Abstract
Objectives: We conducted a retrospective study to evaluate the prognostic factors of elderly patients with small cell lung cancer (SCLC). Patients and methods: The records of elderly patients (≥ 65 years) with histologically-proven SCLC were reviewed. The patients’ information including demographic, clinical and laboratory parameters, staging status on the Veterans Administration Lung Study Group staging system, and treatment modalities were registered. Univariate and multivariate survival analysis was performed by the Kaplan-Meier method and Cox proportional hazards model, respectively. Results: Between January 2004 and December 2012, 247 elderly patients with SCLC were analyzed, 129 patients initially presented with limited stage (LS) and 118 with extensive disease (ES). The median age of the patients was 70.7 years (range, 65-83 years). The median follow-up period for all patients was 22.0 months (range, 1.0-84.0 months) and 39.9 months for the surviving patients (range, 4.7-84.0 months). The median survival time (MST) was 17.3 months, and the 2-year and 3-year OS rates were 36.3% and 22.7%, respectively. The MST, 2-year and 3-year OS rates were 22 months, 45.0% and 30.5% in patients with limited stage, versus 13.4 months, 26.5% and 13.7% in patients having extensive diseases, respectively. Multivariate analysis revealed that disease extent (HR = 3.034; P < 0.001) and the number of chemotherapy cycles (HR = 0.486; P = 0.003) were independent prognostic factors for the OS. Additionally, a normal serum NSE level (HR = 0.447, P = 0.017) at the time of diagnosis was independent positive prognostic factors for patients with LS-SCLC, but not for ES-SCLC. Conclusion: Disease extent and the number of chemotherapy cycles were independent prognostic factors of elderly patients with SCLC. The fit cohort might benefit from positive treatment.
Keywords: Elderly, small cell lung cancer, survival, prognosis factor, overall survival
Introduction
Lung cancer is the most common malignant carcinoma and the leading cause of death due to cancer in the world, and now it is regarded as a senile disease because the median age of patients diagnosed with lung carcinoma is 70 years [1,2]. Small cell lung cancer (SCLC) represents approximately 15% to 20% of all new cases of lung carcinomas [3,4]. The data of Surveillance Epidemiology and End Results (SEER) showed that 68% of patients with SCLC were older than 65 years, and more than 30% were older than 70 years [2]. On the basis of these observations, SCLC represents a significantly serious health problem in the elderly patients.
SCLC is usually classified into two stages: limited disease (LS) and extensive stage (ES) according to the staging system of Veterans Administration Lung Study Group [5]. It is characterized by a high incidence of metastatic disease at presentation, rapid doubling time, and poor survival. A platinum-based chemotherapy (ChT) regimen combined with thoracic radiotherapy (TRT) concurrently or sequentially is the standard treatment for limited-disease, and the median survival time is about 12-16 months and the overall survival is 20% at 5-year approximately [6,7]. For ES diseases, combination ChT remains the standard treatment. TRT is most commonly used as palliative approach, even though consolidative TRT improved the 5-year survival rate and local control in patients who responded to initial ChT [8,9].
Among patients with SCLC, the elderly cohort usually accompanied with poor physiologic status and comorbidities, there was still no high-grade evidence of optimal treatment guide for this special subgroup [10]. Few retrospective studies attempted to evaluate the treatment modalities and identify prognostic factors for elderly patients with SCLC, however, the results could not be satisfied because of highly individual heterogeneity in the study population [11-13]. The current study is aiming to investigate the prognostic factors of elderly patients (≥ 65 years) with SCLC and attempt to afford evidence of treatment selection.
Patients and methods
Patients
Elderly patients (≥ 65 years) diagnosed as SCLC on the basis of cytological or histological proof at Shandong Cancer Hospital between January 2004 and December 2012 were retrospectively reviewed. The clinical data were drawn from their inpatient records. All patients had undergone standardized evaluation, including thoracic and abdominal computed tomography scanning or abdominal ultrasonography, brain magnetic resonance imaging, and bone radionuclide imaging, and the disease stage was reached according to the system of the Veterans’ Administration Lung Study Group [10]. Detailed data including gender, age, weight loss, smoking status, KPS score, the serum levels of biological markers including neuron-specific enolase (NSE) and carcinoembryonic antigen (CEA), and treatment modalities of enrolled patients were recorded.
The study was approved by the institutional review board/ethics committee at Shandong Cancer Hospital.
Treatment
Surgical procedures included wedge resection, lobectomy or pneumonectomy with ipsilateral hilar and mediastinal lymphadenectomy. Chemotherapy regimens were consisted of either cisplatin and etoposide (PE: cisplatin, 30 mg/m2 on days 1-3 and etoposide, 100 mg/m2 on days 1-5 or 100 mg/m2 on days 1-3), or carboplatin/etoposide (CE: carboplatin AUC 5 or 300 mg/m2 on day 1 and etoposide, 100 mg/m2 on days 1-5 or 100 mg/m2 on days 1-3). TRT was administered by three-dimensional conformal radiotherapy technique (3D-CRT), the gross target volume (GTV) included the primary tumour and positive lymph nodes; the clinical target volume (CTV) included the GTV with a 0.8-cm margin and the draining area of positive lymph nodes, and the CTV of patients undergoing complete resection only included bronchial stump, ipsilateral hilum, and adjacent mediastinal lymph nodes; and the planning target volume (PTV) included the CTV with a 1-cm margin. Radiation was delivered with megavoltage linear accelerators. A total dose of 40-60 Gy was administered with 1.8-2 Gy per fraction for 5 days a week. During this period, prophylactic cranial irradiation (PCI) was not institutional policy, and only 22 patients achieving a complete remission (CR) after primary therapy received PCI in all of the groups. PCI dose fractionation was 25 Gy in 10 fractions over 2 weeks.
