Abstract
We aim to investigate the pathological characteristics of liver biopsies and their implications for the prognosis of hepatic epithelioid hemangioendothelioma (HEHE). Clinical data of eight patients (5 male, 3 female) with HEHE were analyzed retrospectively. Expression of CD34, FVIII, AE1/AE3, Hepa-par1, GPC3, CK19 and the proliferation index marker Ki-67 were determined by immunohistochemical staining. The clinical pathological features and effects of treatment on prognosis were investigated. Among the eight patients, four did not exhibit significant symptoms, while four showed symptoms such as abdominal distension, aversion to greasy food and mild fever. Two patients had single liver lesions, while multiple lesions were observed in six cases, in which the tumor cells exhibited spindle, irregular or epithelioid morphology, with scattered, streaked and nested distribution. Individual luminal cells were also visible, containing red cells and accompanied by mucoid or fibrous stroma. All cases were CD34 positive, one case was FVIII factor negative, two cases were AE1/AE3 positive, Ki-67 staining exceeded 15% in two cases, and nuclear fission was visible in two cases. Patients with nuclear fission and Ki-67 > 15% died within 2 years after artery embolization, liver transplantation without relapse was observed in two cases and one case survived with the tumor. The other patients without cellular atypia, without nuclear fission and with Ki-67 < 10% did not relapse during the 2-5 years of follow-up. HEHE can be diagnosed according to hematoxylin and eosin morphology and immunohistochemical characteristics in biopsies before treatment allowing the selection of different treatment protocols based on pathological characteristics.
Keywords: Hepatic epithelioid hemangioendothelioma, pathologic features, Ki-67, arterial embolism, orthotopic liver transplantation
Introduction
Epithelioid hemangioendothelioma (EHE), which was first reported by Weiss and Enzinger in 1982, is a very rare vascular endothelial cell tumor and belongs to mesenchymal tumor with undecided malignant potential. The incidence is approximately 1/10,000,000, with a male-to-female ratio of 3:2, and it mainly occurs in superficial or deep soft tissues, but rarely in the liver [1]. In 1984, Ishak et al. first reported a series of studies, comprising 32 cases of primary hepatic epithelioid hemangioendothelioma (HEHE) [2]. Currently, most of the reports on HEHE are case studies, and pathological diagnosis of the disease is difficult due to unspecific clinical manifestations, leading to a lack of unified treatment principles [3].
CD34 and FVIII are commonly used markers of endothelial cells. The CD34 antigen is an advanced glycation I-type transmembrane protein, and is selectively expressed in human hematopoietic stem cells (HSC), progenitor cells (PC) and endothelial cells [4]. CD34 shows high specificity and sensitivity as a marker of vascular epithelial cell tumors [5]. Previously, FVIII was used as an endothelial cell marker, although the specificity and sensitivity of FVIII is much poorer than that of CD34 [6].
The purpose of this study was to analyze the pathological characteristics of liver biopsies obtained from eight patients with HEHE and to evaluate the value of these characteristics in determining patient prognosis and selecting treatment protocols.
Materials and methods
Clinical data
Clinical data of eight patients who were diagnosed as HEHE based in liver biopsy and received treatment in our hospital between 2010 and 2014 were studied retrospectively.
