells with nuclear staining.
Cells lines and transfection
CNE-1, nasopharyngeal carcinoma cells were purchased from Shanghai Aiyan Biotechnology Co., Ltd. and maintained at 37°C, 5% CO2 in RPMI 1640 complete medium (GIBCO, USA) with 10% fetal bovine serum (FBS, GIBCO, USA). The normal human fibroblast cells (GM05757) were purchased from Coriell Institute (USA) and cultured in the Eagle’s Minimum Essential Medium. To deplete endogenous TROP2 expression, as described previously [7], a pair of SIRNA sequences targeting TROP2 were designed and synthesized by Genepharma Co., Ltd (Shanghai, China). These sequences were: Forward: 5’-GCACGCUCAUCUAUUACCUTT-3’, Reward: 5’-AGGUAAUAGAUGAGCGUGCTT-3’; Negative scramble control sequences were: 5’-UUCUCCGAACGUGUCACGUTT-3’, 5’-ACGUG-ACACGUUCGGAGAATT-3’. Lipofectamine 2000 (Invitrogen) was used to transfect the tumor cells with either scramble (SC) negative control or SIRNA (life technologies) by using the reverse transfection protocol, according to the manufacturer’s instructions.
Quantification of TROP2 expression
In order to evaluate TROP2 expression in mRNA level, Quantitative real-time PCR (QRT-PCR) was utilized. The primers were designed with Primer 4.0 and the forward primer was 3’-gagattcccccgaagttctc-5’ and the reverse primer was 3’-aactcccccagttccttgat-5’. Total RNA was extracted from 23 available primary tumor samples or from cell lines using Recover All Total Nucleic Acid Isolation kit (Ambion). The reverse transcription was performed using Super Script III Reverse Transcriptase (Invitrogen Corp.) according to the manufacturer’s recommendations. QRT-PCR analyses were performed using SYBR Green Master Mix (Applied Biosystems) and the ABI PRISM 7900 Sequence Detection System (Applied Biosystems Inc., Foster City, CA, USA). The relative fold change in mRNA expression was calculated using the 2-ΔΔCt method, where the average of ΔCt values for the amplicon of interest was normalized to that of an endogenous gene (GAPDH), compared with control specimens. Each experiment was performed triplicate.
Western blot was used to detect TROP2 expression in cell lines. The cells were transfected with either SC or SIRNA. The cells were collected and lysed at 48 hours after. As described previously [13]. Blots were incubated with anti-TROP2 antibody (1:1000) for overnight followed by incubation with the second antibodies. Signals were visualized using the ECL Western blotting substrate kit (Pierce, USA).
Cell proliferation assay
The cell proliferation and cytotoxicity of introducing anti-TROP2 SIRNA were assessed by using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) staining kit (Sigma, USA). Cells were reverse transfected with either SC control, anti-TROP2 SIRNA or Lipofectamine 2000 and seeded onto 96-well plates (5×103 cells/per well). Cell viability was measured at 24, 48 and 72 hours post-transfection.
Epstein-Barr virus (EBER) detection
By using a commercial available probe, the presence of latent EBV infection (EBER) in NPC was detected by in situ hybridization on formalin-fixed, paraffin-embedded tissue sections in accordance with the manufacturer’s instruction. Briefly, 4-µm sections were deparaffinised and rehydrated, after predigested with proteinase K, a fluorescein-conjugated EBV (EBER) peptide nucleic acid probe (DAKO-Agilent technologies, China) was applied on the section for 2 hours at 55°C. Hybridization signals were detected by alkaline phosphatase-conjugated antifluorescein, then by 5-bromo-4-chloro-3-indolylphosphate nitroblue tetrazolium as a chromogen. The sample was defined as positive if dark brown staining signal was observed in the tumor nuclei. Based on the staining proportion and intensity, a grade was assigned as 0 (negative); 1+ (< 10% of positive staining); 2+ (10-50% positivity) and 3+ (> 50% positivity).
Statistical analysis
Overall survival (OS) was defined from the time of diagnosis to date of death. Disease-free survival (DFS) was defined from the time of diagnosis to the date of first failure. Survival curves were generated using the Kaplan-Meier method and compared by means of the log-rank test. Associations between TROP2 expression and clinicopathological variables were assessed with the Chi-test. In the univariate model, if factors with prognostic significance, they were then further analysed in a multivariate Cox’s proportional hazards regression model. A P-value of less than 0.05 was considered to be statistically significant.