The follow-up
Patients were followed up every 3 months for the first year, then every 6 months for the following 2 years, and annually thereafter. The follow-up evaluations consisted of history, physical examination, and radiologic examination. Radiologic examination included a chest X-ray, chest CT, abdominal ultrasound or CT. Other necessary examinations were conducted as clinically indicated.
Statistical analysis
The overall survival (OS), calculated from the date of diagnosis to the date of death from any cause or the last known date when the patient was alive, was evaluated using Kaplan Meier method and a comparison between survival curves was done with log-rank test. Multivariate Cox regression analysis with a backward-forward stepwise method assessed the prognostic factors for OS. In the multivariate Cox regression model, all variables with p values < 0.05 in the univariate analysis were included. All statistical tests were two-tailed, and P < 0.05 was considered statistically significant.
Results
Patient characteristics
Between January 2004 and December 2012, 247 patients (≥ 65 years) with SCLC were included in this trial, 129 patients initially presented with LS-SCLC, while 118 patients had extensive stage. The median age of the all patients was 70.7 (range 65-83). The median follow-up period for all patients was 22.0 months (range, 1.0-84.0 months) and 39.9 months for surviving patients (range, 4.7-84.0 months). The characteristics of 247 elderly patients were summarized in Table 1.
Table 1.
Clinical features of elderly patients with SCLC
| Characteristic | Total | % | LS-SCLC | ES-SCLC | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Total | % | Total | % | |||
| Gender | ||||||
| Male | 201 | 81.4 | 103 | 79.8 | 98 | 83.1 |
| Female | 46 | 18.6 | 26 | 20.2 | 20 | 16.9 |
| Age (years) | ||||||
| Range | 65-83 | 65-82 | 65-83 | |||
| Median | 70 | 70 | 71 | |||
| 65-70 | 106 | 42.9 | 60 | 46.5 | 46 | 39.0 |
| ≥ 70 | 141 | 57.1 | 69 | 53.5 | 72 | 61.0 |
| KPS score | ||||||
| ≥ 80 | 173 | 70.0 | 87 | 67.4 | 86 | 72.9 |
| < 80 | 74 | 30.0 | 42 | 32.6 | 32 | 27.1 |
| Wight loss | ||||||
| < 5% | 213 | 86.2 | 115 | 89.1 | 98 | 83.1 |
| ≥ 5% | 34 | 13.8 | 14 | 10.9 | 20 | 16.8 |
| Smoking status | ||||||
| Yes | 166 | 67.2 | 86 | 66.7 | 80 | 67.8 |
| No | 81 | 32.8 | 43 | 33.3 | 38 | 32.2 |
| Cycle of ChT | ||||||
| < 4 | 61 | 25.4 | 32 | 25.8 | 29 | 25.0 |
| ≥ 4 | 179 | 74.6 | 92 | 74.2 | 87 | 75.0 |
| Pleural effusion | ||||||
| Yes | 48 | 19.4 | 4 | 3.1 | 44 | 37.3 |
| No | 199 | 80.6 | 125 | 96.9 | 74 | 62.7 |
| Obstructive | ||||||
| Pneuontise | ||||||
| Yes | 113 | 46.3 | 40 | 31.3 | 73 | 62.9 |
| No | 131 | 53.7 | 88 | 36.1 | 43 | 37.1 |
| Treatment 1 | ||||||
| TRT+ChT | 69 | 48.6 | 36 | 66.7 | 33 | 28.0 |
| ChT alone | 73 | 51.4 | 18 | 33.3 | 55 | 72.0 |
| Treatment 2 | ||||||
| S+ChT±TRT | 43 | 23.2 | 39 | 41.9 | 4 | 4.3 |
| ChT±TRT | 142 | 76.8 | 54 | 58.1 | 88 | 95.7 |
| NSE (ng/mL) | ||||||
| < 17 | 138 | 73.4 | 60 | 62.5 | 79 | 85.9 |
| ≥ 17 | 50 | 26.6 | 36 | 37.5 | 13 | 14.1 |
| CEA (ng/mL) | ||||||
| < 3.4 | 95 | 52.5 | 51 | 52.6 | 44 | 52.4 |
| ≥ 3.4 | 86 | 47.5 | 46 | 47.4 | 40 | 47.6 |
LS-SCLC, limited-stage small cell lung cancer; ES-SCLC, extensive-stage small cell lung cancer; KPS, Karnofsky performance status; ChT, chemotherapy; TRT, thoracic radiation therapy; S, surgery; NSE, neuron-specific enolase; CEA, carcinoembryonic antigen.