Sample treatment
After fixation with 10% formaldehyde, all the liver biopsy samples collected were subjected to dehydration and vifrification before embedding in paraffin wax. Samples were sections (thickness, 40 μm) continuously, and stained with hematoxylin and eosin (HE). Using the EnVision two-step method, the sections were incubated at 60°C for 35 min and dewaxed in a graded series of ethanol solutions with descending concentration, before being placed in 3% hydrogen peroxide for 5 min. Sections were rinsed with PBS three times (5 min per wash) and antigen retrieval was performed using the citric acid/pressure cooker method. Briefly, sections were heated in the pressure cooker at maximum pressure for 2 min, followed by moderate heating for a further 2 min. Sections were then allowed to cool for 10 min before cooling to 37°C with cold water. After washing with PBS three times (5 min per wash), sections were incubated in the presence of mouse anti-human CD34 monoclonal antibody at 4°C overnight. Subsequently, sections were washed with PBS three times (5 min per wash) and incubated in the presence of the secondary detection antibody PV 6000 (universal antibody) at 37°C for 10 min. After washing with PBS three times (5 min per wash), immunostaining was visualized by using the DAB chromogenic substrate. The reaction was terminated by the addition of PBS before sections were dehydrated with a graded series of formaldehyde of descending concentrations, followed by immersion in xylene and sealing. The procedures for staining of CD31, FVIII, Ki-67, AE1/AE3, GPC3 and Hep-Par1 were the same as those described for staining of CD34. All the antibodies were purchased from Zhongshan Company, Beijing.
Results
Clinical information
The eight patients included in this retrospective analysis comprised five males and three females, aged 40 to 66 years, with a mean age of 52 years. Six cases had multiple liver lesions and two cases had single lesions. Four cases did not exhibit significant symptoms, while four cases showed symptoms such as abdominal distension and mild fever. One case had distant metastasis. Two cases received liver transplantation without relapse during the 2-5 years of follow-up; one of these patients had multiple lesions accompanied by symptoms of late stage cirrhosis, while the other case had a single tumor without significant symptoms. One case received tumor resection and radiofrequency ablation, and has survived with the tumor for 4 years. Four cases received radiofrequency ablation alone. Two of these patients, both with multiple tumors were accompanied by symptoms such as low fever, abdominal distension and aversion to greasy food (Table 1).
Table 1.
Clinical information of patients
| Index | Gender | Age | Single/multiple lesions | Manifestations | Liver function | Imaging diagnosis | Treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 66 | Multiple | No | Normal | HCC | Artery embolization | No relapse in 3 years |
| 2 | M | 51 | Single/diameter 6.5 cm | No | Normal | HCC | Liver transplantation | No relapse in 3 years |
| 3 | M | 54 | Single/diameter 4 cm | No | Normal | HCC | Artery embolization | No relapse in 5 years |
| 4 | M | 40 | Multiple | Cirrhosis, abdominal distension | Normal | HCC | Liver transplantation | No relapse in 26 months |
| 5 | M | 60 | Multiple | Low fever abdominal distension | Increased transaminase, hypoproteinosis | Malignant tumor | Artery embolization | Died in 6 months |
| 6 | F | 47 | Multiple | Aversion to greasy food, low fever ‘yan’ zheng | Normal | Hemangioma | Artery embolization | Died after 2 years |
| 7 | F | 54 | Multiple | No | Significantly increased transaminase | Vascular tumor | Artery embolization | Survived after 8-month treatment |
| 8 | F | 44 | Multiple | Abdominal distension | Normal | Metastatic tumor | Resection + Artery embolization | Survived with tumor for 4 years |
Serologic examination results
Transaminase levels were normal in five patients, and slightly elevated in three patients. GGT and ALP were slightly elevated in one patient, total bilirubin was slightly increased in four patients, and AFP was greater than 500 in one patient (tumor markers were not detected in 2 patients) (Table 2).
Table 2.
Laboratory examination results
| No. | ALT (U/L) | AST (U/L) | GGT | ALP | TBIL | AFP | CEA | CA199 |
|---|---|---|---|---|---|---|---|---|
| 1 | 21.4 | 22.1 | 20.6 | 70.3 | 4.9 | 1.27 | 1.58 | 5.05 |
| 2 | 12.5 | 20.7 | 61.2 | 106.3 | 11.7 | |||
| 3 | 16.7 | 29.0 | 45 | 113.7 | 7.0 | 1.41 | 1.54 | 17.5 |
| 4 | 11.6 | 15.2 | 26.9 | 66.9 | 27.1 | 785.2 | 1.41 | 6.88 |
| 5 | 43.6 | 24.9 | 281.3 | 125.1 | 14 | |||
| 6 | 45.2 | 41.3 | 153.5 | 233.6 | 22 | 4.21 | 1.67 | 29.69 |
| 7 | 25.1 | 42.2 | 74.6 | 98.9 | 20.8 | 2.81 | 1.78 | 12.38 |
| 8 | 17.0 | 21.9 | 89.6 | 107.1 | 19.7 | 2.03 | 1.4 | 19.69 |
Imaging data
Imaging revealed a variety of disease manifestations. Two cases had a single tumor, while the other six cases had multiple tumors. Four cases were diagnosed as HCC, two cases were diagnosed as vasogenic tumor, one case was metastatic cancer and one case was a malignant tumor (Figure 1).