Results
Evaluation of TROP2 expression in NPC patients
TROP2 immnuo-activity was determined in 58 archival formalin-fixed and paraffin-embedded NPC samples using immunohistochemistry. TROP2 expression was assessed based on the combination of positive tumor cell staining proportion and intensity. Our observations were consistent with previously reported, when comparing with adjacent normal epithelial cells (showed no or weak staining), the majority of cancer cells showed a homogeneous moderate to strong membranous expression of TROP2. Moderate or strong immuno-activity was observed in 37 of the 58 (63.8%) specimens and none or weak expression was in 21 (36%) specimens. Overall, TROP2 was exhibited overexpression in 64% of NPC samples (Table 1; Figure 1A-C).
Figure 1.
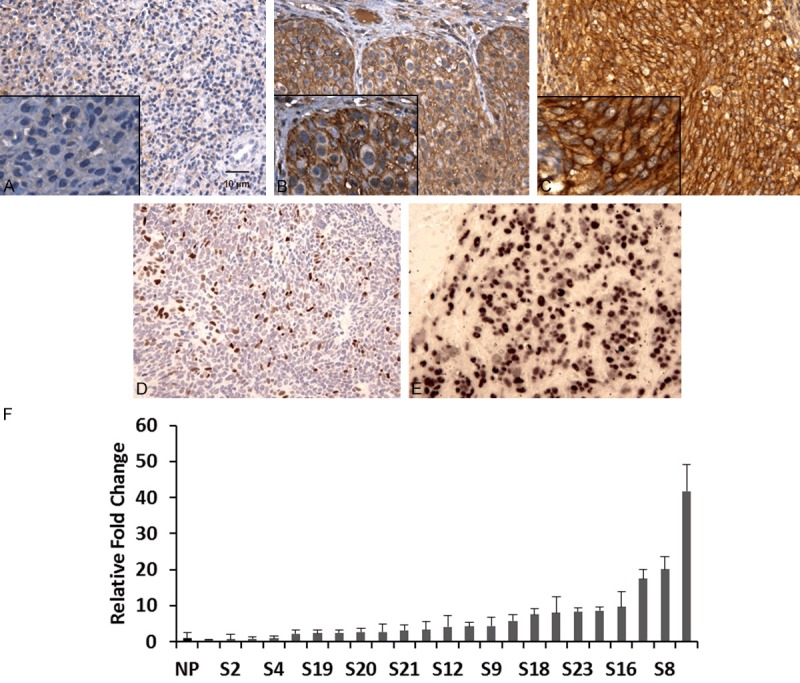
Immunohistochemical staining of TROP2, Ki-67 and in situ hybridization detection of EBV in nasopharyngeal carcinoma. A. None or weak membrane expression of TROP2. B, C. Moderate and strong TROP2 expression in tumor cells (magnification 200× or 400×). D. Ki-67 expression in tumor cells (magnification 200×). E. Purple-blue signal showed EBV infection in NPC tumor cells (magnifications 200×). F. TROP2 over-expressed in 19 out of 23 NPCs by QRT-PCR. P < 0.0001.
Among these 58 samples, TROP2 transcript mRNA expression was further evaluated by QRT-PCR in 23 available samples (with sufficient RNA). By comparing with 6 normal tissues pooled RNA (squamous mucosa with chronic inflammation and lymphoid hyperplasia), 19 out of 23 cases had 2.3- to 41.8- fold higher expression levels of TROP2 in NPC samples (Figure 1F), demonstrating that TROP2 was indeed significantly overexpressed in NPC at both protein and mRNA levels.
Expression of Ki-67 and EBV in NPC
Tumor nuclei immuostaining of Ki-67 was observed in 35 of 58 (60.3%) of NPC specimens (Table 1; Figure 1D). Overexpression of Ki-67 was correlated significantly with membrane accumulation of TROP2 protein in NPC tumor cells (Table 2; P = 0.02), 28 of 39 (71.7%) of the highly TROP2 expressed samples have increased expression of Ki-67, indicating TROP2 more likely expressed in the highly proliferative tumor cells.
Table 2.