Survival
At the end of follow-up, 52 patients were still alive. The median survival time (MST) was 17.3 months, and the 2-year and 3-year OS rates were 36.3% and 22.7%, respectively, for the entire cohort of patients (Figure 1A). The MST, the OS rates at 2-year and 3-year were 22 months, 45.0% and 30.5% in patients with limited stage, versus 13.4 months, 26.5% and 13.7% in patients with extensive stage, respectively (P < 0.001). The MST, 2-year and 3-year OS rates in the patients who accepted ≥ 4 cycles of ChT were 20.7 months, 45.1% and 29.2%, and those of patients who accepted < 4 cycles of ChT were 11.0 months, 13.5% and 4.5%, respectively (P < 0.001). Additionally, the analysis showed that the survival of patients with elevated NSE level (≥ 17 ng/mL) or elevated LDH level (≥ 245 u/l) was worse than that of patients with normal NSE level (< 17 ng/mL) or normal LDH level (< 245 u/l), p value were 0.003 and 0.027, respectively (Figure 1). The survival data of the whole group were detailed in Table 2.
Figure 1.
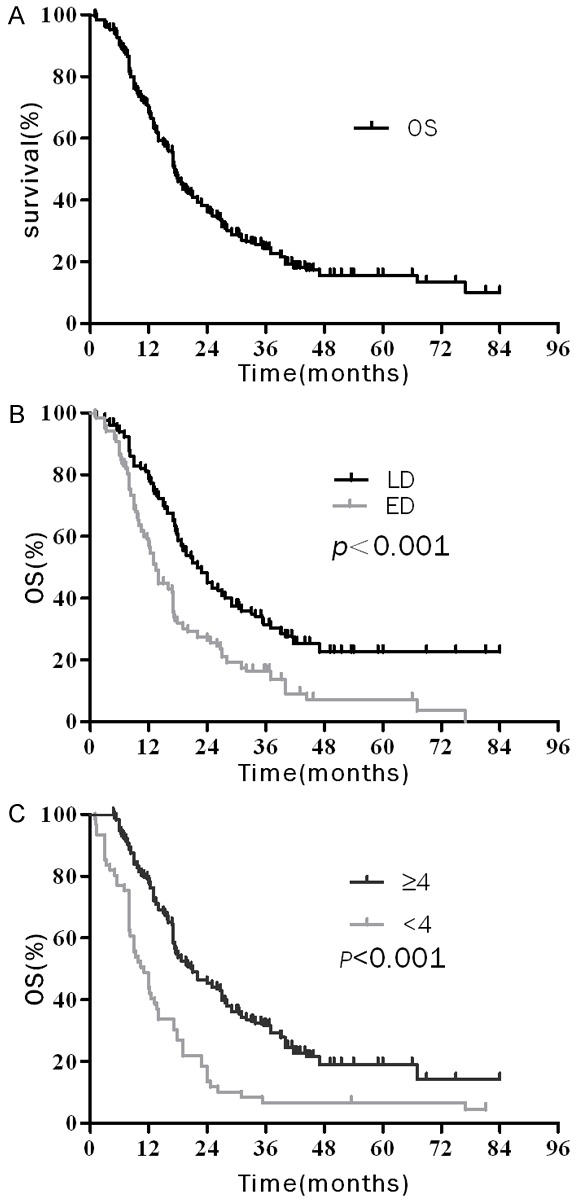
Over survival (OS) curves of elderly patients with SCLC: A. OS curves of all elderly patients; B. OS curves of patients according to disease extent (C) OS curves of patients according to number cycles of chemotherapy (D) OS curves of patients according to serum NSE level.
Table 2.
Univariate analysis and multivariate analysis of the prognostic factors on OS in patients with total SCLC
| Factors | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| MST (m) | 2-y OS (%) | 3-y OS (%) | P | HR | 95% CI | P | |
| Gender | |||||||
| Male | 17 | 33.5 | 19.1 | ||||
| Female | 22 | 46.2 | 38.8 | 0.077 | |||
| Age (years) | |||||||
| 65-70 | 19.7 | 43.0 | 25.9 | 0.296 | |||
| ≥ 70 | 17.0 | 31.4 | 22.3 | ||||
| KPS | |||||||
| ≥ 80 | 17.2 | 40.1 | 25.2 | ||||
| < 80 | 17.3 | 34.6 | 20.0 | 0.092 | |||
| Wight loss | |||||||
| < 5% | 17.7 | 37.1 | 22.7 | ||||
| ≥ 5% | 13.0 | 28.1 | 19.0 | 0.166 | |||
| Smoking | |||||||
| Yes | 17.0 | 37.5 | 22.9 | ||||
| No | 17.9 | 33.7 | 20.5 | 0.888 | |||
| Cycle of ChT | |||||||
| < 4 | 11.0 | 13.5 | 4.5 | 0.301- | |||
| ≥ 4 | 20.7 | 45.1 | 29.2 | < 0.001 | 0.486 | 0.786 | 0.003 |
| Pleural effuion | |||||||
| Yes | 14.0 | 30.0 | 12.3 | ||||
| No | 17.9 | 37.7 | 23.8 | 0.251 | |||
| Disease extent | |||||||
| LS | 22.0 | 45.0 | 30.5 | 2.022- | |||
| ES | 13.4 | 26.5 | 13.7 | < 0.001 | 3.034 | 4.552 | < 0.001 |
| Obstructive | |||||||
| Pneuontise | |||||||
| Yes | 14.0 | 32.2 | 20.1 | ||||
| No | 19.0 | 39.6 | 24.6 | 0.082 | |||
| NSE (ng/mL) | |||||||
| ≥ 17 | 17.0 | 32.1 | 18.9 | 0.642- | |||
| < 17 | 26.0 | 50.4 | 34.4 | 0.003 | 1.111 | 1.924 | 0.706 |
| CEA (ng/mL) | |||||||
| ≥ 3.4 | 17.0 | 33.5 | 22.4 | ||||
| < 3.4 | 17.4 | 42.8 | 23.9 | 0.552 | |||
OS, overall survival; SCLC, small cell lung cancer; KPS, Karnofsky performance status; ChT, chemotherapy; LS, limited-stage; ES, extensive-stage; NSE, neuron-specific enolase; CEA, carcinoembryonic antigen.