Figure 1.
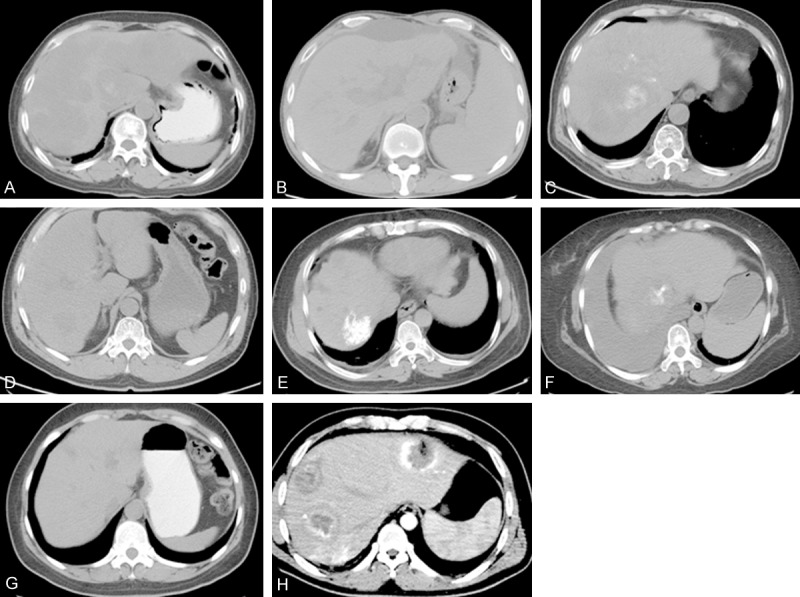
A. Multiple infused low-density lesions are visible in the CT scan. The patient was diagnosed as vascular tumor, possibly malignant. B. Patchy low-density shadow with unclear edge and uneven density visible in liver segment VII/VI; strongly indicative of liver cancer. C. Low-density shadow with unclear edge and uneven density visible in liver segment VIII. D. Small patchy low-density shadow with clear edge and uneven density visible in liver segment VI; speculated to be vasogenic tumor. E. Multiple patchy low-density shadow with clear edge and even density visible in liver segment VIII. F. Patchy low-density shadow with unclear edge, uneven density and numerous ascites visible in liver segments IV and VIII. G. Multiple low-density shadows with clear edge and uneven density visible at the junction of liver segments IV and II/III. H. Multiple low-density shadows in the liver in the enhanced parenchyma phase. Significant circle enhancement with uneven density and irregular low-density necrotic regions visible in the parenchyma phase; speculated to be liver cancer.
Histological characteristics
The tumor cells grew along the original hepatic sinusoidal endothelium and vein endothelium. In some regions, the original hepatic plates were completely replaced by the tumors. Part of the tumor cells generated polypoid protrusions in blood vessels, where the tumor cells were dendritic, spindle or irregular shaped, with abundant eosinophilic cytoplasm and nucleus deviation. Some tumor cells were characteristically manifested as luminal individual cells containing single or a few red cells. One case displayed significant interstitial sclerosis, in which the tumor tissues were alternated with sclerotic region due to less cells and epithelial cell region with more cells. In one case, the interstitium was accompanied by significant hyaline degeneration. Two cases displayed significant tumor cell atypia, in which multinuclear tumor giant cells were visible. And mitosis was visible in two cases (3/50 HPF and 1/50 HPF). The tumor cell atypia was not significant and mitosis was rarely seen in other cases (Figure 2).