TROP2 expression with clinicopathological parameters
| TROP2 expression | |||
|---|---|---|---|
|
|
|||
| Parameter | Low (%) | High (%) | P value |
| Sex (n = 58) | |||
| Male | 14 (33.3) | 28 (66.7) | 0.86 |
| Female | 5 (31.3) | 11 (68.7) | |
| Age (median) | |||
| < 45 | 10 (34.5) | 19 (60.9) | 0.97 |
| ≥ 45 | 9 (31.0) | 20 (69.0) | |
| Tumor stage | |||
| T1-2 | 7 (36.8) | 12 (63.2) | 0.87 |
| T3-4 | 12 (30.8) | 27 (69.2) | |
| lymph node status | |||
| No | 11 (52.4) | 10 (47.6) | 0.035 |
| Yes | 8 (21.6) | 29 (78.4) | |
| Distant metastasis | |||
| No | 14 (30.4) | 32 (69.6) | 0.69 |
| Yes | 5 (41.7) | 7 (58.3) | |
| Ki-67 | |||
| ≤ 10 | 12 (52.2) | 11 (47.8) | 0.023 |
| > 10 | 7 (20.0) | 28 (80.0) | |
| EBER | |||
| 0-1 | 5 (29.4) | 12 (70.6) | 0.91 |
| 2-3 | 14 (34.1) | 27 (65.9) | |
NPC has a strong correlation with EBV infection [14]. Our in situ hybridization (ISH) data demonstrated that the majority of tumors were EBER positive (41 of 58; 70.7%) (Table 1; Figure 1E) (defined as ≥ 10% positively signal in tumor nuclei). However, there was not a statistical association between overexpression of TROP2 and positive EBV status in this cohort (Table 2; P = 0.91).
Correlation of TROP2 expression with clinicopathological features
Using univariate analysis, TROP2 status was observed to be correlated significant for overall survival (OS) and disease-free survival (DFS). As observed in Figure 2, Kaplan-Meier survival curves showed that patients with high TROP2 expression had worse clinical outcome than those with low TROP2 expression by log-rank test (P = 0.026 and P = 0.021, respectively). When compared TROP2 expression to gender, age, tumor stage, lymph node metastases, distant metastases, Ki-67 and EBER expression, overexpression of TROP2 significantly correlated with patients with distant metastases, proliferation indicator and EBV infection, but not with sex, age and lymph node status (Table 3). By using Cox proportional hazard regression model, multivariate analysis was performed. TROP2 overexpression together with Ki-67 and distant metastases were independent prognostic factors for poor disease-free survival (Table 4; TROP2; P = 0.031; Hazard ratio: 4.81; 95% CI between 2.097 to 21.07). Likewise, TROP2 was also significantly correlated with overall survival when controlled for the clinical prognostic factors (Table 4; P = 0.04). The hazard ratio for the effect of TROP2 on survival when controlled for clinical factors was 4.56.
Figure 2.
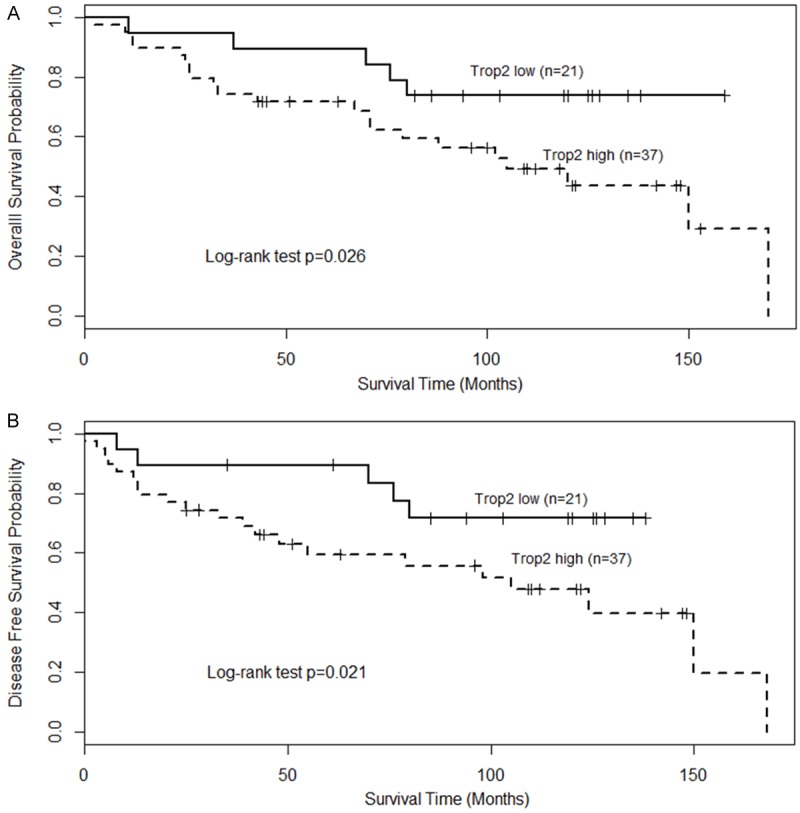
Prognostic significance of TROP2 in patients with nasopharyngeal carcinoma. Kaplan-Meier analysis was performed for overall survival and disease free survival. TROP2 low: score as 0-1; TROP2 high (over-expression): score as 2-3. Patients with high expression of TROP2 exhibited significantly worse overall survival (A) and shortened disease-free survival (B) than that with low expression of TROP2 as defined by log-rank test.