In the subgroup patients with limited stage, those who accomplished ≥ 4 cycles of chemotherapy, accepted surgery-included treatment or with normal serum NSE had higher overall survival rates than patients who accepted < 4 cycles of chemotherapy, non-surgical treatment modalities or with elevated serum NSE (Figure 2), the data were detailed in Table 3. In the subgroup of ES-SCLC, the overall survival of the patients with weight loss < 5%, ≥ 4 cycles of chemotherapy or TRT were significantly higher than that for patients with weight loss ≥ 5%, < 4 cycles of chemotherapy or non-TRT (Figure 3; Table 3).
Figure 2.
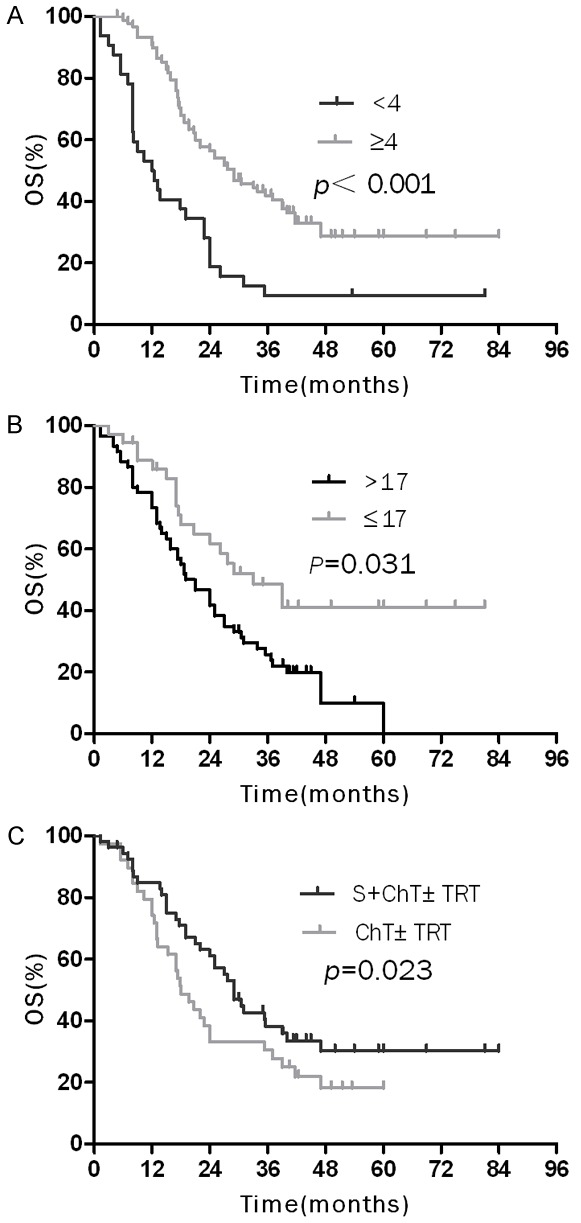
Over survival (OS) curves of elderly patients with LD-SCLC: A. OS curves of patients according to number cycles of chemotherapy; B. OS curves of patients according to serum NSE level; C. OS curves of patients who underwent surgery followed by chemotherapy ± thoracic radiation therapy and chemotherapy ± thoracic radiation therapy.
Table 3.