Figure 2.
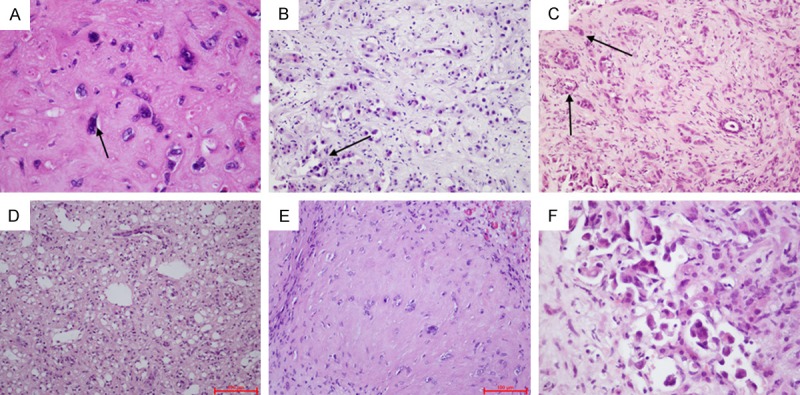
A. Diffuse tumor leads to atrophy and disappearance of hepatic plates. B. Tumor cells grow along the lumen, with sprouting-like protrusions facing inwardly. C. Tumor cells showing a duct-like arrangement are easily confused with the intrahepatic cholangiocarcinoma. D. Tumor cells grow along the sinus hepaticus, surrounded by residual liver cells and single tubular epithelial cells. E. Abundant sclerosing stroma, with scattered individual epithelial cells. F. Deeply stained and deformed heterocysts with large nuclei are visible.
Immunohistochemistry
Immunohistochemical analysis showed that all cases were CD34 and CD31 positive, one case was FVIII was negative and the Ki67 proliferation index ranged from 1% to 40%, while Hep-Par-1, GPC3 and CK19 were negative (Figure 3).
Figure 3.
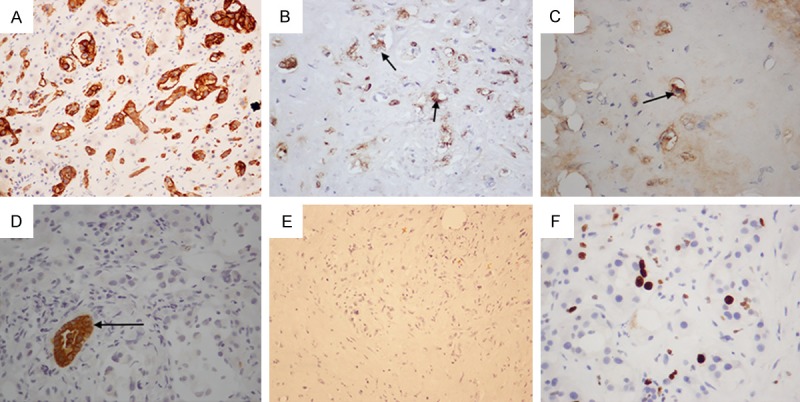
A. CD34 staining appears strongly positive. B. Positive FVIII staining of tumor cells. C. AE1/AE3 positive staining (2 cases). D. The duct-like arrangement of tumor cells are CK19 negative, and surrounded by CK19 positive residual biliary epithelial cells. E. Hep-Par-1 negative. F. The Ki-67 proliferation index is approximately 5%.
Histological characteristics and prognosis
In this study, two patients died, both had Ki-67 proliferation indexes > 15% and tumor giant cells were visible in one case. Among the other six cases, tumor giant cells were visible in one case. Patients with Ki-67 proliferation indexes of 15% did not relapse in the 26-month follow-up period after liver transplantation. The cell atypia was not significant in the other cases with Ki-67 proliferation indexes of between 1% and 5%. Three cases did not appear relapse within the 2-5 years after artery embolization alone. The Ki-67 proliferation index was 5% in one case, with no significant cell did atypia. The patient without nuclear fission survived with the tumor for 4 years with good results achieved by artery embolization and partial liver resection (Table 3).