Table 3.
Univariate analysis of prognostic parameters
| OS | DFS | |
|---|---|---|
|
|
|
|
| Parameter | P value | P value |
| Sex | ||
| (Male/female) | 0.561 | 0.503 |
| Age | ||
| (≥ 45 vs. < 45) | 0.109 | 0.167 |
| T stage | ||
| (pI-II vs. pIII-IV) | 0.221 | 0.084 |
| lymph node status | ||
| (Y/N) | 0.740 | 0.602 |
| Distant metastasis | ||
| (Y/N) | 0.029 | 0.009 |
| TROP2 overexpression | ||
| (Y/N) | 0.026 | 0.021 |
| Ki-67 overexpression | ||
| (Y/N) | 0.019 | 0.012 |
| EBER expression | ||
| (Y/N) | 0.025 | 0.057 |
DFS-disease-free survival, OS-overall survival.
Table 4.
Multiivariate analyses of prognostic parameters
| DFS | OS | |||
|---|---|---|---|---|
|
|
|
|||
| Parameter | P value | HR (95% CI) | P value | HR (95% CI) |
| T stage | ||||
| (pI-II vs. pIII-IV) | 0.096 | 2.59 (0.843-7.98) | 0.233 | 2.02 (0.643-6.257) |
| lymph node status | ||||
| (Y/N) | 0.604 | 1.35 (0.441-4.163) | 0.741 | 1.21 (0.391-3.764) |
| Distant metastasis | ||||
| (Y/N) | 0.016 | 3.78 (1.278-11.18) | 0.040 | 2.87 (1.048-7.885) |
| TROP2 overexpression | ||||
| (Y/N) | 0.031 | 4.81 (1.097-21.07) | 0.037 | 4.56 (1.043-19.92) |
| Ki-67 overexpression | ||||
| (Y/N) | 0.020 | 3.79 (1.242-11.62) | 0.033 | 3.45 (1.26-10.54) |
| EBER expression | ||||
| (Y/N) | 0.065 | 0.42 (0.163-1.057) | 0.030 | 0.36 (0.143-0.923) |
DFS-disease-free survival, OS-overall survival, HR-Hazard ratio, CI-confidence interval.
TROP2 promotes cell proliferation in NPC cells
Given the evidence that TROP2 overexpressed in NPC samples and was associated with poor clinical outcomes, we further investigated the biological significance of depletion of TROP2. CNE-1 cells were transfected with scramble controls (SC) or anti-TROP2 SIRNA. First, we demonstrated that untreated CNE-1 cells exhibited elevated TROP2 expression compared with a normal cell control (Figure 3A), 3.8-folds increasing of TROP2 expression were observed, consistent with a previously study [7]. Furthermore, introduction of anti-TROP2 SIRNA caused significant reduction of TROP2 expression in both mRNA and protein levels (Figure 3A, 3B).
Figure 3.