Univariate analysis and multivariate analysis of the prognostic factors on OS in patients with LS-SCLC and ES-SCLC
| Factors | LS-SCLC | ES-SCLC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||||
|
|
|
|
|
|||||||||||
| MST (m) | 2-y OS (%) | 3-y OS (%) | P | HR | 95% CI | P | MST (m) | 2-y OS (%) | 3-y OS (%) | P | HR | 95% CI | P | |
| Gender | ||||||||||||||
| Male | 21.0 | 42.1 | 26.3 | 13.0 | 24.3 | 11.2 | ||||||||
| Female | 33.9 | 52.6 | 43.1 | 0.467 | 17.0 | 31.8 | 0.0 | 0.068 | ||||||
| Age (years) | ||||||||||||||
| 65-70 | 27.6 | 54.0 | 32.8 | 12.0 | 26.0 | 13.2 | ||||||||
| ≥ 70 | 19.0 | 37.7 | 26.6 | 0.268 | 14.0 | 25.3 | 10.9 | 0.941 | ||||||
| KPS | ||||||||||||||
| ≥ 80 | 20.7 | 42.5 | 23.7 | 13.0 | 23.7 | 10.2 | ||||||||
| < 80 | 24.0 | 50.0 | 30.6 | 0.816 | 14.0 | 26.3 | 15.4 | 0.361 | ||||||
| Wight loss | ||||||||||||||
| < 5% | 29.0 | 50.0 | 28.8 | 14.0 | 29.4 | 15.1 | 0.319- | |||||||
| ≥ 5% | 22.0 | 43.5 | 23.8 | 0.680 | 12.0 | 6.2 | 0.0 | 0.043 | 0.576 | 1.038 | 0.067 | |||
| Smoking | ||||||||||||||
| Yes | 21.0 | 38.3 | 22.3 | 12.0 | 25.7 | 11.6 | ||||||||
| No | 22.8 | 45.9 | 33.1 | 0.593 | 13.4 | 25.5 | 19.8 | 0.888 | ||||||
| Cycle of ChT | ||||||||||||||
| < 4 | 12.0 | 18.8 | 9.4 | 0.184- | 9.8 | 3.7 | 0.0 | 0.315- | ||||||
| ≥ 4 | 27.6 | 55.8 | 40.0 | < 0.001 | 0.348 | 0.658 | 0.001 | 16.0 | 33.6 | 17.3 | 0.003 | 0.529 | 0.889 | 0.016 |
| Pleural effuion | ||||||||||||||
| Yes | 20.7 | 33.3 | 0.0 | 11.7 | 29.6 | 8.6 | ||||||||
| No | 22.0 | 45.3 | 30.3 | 0.718 | 14.0 | 24.6 | 12.7 | 0.286 | ||||||
| Obstructive | ||||||||||||||
| Pneuontise | ||||||||||||||
| Yes | 17.7 | 43.7 | 29.7 | 13.0 | 24.1 | 11.0 | ||||||||
| No | 22.8 | 44.4 | 30.4 | 0.896 | 16.0 | 27.8 | 9.7 | 0.399 | ||||||
| Treatment 1 | ||||||||||||||
| TRT+ChT | 24.0 | 43.4 | 25.7 | 17.0 | 24.4 | 10.5 | 0.840- | |||||||
| ChT alone | 17.3 | 48.4 | 26.3 | 0.577 | 9.8 | 11.9 | 5.9 | 0.035 | 1.380 | 2.268 | 0.204 | |||
| Treatment 2 | ||||||||||||||
| S+ChT±TRT | 27.0 | 53.7 | 42.3 | 0.627- | ||||||||||
| ChT±TRT | 19.0 | 40.9 | 28.0 | 0.023 | 0.512 | 2.724 | 0.474 | |||||||
| NSE (ng/mL) | ||||||||||||||
| ≥ 17 | 21.0 | 43.3 | 24.7 | 0.231- | 12.3 | 22.8 | 11.9 | |||||||
| < 17 | 29.0 | 59.2 | 39.4 | 0.031 | 0.447 | 0.865 | 0.017 | 13.4 | 23.1 | 7.7 | 0.838 | |||
| CEA (ng/mL) | ||||||||||||||
| ≥ 3.4 | 22.0 | 39.2 | 26.9 | 14.0 | 26.5 | 12.6 | ||||||||
| < 3.4 | 29.0 | 57.6 | 20.4 | 0.211 | 13.0 | 23.0 | 5.7 | 0.397 | ||||||
OS, overall survival; LS-SCLC, limited-stage small cell lung cancer; ES-SCLC, extensive-stage small cell lung cancer; KPS, Karnofsky performance status; ChT, chemotherapy; TRT, thoracic radiation therapy; S, surgery; NSE, neuron-specific enolase; CEA, carcinoembryonic antigen.
Figure 3.
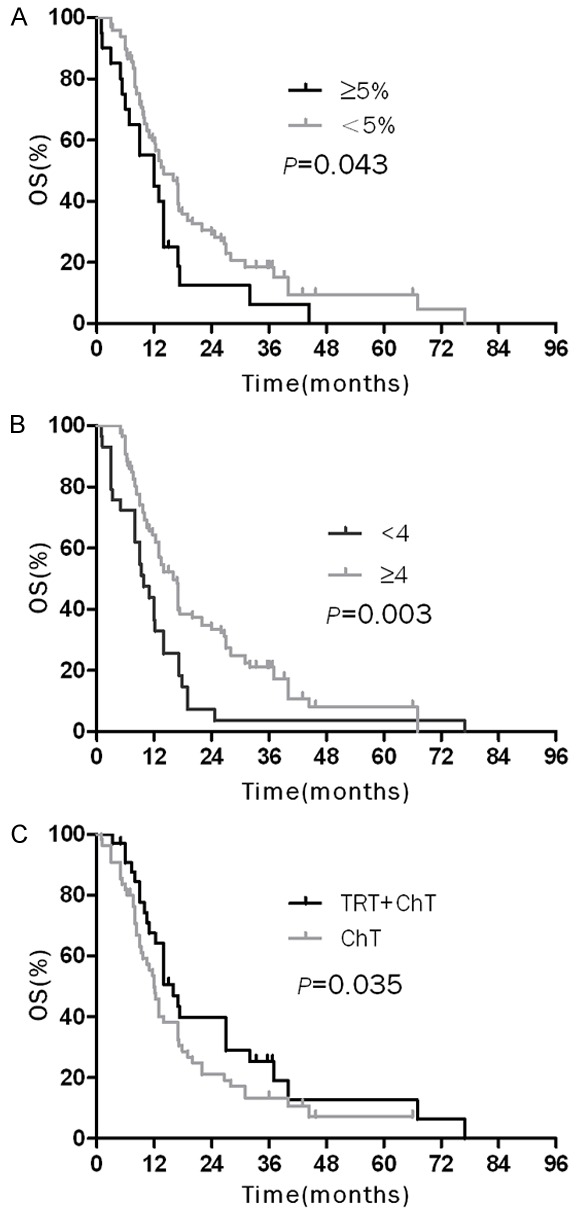
Over survival (OS) curves of elderly patients with ED-SCLC: A. OS curves of patients according to weight loss; B. OS curves of patients according to number cycles of chemotherapy; C. OS curves of patients who underwent chemotherapy combined with thoracic radiation therapy and only chemotherapy.