Table 3.
Relationship between histological morphology and prognosis
| Index | Tumor cell atypia | Nuclear division (/50HPF) | Ki-67 | Metastasis | Treatment | Prognosis |
|---|---|---|---|---|---|---|
| 1 | No | No | 5% | No | Artery embolization | No relapse in 3 years |
| 2 | No | No | 5% | No | Liver transplantation | No relapse in 3 years |
| 3 | No | No | 1% | No | Artery embolization | No relapse in 5 years |
| 4 | Visible tumor giant cells | No | 5% | No | Liver transplantation | No relapse in 26 months |
| 5 | Visible tumor giant cells | 1 | 40% | No | Artery embolization | Died in 6 months |
| 6 | No | 3 | 15% | Yes | Artery embolization | Died in 20 months |
| 7 | Visible tumor giant cells | No | 15% | No | Artery embolization | Survived after 8-month treatment |
| 8 | No | No | 5% | No | Resection + Artery embolization | Survived with the tumor for 4 years |
Discussion
EH is a rare vasogenic tumor that has been rarely reported since its first identification in 1982. In 2013, EH was assigned as a malignant vasogenic tumor by the World Health Organization. It may occur in various organs, and is more common in the finger/toe tips, lung, and small intestine, but rarely occurs in the liver. Furthermore, it may occur at different ages, but is more common in adults aged 20 years or older, with a slightly higher incidence in females. The clinical symptoms of EH are not significant, although mild pain a common symptom. Tumors in the lumen of large vessels may induce correlated symptoms due to obstruction of the vessels [7]. As a non-invasive method, imaging plays an important role in the diagnosis of HEH, although the accuracy is unsatisfactory. Among the eight patients included in this retrospective study, four were diagnosed as HCC, one as metastatic cancer and one as malignant tumor, while vasogenic tumors were suspected in only two patients. Imaging can be used to investigate the presence of single or multiple lesions in the liver, while enhanced CT can be used to determine the benign or malignant tendencies, although this technique cannot be used to confirm the diagnosis, where many hepatic EHs are generally considered as metastatic EHs [8,9].
Currently, most of the studies on HEHE are case reports, while reports of studies involving multiple cases are rare [1,10-13]. The pathogenesis HEHE is still unclear, although the incidence can be increased by many factors, such as oral contraceptives, alcohol, viral infection and chronic liver diseases. Recent studies have shown that there HEHE is associated with genetic allotopia at t(1;3)(p36.3;q25), and WWTR1-CAMTA1 or YAP1-TFE3 gene fusions are detected in some cases of HEHE, although the mechanism by which these traits influence the pathogenesis of HEHE remains to be elucidated [14,15]. HEHE lacks specific clinical symptoms, and only a subpopulation of patients exhibit symptoms such as abdominal distension, aversion to greasy food, and mild fever. Approximately 10% to 20% of the patients display elevated transaminases and other signs of liver dysfunction [16]. In this study, mild liver dysfunction was detected in four patients, and only one patient had AFP elevation (> 500).
Generally, HEHE is manifested as a gray solid mass in the liver, without a capsule, with unclear edges, and multiple lesions are often observed. Multiple lesions have been reported in the right hepatic lobe in some studies. Under the microscope, the tumor cells are spindle-like, irregular and epithelial in shape, and grow along the hepatic sinusoid endothelium and the lacuna vasorum of vessels such as the central and portal veins, with abundant sclerosing stroma, and accompanied by mucoid or fibrous stroma, while mitotic cells are rarely observed [17,18].