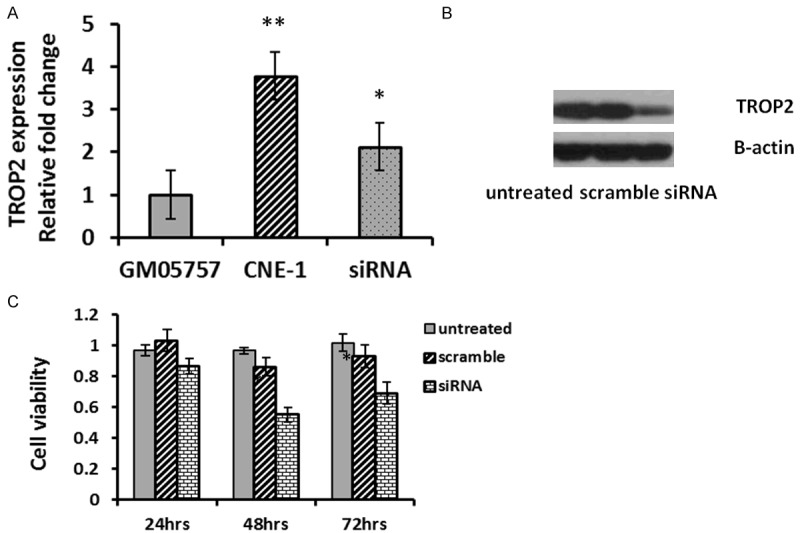
TROP2 promotes cell proliferation in NPC cells. A. TROP2 expression was detected by real-time RT-PCR. Overexpression of TROP2 was observed in CNE-1 cells, while a reduction was exhibited after the cells transfected with anti-TROP2 SIRNA. B. Western blotting of TROP2 in CNE-1 cells was determined 48 hours post-transfection. C. Knock-down TROP2 reduced cell viability measuring by the MTT assay 24-72 hours after transfection. Each datum represents the mean fold change ± SE in triplicates.
The SIRNA transfected cells showed significantly decreased cell viability compared to controls starting from 24 hours post-transfection, continuously declined at 48 (55%) hours and 72 (62%) hours post-transfection (Figure 3C).
Discussion
In the present study, we observe for the first time that a correlation between TROP2 expression with patient clinical outcome in nasopharyngeal carcinoma. We provide evidence that TROP2 was overexpressed in 64% of NPC patients, with none or low expression in normal, suggesting that TROP2 plays an important role in this aggressive malignancy and associates with the development of NPC. Moreover, overexpression of TROP2 significantly correlates with poor overall survival and disease-free survival. Particularly, patients with higher TROP2 expression exhibit a closely relationship with higher proliferation index (Ki-67) and promote tumor cell proliferation in vitro. Furthermore, overexpression of TROP2 along with Ki-67 and distant metastasis have been considered as independent prognostic predictors for NPC patients. Our data further confirm the observations in many other studies in various cancer phenotypes.
Ki-67 has been reported extensively in numerous of cancers as a tumor proliferation index. Liu T at al. [7] showed that TROP2 was overexpressed in cervical cancer and the overexpression was closely related to the high expression of Ki-67 and poor prognosis. Our data in line with their observations, suggesting that TROP2 expression could correlate with the cancer aggressive behaviour. However, the precise mechanism of TROP2 in the development and progression of NPC remains unclear.
TROP2 plays a critical role for tumor growth. It can be phosphorylated by protein kinase C (PKC) [15], resulting in transient increase in intracellular calcium levels [16] and leading to cell proliferation. Another mechanism is that TROP2 may control cleavage of membrane-bound proteins [17]. Upon cleavage, the intracellular domain can enter the nucleus and regulate target genes (e.g. c-myc) to promote cell proliferation [18]. Extensive studies have shown that TROP2 regulates proliferation not only by effecting ERK pathway, but also mediating their downstream transcription cell cycle genes [19], such as cyclin D1, cyclin E, cyclin-dependent kinase (CDK) 4, CDK2, and up-regulation of NF-KB [20]. This regulating capacity relies on the phosphorylation on an S303 site of protein kinase C in the cytoplasmic region of TROP2 [20], which leads to the activation of MAPK/ERK pathway, resulting in tumor invasion and metastasis [21].