Factors predictive of the OS
The clinical factors were assessed to determine their prognostic value. With regard to OS, multivariate analysis revealed that extensive-stage disease [hazard ratio (HR) = 3.034, P < 0.001], and < 4 cycles of chemotherapy (HR = 0.486, P = 0.003) were independent negative prognostic factors of OS (Table 2); In the subgroup of patients with LS-SCLC, a normal serum NSE level (HR = 0.447, P = 0.017) at the time of diagnosis was independent positive prognostic factors of OS, and < 4 cycles of chemotherapy (HR = 0.348, P = 0.001) was independent negative prognostic factors of OS (Table 3); In the subgroup of extensive stage, ≥ 4 cycles of chemotherapy (HR = 0.529, P = 0.016) was the only independent prognostic factors of OS (Table 3).
Discussion
Generally, elderly patients with SCLC have significantly more comorbidities, smaller bone marrow reserve, poor performance status that affect worse tolerability to chemotherapy, therefore, the optimal treatment for elderly SCLC patients is still controversial and prognostic indicators are heterogeneous [10-14]. According to analyze the 247 elderly patients with SCLC, the retrospective study revealed that extensive-stage disease and < 4 cycles of chemotherapy were independent negative prognostic factors for the elderly patients with SCLC. Additionally, a normal serum NSE level (HR = 0.53, P = 0.021) at the time of diagnosis was independent positive prognostic factors for the elderly patients with limited stage SCLC.
The prognostic role of age in elderly patients with SCLC could not reach a consensus. One retrospective study conducted by Safont revealed that age was likely to affect the OS of elderly patients with LD-SCLC, the MST was 60.3 weeks in the subgroup of patients with 65-70 years, while it was 49.4 weeks in patients with ≥ 70 years, P = 0.005 [12]. However, several previous studies did not show a significant relationship between age and survival status [14-17]. In the current analysis, 106 patients (42.9%) were 65-70 years old and 141 patients (57.1%) were aged ≥ 70 years, the 2y-OS was 43.0 % in the group aged with 65-70 years and that was 31.4 % in the group aged with ≥ 70 years. The prognostic role of age for the survival in elderly patients was not established (P = 0.296). Therefore, in the routine clinical practice the treatment should be based on biological rather than chronological age.
It is well accepted that the extent of disease has traditionally been included in prognostic evaluation for SCLC [18-21]. A retrospective study included 284 patients with LS-SCLC and 328 patients with ES-SCLC between 1974 and 1986 showed that median survival time of patients with limited-stage disease (53 weeks) was significantly better than that those with extensive disease (35 weeks, P < 0.00005) [21]. The current study manifested that the extreme differences in survival between patients with LS and ES-SCLC (2-year and 3-year OS rate of 45.0 and 30.5% versus 26.5 and 13.7%, P < 0.001). So the discovery of the disease in the shortest possible time is vital for SCLC patients.
As SCLC is a systemic disease, chemotherapy plays a key role in the treatment. Four to six cycles of a platinum-based chemotherapy doublet is recommended usually, and many studies have demonstrated that less than four cycles of chemotherapy could incur a poor prognosis. The result of Intergroup Trial 0096 showed that elderly patients (age > 70 years) had non-inferior response and survival figures compared with younger patients if the elderly subgroup could get through four cycles of chemotherapy [11]. A randomized trial by Maryska concluded that the survival of elderly patients who could complete at least 4 cycles of chemotherapy was better than that less than 4 cycles of chemotherapy, the MST was 11.5 months versus 3.6 months, P < 0.0001 [13]. This conclusion was similar to our current study.
Chemotherapy combined with TRT was assumed as the standard treatment for the management of LS-SCLC [10], surgery has been known to be a valuable local treatment app-roach for LS-SCLC since the last three decades. Nevertheless, the role of surgery as a part of multimodality treatment remains uncertain in elderly patients. A matched-pair analysis comparing 67 patients who underwent surgery followed by adjuvant ChT and 67 patients who received conventional non-surgical management revealed a 5-year OS rate of 27% for the surgical group and 4% for the matched non-surgical cohort [22]. Other several trials have demonstrated that surgery followed by adjuvant ChT results in a favorable survival rate for early stage disease [23-25]. In this retrospective study, the MST and 3-year OS rates for the surgical subgroup were 27.0 months and 42.3% respectively, and those for patients who received non-surgical therapy were 19.0 months and 28.0% respectively (P = 0.023). Interestingly, surgery was not revealed as the independent prognostic indicator for the OS in multivariate analysis. The reason for this discrepancy might be a relatively higher percentage of elderly patients with stage III SCLC at the diagnosis and relatively small sample size in our study.