Histological examination is the gold standard for HEHE diagnosis. Due to diverse morphologies of the tumor cells as well as different substance and matrix responses, HEHE is often confused with other types of damage, leading to difficult pathological diagnosis. Therefore, HEHE should be differentiated from other hemangiomas, cholangiocarcinomas, hepatocellular carcinomas, metastatic carcinomas, sarcomas or mixed tumors [14,19]. Differentiation of hemangiomas from the common tumors can be achieved as follows: (1) Epithelioid angiosarcoma (EA), in which the tumor cells present epithelial differentiation, and cytoplasmic protrusions are visible. However, the tumor cells are diffuse, with significant nuclear atypia, numerous mitotic cells, significant hemorrhage and necrosis, more primitive neoplastic vascular differentiation as well as an irregular interconnected sinusoid vessel network. There are neither typical intercellular lumens nor dense sclerosis, and the Ki-67 index is normally > 10%, while this is normally < 5% in HEHE. (2) Hepatocellular carcinoma (HCC), scirrhous hepatocellular carcinoma (SHCC) and fibrolamellar HCC (FLC) are often confused with HEHE due to the presence of abundant sclerosing stroma. In SHCC, the significant interfibrillar substance is distributed along the sinusoid-like space, with different degrees of trabecular atrophy, presenting typical HCC morphologies. FLC commonly occurs in young people, and is composed of large polygonal cells. Its hallmark characteristics include: abundant eosinophilic cytoplasm, large vacuole nucleus and lamellar fibrous tissues. For both the SHCC and FLC, immunochemical analysis is positive for the HCC phenotype and negative for vasogenic markers. (3) In intrahepatic cholangiocarcinoma (ICC), poor differentiation, deformed duct-like and streak structures or scattered individual cells with significant atypia are visible in the fibrous stroma. Thus, the HE results of liver biopsy in ICC are easily confused with those of HEHE. However, bile duct epithelial markers, such as CK7 and CK19, are expressed in ICC. (4) In metastatic cancer the primary tumor is normally visible in metastatic cancer, while immunohistochemistry is conducive to differentiation diagnosis. Reports reveal that the immunohistochemical characteristics of HEHE include: Vimentin positive rate of 100%, CD34 positive rate of 93.3%, FVIII positive rate of 90.0% and CD31 positive rate of 75.0% [3]. Among the eight patients included in this study, all were found to be both CD34 and CD31 positive, one patient was FVIII negative, one patient was AE1/AE3 positive, and all were GPC3, Hep-Par1 and CK19 negative.
Current studies have demonstrated that the biological behavior as well as the prognosis of HEH can vary dramatically. Some studies revealed a 5-year survival rate of approximately 59% in patients with tumor diameter > 3 cm and mitotic counts > 3/50 HPE, which was significantly lower than patients with tumor diameter < 3 cm and without mitosis and atypia (approximately 100%) [4]. Two of the patients included in this study died; both had Ki-67 > 15%, with one case was accompanied by tumor giant cells, and another case exhibiting mitotic, indicating that the presence of a high Ki-67 proliferation index, cellular atypia and mitosis is suggestive of a poor prognosis. Two patients with single tumors did relapse their 3-year and 5-year follow-up periods, respectively, suggesting a good prognosis of patients with single tumor.
