Abstract
In pregnancy, the immunologic system plays an important role that ensures normal pregnancy development and can as well promote the development of complications. Pregnancy success appears to rely on a discrete balance between the Th cytokines, which are involved in fetal growth and development. Preeclampsia and gestational diabetes are known complications associated with pregnancy. However, the source of the increased IL-17 cytokine in preeclampsia and other pregnancy associated diseases still remains unclear amidst numerous inconsistencies. The recent identification of innate lymphoid cells (ILC) has raised more doubts about the sources of most of the Th associated cytokines. We investigated the source of peripheral IL-17 levels in preeclamptic, gestational diabetics and chronic diabetics compared to healthy pregnancy subjects. To evaluate the source of the increased IL-17 cytokine among preeclampsia, chronic diabetic and gestational diabetic patients we investigated the proportion of Th17 cell populations in peripheral blood mononuclear cells using flow cytometry as well as analyzing levels of IFN-γ, IL-17, IL-1β and HMGB1. This study found that the Th17 cell populations in peripheral blood of preeclamptic, gestational nor chronic diabetes during pregnancy did not correlate with the increased IL-17. We report that the increased IL-17 levels observed in patients with preeclampsia, gestational diabetes and chronic diabetes are associated with innate lymphoid cells 3 (ILC3) and may pose threats to the fetus if disregulated.
Keywords: Gestational diabetes, innate lymphoid cells, Th17 cells, preeclampsia
Introduction
Pregnancy is a state of immune tolerance that prevents maternal rejection of the fetus. Preeclampsia and gestational diabetes are the most common major medical complications of pregnancy. Preeclampsia represents the leading cause of both fetal and maternal morbidity and mortality, affecting 5-10% of all pregnancies and can result in a variety of maternal and fetal complications, including seizures, stroke, hepatic failure, renal failure, intrauterine growth retardation, fetal distress, premature delivery, and death [1]. Even though the cause of preeclampsia remains a mystery, it is vastly becoming associated with chronic immune activation [2,3]. Gestational diabetes on the other hand affects 3-5 percent of pregnancies, resulting in a variety of complications that primarily affect the fetus, including macrosomia, stillbirth, jaundice, and respiratory distress syndrome [4]. The maternal immune system plays a vital role in the establishment and maintenance of pregnancy and successful birth [5,6]. It is been established that gestational diabetes [7] preeclampsia and preterm birth are all asso-ciated with altered maternal immune parameters, and there is a compelling body of evidence that immunological factors play a major role in the causal pathways underlying these diseases [5,8].
It has been previously speculated that the increased IL-17 in pregnancy associated diseases sources from CD4+ T cells [9]. While CD4(+) and CD8(+) T cells are important sources of IL-17 cytokine, recent evidence has suggested that γδ T cells and a number of families of innate lymphoid cells (ILCs) can secrete IL-17 and related cytokines [10]. The ILCs are a group of hematopoietic cells devoid of antigen receptors that have important functions in lymphoid organogenesis, in the defense against extracellular pathogens, and in the maintenance of the epithelial barrier [11]. The importance of these cells previously unappreciated ILCs lie in their ability to produce many TH cell-associated cytokines. On the basis of their functional and developmental characteristics, three main groups of ILC have been identified: ILC1, ILC2 and ILC3 [12,13]. The group 1 ILCs express the transcription factor T-bet and secrete T helper type-1- (Th1-) related cytokines, notably IFNγ and tumor necrosis factor (TNF). The group 2 ILCs is dependent on the transcription factor RORα, expresses Gata-3 and the chemokine receptor homologous molecule (CRTH2) and produce Th2-related cytokines IL-5, IL-9, IL-13. The ILC3 express the transcription factor RORγt and synthesize interleukin- (IL-) 17, IL-22, and under specific stimuli, interferon-γ [11,14]. Evidence is emerging that these newly recognized ILCs have been detected in disease models. Human ILCs have been found at inflammatory sites such as the nasal tissue in rhinosinusitis [15], the gastrointestinal tract in Crohn’s disease [16] and the skin in atopic dermatitis [17]. This family of innate cells has also been identified in human tissues [15] and peripheral blood of asthmatics [18] as well as human cord blood.
Even though the function, peripheral blood expression, and physiologic importance of IL-17 is not well established in pregnancy and pregnancy associated diseases, it has been found that IL-17 can play both pathological and protective roles in pregnancy associated diseases [10]. Many researchers have reported that the IL-17 produced during pregnancy is known to induce hypertension [19] by stimulating oxidative stress [20]. The study was aimed at identifying the source of the increased IL-17 in preeclampsia and other pregnancy associated diseases. This paper reports for the first time that the increased IL-17 detected in preeclamptic, gestational diabetics and chronic diabetics may not be produced by TH17 cells as have been previously reported but by ILC3.
Materials and methods
Patients
Informed written consent was obtained from all patients included in this study. Sixty-three (63) pregnant Chinese women at 38 week gestation period receiving ante natal care from the Fourth Affiliated People’s Hospital of Jiangsu University consisting of 15 chronic diabetics, 14 gestational diabetics and 17 preeclampsia patients whose age ranged from 19 to 41 years consented to be included in this study. Preeclampsia was defined as the onset of new hypertension in the second half of pregnancy (blood pressure > 140/90 mm Hg) and new proteinuria (> 300 mg in a 24-h urine collection in the proven absence of a urinary tract infection) with a return to normal postnatally [21].