For ES-SCLC patients, the role of radiotherapy was controversial. A retrospective study in 1999 first demonstrated that TRT could improve the 5-year OS rate for a favorable patient subgroup of complete responders compared with ChT alone (9.1% vs 3.7%), and it decreased the local recurrence rate [8]. A phase III randomized controlled trial at 42 hospitals manifested that thoracic radiotherapy after chemotherapy improved 2-year survival for patients with ES-SCLC (P = 0.004) [26]. Our retrospective study also confirmed the positive role of TRT in ES-SCLC [9]. However, for the elderly, the role of TRT as palliative or curative intent is inconclusive. Our current study demonstrated that comparing with ChT alone, ChT combined with TRT almost doubled the median survival from 9.8 months to 17.0 months, 3-year OS rates were increased from 5.9% and 10.5%, respectively (P = 0.0035), but it was not an independent positive factor of prognosis for elderly patients. So much caution should be exercised when thoracic radiotherapy is planned for elderly patients with ES-SCLC.
NSE, an enolase isoenzyme, has been regarded as a sensitive tumor marker in SCLC. Serum NSE levels are elevated in 61-83% of SCLC patients [27]. Studies have indicated that the NSE level is associated with disease stage, response to therapy, and survival. It can also be used to monitor disease progression [19,27,28]. In our study, the subgroup of patients with elevated NSE levels had poor survival status. And multivariate analysis confirmed that the serum NSE level was an independent prognostic marker of elderly patients with LS-SCLC.
Our study has several limitations. First, the study is retrospective and biases were inevitable, even though the multivariate analysis can aid in decreasing these biases to a certain degree. Second, the number of patients was moderate, and the elderly patients of our study who all have accepted treatment are not completely on behalf of the general elderly patients. Third, other important prognostic indicators such as co-morbidities, toxicity, socioeconomic, emotional and cognitive conditions, functional and nutritional status which may be benefit of improving treatment suitability were not included in the study. The last but not least, a certain proportion of patients did not receive prophylactic cranial irradiation. This might cause some inconsistency with other similar investigations which precluded the generalization of the results in our study.
Conclusion
Our study manifested disease extent and chemotherapy cycle were strongly associated with survival rate of the elderly patient with SCLC. We hope these prognostic factors will be useful for the better evaluation of treatment outcome in future clinical trials and the guidance of an individual treatment.
Acknowledgements
This study was supported by Grant NO.ZR20011HM089 from the Natural Science Foundation of Shandong Province to Li Kong.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015.
- 3.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 4.Bayman NA, Sheikh H, Kularatne B, Lorigan P, Blackhall F, Thatcher N, Faivre-Finn C. Radiotherapy for small-cell lung cancer-Where are we heading? Lung Cancer. 2009;63:307–314. doi: 10.1016/j.lungcan.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4:31–42. [PubMed] [Google Scholar]
- 6.Ihde DC. Chemotherapy of lung cancer. N Engl J Med. 1992;327:1434–1441. doi: 10.1056/NEJM199211123272006. [DOI] [PubMed] [Google Scholar]
- 7.Schild SE, Bonner JA, Hillman S, Kozelsky TF, Vigliotti AP, Marks RS, Graham DL, Soori GS, Kugler JW, Tenglin RC, Wender DB, Adjei A. Results of a phase II study of high-dose thoracic radiation therapy with concurrent cisplatin and etoposide in limited-stage small-cell lung cancer (NCCTG 95-20-53) J. Clin. Oncol. 2007;25:3124–3129. doi: 10.1200/JCO.2006.09.9606. [DOI] [PubMed] [Google Scholar]
- 8.Jeremic B, Shibamoto Y, Nikolic N, Milicic B, Milisavljevic S, Dagovic A, Aleksandrovic J, Radosavljevic-Asic G. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J. Clin. Oncol. 1999;17:2092–2099. doi: 10.1200/JCO.1999.17.7.2092. [DOI] [PubMed] [Google Scholar]
- 9.Zhu H, Zhou Z, Wang Y, Bi N, Feng Q, Li J, Lv J, Chen D, Shi Y, Wang L. Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer. 2011;117:5423–5431. doi: 10.1002/cncr.26206. [DOI] [PubMed] [Google Scholar]
- 10.Pallis AG, Shepherd FA, Lacombe D, Gridelli C. Treatment of small-cell lung cancer in elderly patients. Cancer. 2010;116:1192–1200. doi: 10.1002/cncr.24833. [DOI] [PubMed] [Google Scholar]