Over expression of TROP2 has been correlated to increased metastasis in many cancers, such as colorectal cancer [6], pancreatic cancer [10], lung cancer [19], laryngeal squamous cell carcinoma [9], and prostate cancer [22]. In prostate cancer, overexpression of TROP2 in cancer cell lines relocates RACK1 and interacts with integrin b1, resulting in decreased cell adhesion to fibronectin [22]. Our results are in line with their observation, in that knock down TROP2 cause significant reduction of cell viability (Figure 3C). TROP2 has been proposed to be a potential therapeutic target either alone or in combination with other agents [23]. Anti-TROP2 antibody (hRS7) has been used in pre-clinical trials [24].
TROP2 signaling is a rapidly expanding area. There is likely a biologic basis for Epstein-Barr virus (EBV) infection with TROP2 overexpression. EBV status has closely association with non-keratinizing nasopharyngeal carcinomas [25]. Genetic alterations in host cells [26], oncogenic properties of EBER1, multiple EBV latent genes infections [27] and inflammatory stroma stimulated by TGF-b1 [28] or other genes have been reported to be crucial events in NPC. In the current cohort of 58 patients, EBER status has statistically significant for improved overall survival compared to EBER negative patients (P = 0.025), but no significant for disease free survival (P = 0.06). However, there is bore no relationship to TROP2 over expression (P = 0.91). One explanation may be related to the small sample size or the criteria used to define over-expression. Nonetheless, further studies in a large cohort of samples may validate the exploratory findings.
In conclusion, our findings have demonstrated that overexpression of TROP2 appears to be an independent predictor for poor clinical outcome in NPC. The strong correlation of overexpression of TROP2 with Ki-67 and distant metastases indicates a potentially therapeutic strategies targeting TROP2 for NPC patients.
Acknowledgements
The authors acknowledge the financial support of the Science and Technology R&D program of Jilin Province, China (20150101140JC).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Gregory CD, Kirchgens C, Edwards CF, Young LS, Rowe M, Forster A, Rabbitts TH, Rickinson AB. Epstein-Barr virus-transformed human precursor B cell lines: altered growth phenotype of lines with germ-line or rearranged but nonexpressed heavy chain genes. Eur J Immunol. 1987;17:1199–1207. doi: 10.1002/eji.1830170818. [DOI] [PubMed] [Google Scholar]
- 3.Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, Gastl G, Spizzo G. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–1295. doi: 10.1038/sj.bjc.6604677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szala S, Froehlich M, Scollon M, Kasai Y, Steplewski Z, Koprowski H, Linnenbach AJ. Molecular cloning of cDNA for the carcinoma-associated antigen GA733-2. Proc Natl Acad Sci U S A. 1990;87:3542–3546. doi: 10.1073/pnas.87.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhlmann G, Spizzo G, Gostner J, Zitt M, Maier H, Moser P, Gastl G, Zitt M, Muller HM, Margreiter R, Ofner D, Fong D. TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol. 2009;62:152–158. doi: 10.1136/jcp.2008.060590. [DOI] [PubMed] [Google Scholar]
- 6.Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–3063. doi: 10.1158/1078-0432.CCR-05-1961. [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Liu Y, Bao X, Tian J, Liu Y, Yang X. Overexpression of TROP2 predicts poor prognosis of patients with cervical cancer and promotes the proliferation and invasion of cervical cancer cells by regulating ERK signaling pathway. PLoS One. 2013;8:e75864. doi: 10.1371/journal.pone.0075864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong D, Spizzo G, Gostner JM, Gastl G, Moser P, Krammel C, Gerhard S, Rasse M, Laimer K. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21:186–191. doi: 10.1038/modpathol.3801001. [DOI] [PubMed] [Google Scholar]
- 9.Wu H, Xu H, Zhang S, Wang X, Zhu H, Zhang H, Zhu J, Huang J. Potential therapeutic target and independent prognostic marker of TROP2 in laryngeal squamous cell carcinoma. Head Neck. 2013;35:1373–1378. doi: 10.1002/hed.23138. [DOI] [PubMed] [Google Scholar]