Currently, there is a lack of unified treatment principles for HEHE. Surgical resection is preferred option in most cases; however, some reports indicate that incomplete tumor resection can aggravate the disease. A recent study showed that HEHE is often accompanied by inert growth, suggesting that the biological behavior of each case of HEHE should be assessed to filter the treatment protocols and select the best choice [20,21]. This study also showed that different treatment protocols might have different effects on prognosis. Two patients receiving liver transplantation survived without relapse and good quality of life. Two out of five patients receiving artery embolization died, while one patient receiving partial liver resection plus artery embolization survived with the tumor and good quality of life. Analysis of the clinical pathological characteristics of the two patients who died indicated a poor prognosis for patients with significant tumor cell atypia, abnormal mitosis and Ki-67 index > 15% in biopsy before treatment. In such cases, treatment protocols should be selected cautiously. In contrast, patients with Ki-67 index < 10%, without mitosis and atypia did not occur relapse during the 2-5 years of follow-up after artery embolization. Many studies show that HEHE is not a liver transplantation indication. However, accumulating evidence shows that liver transplantation can significantly improve the HEH survival rate, and is the most effective treatment for patients with multiple unresectable HEHE. In contrast, extrahepatic metastases are not a contraindication for liver transplantation. Some studies revealed that there were no significant differences in the 1-, 5- and 10-year survival rates after liver transplantation between HEHE patients with and without distant metastases [22]. HEHE appears to be insensitive to chemotherapy. The latest studies indicate that anti-tumor angiogenesis drugs obstructing VEGF or its receptors can play a role in the treatment of HEHE, although this approach requires clinical staging and detection of corresponding gene mutations [23].
In summary, liver biopsy before treatment is helpful in HEHE diagnosis and the selection of the most appropriate the treatment protocols.
Acknowledgements
Supported by Beijing Youan Hospital Hepatic Disease & HIV Fund 20150106.
Disclosure of conflict of interest
None.
References
- 1.Kim M, Chang J, Choi H, Oh IJ, Park CK, Kim YC, Choi YD, Yun JS, Song SY, Na KJ. Pulmonary epithelioid hemangioendothelioma misdiagnosed as a benign nodule. World J Surg Oncol. 2015;13:107. doi: 10.1186/s12957-015-0518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron PW, Amankonah T, Cubas RF, Kore AH, Elihu A, de Vera ME, Perez MC. Diffuse hepatic epithelioid hemangioendothelioma developed in a patient with hepatitis C cirrhosis. Case Rep Transplant. 2014;2014:694903. doi: 10.1155/2014/694903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mistry AM, Gorden DL, Busler JF, Coogan AC, Kelly BS. Diagnostic and therapeutic challenges in hepatic epithelioid hemangioendothelioma. J Gastrointest Cancer. 2012;43:521–5. doi: 10.1007/s12029-012-9389-y. [DOI] [PubMed] [Google Scholar]
- 4.Zigdon-Giladi H, Rudich U, Michaeli Geller G, Evron A. Recent advances in bone regeneration using adult stem cells. World J Stem Cells. 2015;7:630–40. doi: 10.4252/wjsc.v7.i3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, Milne AN, Huysentruyt CJ, Verdijk MA, van Asseldonk MM, Suurmeijer AJ, Bras J, Palmedo G, Groenen PJ, Mentzel T. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. doi: 10.1186/1746-1596-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akdur NC, Donmez M, Gozel S, Ustun H, Hucumenoglu S. Intravascular papillary endothelial hyperplasia: histomorphological and immunohistochemical features. Diagn Pathol. 2013;8:167. doi: 10.1186/1746-1596-8-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridge JA, Hogendoorn P Cancer, IAfRo, Organization. WH, Pathology. IAo. WHO Classification of Tumors of Soft Tissue and Bone. France: IARC; 2013. [Google Scholar]