- 11.Yuen AR, Zou G, Turrisi AT, Sause W, Komaki R, Wagner H, Aisner SC, Livingston RB, Blum R, Johnson DH. Similar outcome of elderly patients in intergroup trial 0096: Cisplatin, etoposide, and thoracic radiotherapy administered once or twice daily in limited stage small cell lung carcinoma. Cancer. 2000;89:1953–1960. doi: 10.1002/1097-0142(20001101)89:9<1953::aid-cncr11>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Safont MJ, Artal-Cortes A, Sirera R, Gomez-Codina J, Gonzalez-Larriba JL, Barneto I, Carrato A, Isla D, Rosell R, Camps C. Retrospective study of efficacy and toxicity on patients older than 70 years within a randomized clinical trial of two cisplatin-based combinations in patients with small-cell lung cancer. Lung Cancer. 2009;63:83–87. doi: 10.1016/j.lungcan.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Janssen-Heijnen ML, Maas HA, Koning CC, van der Bruggen-Bogaarts BA, Groen HJ, Wymenga AN. Tolerance and benefits of treatment for elderly patients with limited small-cell lung cancer. J Geriatr Oncol. 2014;5:71–77. doi: 10.1016/j.jgo.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Caprario LC, Kent DM, Strauss GM. Effects of chemotherapy on survival of elderly patients with small-cell lung cancer: analysis of the SEER-medicare database. J Thorac Oncol. 2013;8:1272–1281. doi: 10.1097/JTO.0b013e3182a007ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jara C, Gomez-Aldaravi JL, Tirado R, Meseguer VA, Alonso C, Fernandez A. Small-cell lung cancer in the elderly--is age of patient a relevant factor? Acta Oncol. 1999;38:781–786. doi: 10.1080/028418699432941. [DOI] [PubMed] [Google Scholar]
- 16.Poplin E, Thompson B, Whitacre M, Aisner J. Small cell carcinoma of the lung: influence of age on treatment outcome. Cancer Treat Rep. 1987;71:291–296. [PubMed] [Google Scholar]
- 17.Quon H, Shepherd FA, Payne DG, Coy P, Murray N, Feld R, Pater J, Sadura A, Zee B. The influence of age on the delivery, tolerance, and efficacy of thoracic irradiation in the combined modality treatment of limited stage small cell lung cancer. Int J Radiat Oncol Biol Phys. 1999;43:39–45. doi: 10.1016/s0360-3016(98)00373-3. [DOI] [PubMed] [Google Scholar]
- 18.Paesmans M, Sculier JP, Lecomte J, Thiriaux J, Libert P, Sergysels R, Bureau G, Dabouis G, Van Cutsem O, Mommen P, Ninane V, Klastersky J. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer. 2000;89:523–533. doi: 10.1002/1097-0142(20000801)89:3<523::aid-cncr7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Bremnes RM, Sundstrom S, Aasebø U, Kaasa S, Hatlevoll R, Aamdal S. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer. 2003;39:303–313. doi: 10.1016/s0169-5002(02)00508-1. [DOI] [PubMed] [Google Scholar]
- 20.Arinc S, Gonlugur U, Devran O, Erdal N, Ece F, Ertugrul M, Derince D, Oruc O, Hazar A. Prognostic factors in patients with small cell lung carcinoma. Med Oncol. 2010;27:237–241. doi: 10.1007/s12032-009-9198-8. [DOI] [PubMed] [Google Scholar]
- 21.Sagman U, Maki E, Evans WK, Warr D, Shepherd FA, Sculier JP, Haddad R, Payne D, Pringle JF, Yeoh JL, et al. Small-cell carcinoma of the lung: derivation of a prognostic staging system. J. Clin. Oncol. 1991;9:1639–1649. doi: 10.1200/JCO.1991.9.9.1639. [DOI] [PubMed] [Google Scholar]
- 22.Badzio A, Kurowski K, Karnicka-Mlodkowska H, Jassem J. A retrospective comparative study of surgery followed by chemotherapy vs. non-surgical management in limited-disease small cell lung cancer. Eur J Cardiothorac Surg. 2004;26:183–188. doi: 10.1016/j.ejcts.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, Zhou Z, Xue Q, Zhang X, He J, Wang L. Treatment modality selection and prognosis of early stage small cell lung cancer: retrospective analysis from a single cancer institute. Eur J Cancer Care (Engl) 2013;22:789–796. doi: 10.1111/ecc.12082. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber D, Rineer J, Weedon J, Vongtama D, Wortham A, Kim A, Han P, Choi K, Rotman M. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer. 2010;116:1350–1357. doi: 10.1002/cncr.24853. [DOI] [PubMed] [Google Scholar]
- 25.Hanagiri T, Sugio K, Baba T, Ichiki Y, Yasuda M, Uramoto H, Ohga T, Takenoyama M, Yasumoto K. Results of surgical treatment for patients with small cell lung cancer. J Thorac Oncol. 2009;4:964–968. doi: 10.1097/JTO.0b013e3181a8cd84. [DOI] [PubMed] [Google Scholar]
- 26.Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, Keijser A, Faivre-Finn C, Senan S. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385:36–42. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 27.Fizazi K, Cojean I, Pignon JP, Rixe O, Gatineau M, Hadef S, Arriagada R, Baldeyrou P, Comoy E, Le Chevalier T. Normal serum neuron specific enolase (NSE) value after the first cycle of chemotherapy: an early predictor of complete response and survival in patients with small cell lung carcinoma. Cancer. 1998;82:1049–1055. doi: 10.1002/(sici)1097-0142(19980315)82:6<1049::aid-cncr6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Bonner JA, Sloan JA, Rowland KM Jr, Klee GG, Kugler JW, Mailliard JA, Wiesenfeld M, Krook JE, Maksymiuk AW, Shaw EG, Marks RS, Perez EA. Significance of neuron-specific enolase levels before and during therapy for small cell lung cancer. Clin Cancer Res. 2000;6:597–601. [PubMed] [Google Scholar]