- 10.Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, Gastl G, Spizzo G. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–1295. doi: 10.1038/sj.bjc.6604677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fornaro M, Dell’Arciprete R, Stella M, Bucci C, Nutini M, Capri MG, Alberti S. Cloning of the gene encoding Trop-2, a cell-surface glycoprotein expressed by human carcinomas. Int J Cancer. 1995;62:610–618. doi: 10.1002/ijc.2910620520. [DOI] [PubMed] [Google Scholar]
- 12.Stoyanova T, Goldstein AS, Cai H, Drake JM, Huang J, Witte ON. Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via beta-catenin signaling. Genes Dev. 2012;26:2271–2285. doi: 10.1101/gad.196451.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan G, Zhang D, Zheng Y, Wen L, Yu D, Lu Y, Zhao Y. microRNA-423-3p promotes tumor progression via modulation of AdipoR2 in laryngeal carcinoma. Int J Clin Exp Pathol. 2014;7:5683–5691. [PMC free article] [PubMed] [Google Scholar]
- 14.Kwok H, Wu CW, Palser AL, Kellam P, Sham PC, Kwong DL, Chiang AK. Genomic diversity of Epstein-Barr virus genomes isolated from primary nasopharyngeal carcinoma biopsy samples. J Virol. 2014;88:10662–10672. doi: 10.1128/JVI.01665-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanger TM, Dewitt S, Collins A, Maitland NJ, Poghosyan Z, Knauper V. Differential regulation of TROP2 release by PKC isoforms through vesicles and ADAM17. Cell Signal. 2015;27:1325–1335. doi: 10.1016/j.cellsig.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Ripani E, Sacchetti A, Corda D, Alberti S. Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer. 1998;76:671–676. doi: 10.1002/(sici)1097-0215(19980529)76:5<671::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Guerra E, Trerotola M, Aloisi AL, Tripaldi R, Vacca G, La Sorda R, Lattanzio R, Piantelli M, Alberti S. The Trop-2 signalling network in cancer growth. Oncogene. 2013;32:1594–1600. doi: 10.1038/onc.2012.151. [DOI] [PubMed] [Google Scholar]
- 18.Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, Gires O. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162–171. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 19.Lin JC, Wu YY, Wu JY, Lin TC, Wu CT, Chang YL, Jou YS, Hong TM, Yang PC. TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma. EMBO Mol Med. 2012;4:472–485. doi: 10.1002/emmm.201200222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra E, Trerotola M, Dell’Arciprete R, Bonasera V, Palombo B, El-Sewedy T, Ciccimarra T, Crescenzi C, Lorenzini F, Rossi C, Vacca G, Lattanzio R, Piantelli M, Alberti S. A bicistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Res. 2008;68:8113–8121. doi: 10.1158/0008-5472.CAN-07-6135. [DOI] [PubMed] [Google Scholar]
- 21.Cubas R, Zhang S, Li M, Chen C, Yao Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer. 2010;9:253. doi: 10.1186/1476-4598-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte ON. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci U S A. 2008;105:20882–20887. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDougall AR, Tolcos M, Hooper SB, Cole TJ, Wallace MJ. Trop2: from development to disease. Dev Dyn. 2015;244:99–109. doi: 10.1002/dvdy.24242. [DOI] [PubMed] [Google Scholar]
- 24.Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res. 2011;17:3157–3169. doi: 10.1158/1078-0432.CCR-10-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsao SW, Tsang CM, To KF, Lo KW. The role of Epstein-Barr virus in epithelial malignancies. J Pathol. 2015;235:323–333. doi: 10.1002/path.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo S, Ho WK, Wei WI. Outcome of patients with positive Epstein-Barr virus serologic status in the absence of nasopharyngeal carcinoma in Hong Kong. Arch Otolaryngol Head Neck Surg. 2004;130:770–772. doi: 10.1001/archotol.130.6.770. [DOI] [PubMed] [Google Scholar]
- 27.Ma SD, Yu X, Mertz JE, Gumperz JE, Reinheim E, Zhou Y, Tang W, Burlingham WJ, Gulley ML, Kenney SC. An Epstein-Barr Virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J Virol. 2012;86:7976–7987. doi: 10.1128/JVI.00770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsang CM, Zhang G, Seto E, Takada K, Deng W, Yip YL, Man C, Hau PM, Chen H, Cao Y, Lo KW, Middeldorp JM, Cheung AL, Tsao SW. Epstein-Barr virus infection in immortalized nasopharyngeal epithelial cells: regulation of infection and phenotypic characterization. Int J Cancer. 2010;127:1570–1583. doi: 10.1002/ijc.25173. [DOI] [PubMed] [Google Scholar]