- 8.Bouslama K, Houissa F, Ben Rejeb M, Bouzaidi S, Moualhi L, Mekki H, Dabbeche R, Salem M, Najjar T. Malignant epithelioid hemangioendothelioma: a case report. Oman Med J. 2013;28:135–7. doi: 10.5001/omj.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres LR, Timbo LS, Ribeiro CM, Galvao Filho MM, Verrastro CG, D’Ippolito G. Multifocal and metastatic hepatic hemangioendothelioma: case report and literature review. Radiol Bras. 2014;47:194–6. doi: 10.1590/0100-3984.2012.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallotti MC, Nannini M, Agostinelli C, Leoni S, Scioscio VD, Mandrioli A, Lolli C, Saponara M, Pileri S, Bolondi L, Biasco G, Pantaleo MA. Long-term durable response to lenalidomide in a patient with hepatic epithelioid hemangioendothelioma. World J Gastroenterol. 2014;20:7049–54. doi: 10.3748/wjg.v20.i22.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neofytou K, Chrysochos A, Charalambous N, Dietis M, Petridis C, Andreou C, Petrou A. Hepatic epithelioid hemangioendothelioma and the danger of misdiagnosis: report of a case. Case Rep Oncol Med. 2013;2013:243939. doi: 10.1155/2013/243939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupinacci RM, Rocha Mde S, Herman P. Hepatic epithelioid hemangioendothelioma: an unusual lesion of the liver. Clin Gastroenterol Hepatol. 2012;10:e15–6. doi: 10.1016/j.cgh.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Deng Y, Zhou Y, Cheng N. Laparoscopic liver biopsy in the diagnosis of hepatic epithelioid hemangioendothelioma: A case report. Oncol Lett. 2014;8:1317–9. doi: 10.3892/ol.2014.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasudevan G, Nayal B, Nagel B, Rao L. Hepatic Epithelioid Hemangioendothelioma in an Eight Year old - A Case Report. J Clin Diagn Res. 2014;8:Fd01–2. doi: 10.7860/JCDR/2014/9910.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel NR, Salim AA, Sayeed H, Sarabia SF, Hollingsworth F, Warren M, Jakacky J, Tanas M, Oliveira AM, Rubin BP, Lazar AJ, Lopez-Terrada D, Wang WL. Molecular characterization of epithelioid haemangioendotheliomas identifies novel WWTR1-CAMTA1 fusion variants. Histopathology. 2015;67:699–708. doi: 10.1111/his.12697. [DOI] [PubMed] [Google Scholar]
- 16.Zhao XY, Rakhda MI, Habib S, Bihi A, Muhammad A, Wang TL, Jia JD. Hepatic epithelioid hemangioendothelioma: A comparison of Western and Chinese methods with respect to diagnosis, treatment and outcome. Oncol Lett. 2014;7:977–83. doi: 10.3892/ol.2014.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad N, Adams DM, Wang J, Prakash R, Karim NA. Hepatic epithelioid hemangioendothelioma in a patient with hemochromatosis. J Natl Compr Canc Netw. 2014;12:1203–7. doi: 10.6004/jnccn.2014.0119. [DOI] [PubMed] [Google Scholar]
- 18.Li XM, Lin XY, Xu HT, Yu JH, Wang L, Fan CF, Liu Y, Wang EH. Mediastinal epithelioid hemangioendothelioma with abundant spindle cells and osteoclast-like giant cells mimicking malignant fibrous histiocytoma. Diagn Pathol. 2013;8:103. doi: 10.1186/1746-1596-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562–82. doi: 10.1002/(sici)1097-0142(19990201)85:3<562::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Thomas RM, Aloia TA, Truty MJ, Tseng WH, Choi EA, Curley SA, Vauthey JN, Abdalla EK. Treatment sequencing strategy for hepatic epithelioid haemangioendothelioma. HPB (Oxford) 2014;16:677–85. doi: 10.1111/hpb.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oshima N, Terajima H, Hosotani R. Surgical Therapy for a Solitary Form of Hepatic Epithelioid Hemangioendothelioma: A Long-Term Survival Case. Case Rep Gastroenterol. 2009;3:214–21. doi: 10.1159/000227734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remiszewski P, Szczerba E, Kalinowski P, Gierej B, Dudek K, Grodzicki M, Kotulski M, Paluszkiewicz R, Patkowski W, Zieniewicz K, Krawczyk M. Epithelioid hemangioendothelioma of the liver as a rare indication for liver transplantation. World J Gastroenterol. 2014;20:11333–9. doi: 10.3748/wjg.v20.i32.11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sangro B, Inarrairaegui M, Fernandez-Ros N. Malignant epithelioid hemangioendothelioma of the liver successfully treated with Sorafenib. Rare Tumors. 2012;4:e34. doi: 10.4081/rt.2012.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]