Gestational diabetes as defined by World Health Organization is hyperglycaemia with blood glucose values above normal but below those diagnostic of diabetes, occurring only during pregnancy. Chronic diabetes was defined as having fasting blood glucose level of 7 mmol/l or higher, and on medication for raised blood glucose or with a history of diagnosis of diabetes for three months and more. Seventeen [17] healthy pregnant women without any clinical signs of diabetes and high blood pressure were simultaneously included as control was recruited into this study. Control group women had no history of any still birth, preterm and post-term labor, gestational and chronic diabetes, ectopic pregnancy, preeclampsia and abnormal pregnancy. The characteristics of these pregnant women are summarized in Table 1. Enrollment took place between August 2013 and November 2014. This study was approved by the ethical committee of Jiangsu University.
Table 1.
The demographic characteristics of the patients are summarized below
| Groups | N | Age |
|---|---|---|
| Control | 17 | 25.01 ± 3.20 |
| PE | 17 | 26.12 ± 5.82 |
| CD | 15 | 29.34 ± 6.01 |
| GD | 14 | 31.04 ± 3.62 |
Cell preparation
Peripheral blood samples were collected from the study subjects and healthy pregnant volunteers. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density-gradient centrifugation. Cells at the interface were harvested, washed twice, and resuspended in phosphate buffered saline (PBS). Viable cells were counted by the trypan blue staining method. More than 95% of the cells were viable. The cell suspensions were divided into two equal aliquots; one was immediately used for the experiments. The other was cryopreserved at -80°C and thawed for testing at a later date.
Flow cytometry
For analysis of Th17 cells, PBMC (2×106 cells/ml) were suspended in complete culture medium that contained RPMI 1640 with L glutamine, penicillin (100 U/ml), streptomycin (10 mg/ml) and 10% fetal bovine serum (FBS). This cell suspension (2×106 cells/ml) was transferred to the 24-well plates. Phorbol 12-myristate 13-acetate (PMA, 150 ng/ml) and ionomycin (1 μM) were added to each well in the presence of monensin (500 ng/ml) for 12 hours (all purchased from Sigma, USA). Then, cells were incubated at 37°C in a humidified 5% CO2 atmosphere. After 12 hours, cells were washed with PBS at 1500 rpm for 5 minutes. After stimulation of PBMC in vitro, the cells were incubated with fluorescein isothiocyanate (FITC) anti-human CD4 at 4°C for 30 minutes. After surface staining, the cells were fixed and permeabilized with BD Cytofix/Cytoperm solution and stained with phycoerythrin (PE) anti-human IL-17A. The isotype control antibody was used in all cases. Data acquisition and analysis were performed on FACSCalibur flow cytometer (Becton Dickinson, CA, USA) using CellQuest software (Becton Dickinson, CA, USA).
Lymphocyte cell counts
Maternal venous blood was collected before delivery. Blood samples were processed within 2 hours after collection. Whole blood (EDTA as anticoagulant) was used to perform hematologic studies. Blood serum and plasma were separated from blood cells and aliquots were stored at -80°C until assayed.
Enzyme-linked immunosorbent assay (ELISA)
For quantitative detection of IFN-γ, IL-17, IL-1β and high mobility group box 1 (HMGB1) in plasma, commercially available ELISA kits were used according to manufacturer’s instruction (Biovender). All samples were run in batches to minimize inter-assay variability. All samples were measured in triplicates and the mean concentration was calculated from the standard curve.
Statistical analysis
The statistical analysis was performed with GraphPad Prism, Version 5.0, software (San Diego, CA, USA). Correlations between variables were determined by Spearman’s correlation coefficient. The rest of the data were analyzed by the independent Student’s t test and Anova was used to compare differences between the groups. Differences were considered statistically significant when the P-value <0.05.
Results
Characteristics of pregnant women enrolled for the study
Patients recruited for this study had all attained at least 38 weeks of gestation. No significant difference was observed in the ages of the study participants. Data presented as means ± SE. PE: Preeclampsia, CD: Chronic diabetes, GD: Gestational diabetes.
Elevated lymphocyte counts among the PE, CD and GD patients
Maternal venous blood was collected before delivery. Blood samples were processed within 2 hours after collection. Lymphocyte cell counts were determined by the blood count analyzer and calculated as % lymphocyte count. The level of individual lymphocyte counts was significantly elevated in preeclampsia patients as compared to healthy pregnant controls (P<0.05; Figure 1). As shown in Figure 1, the proportion of lymphocyte cell counts in patients with gestational diabetes was also significantly higher compared to patients with chronic diabetes (P<0.05). Moreover, the proportion of lymphocyte cell counts in patients with preeclampsia and gestational diabetes showed significant difference (P<0.001). There were significant differences between the proportions of lymphocytes among the various study groups (P<0.0438).
Figure 1.
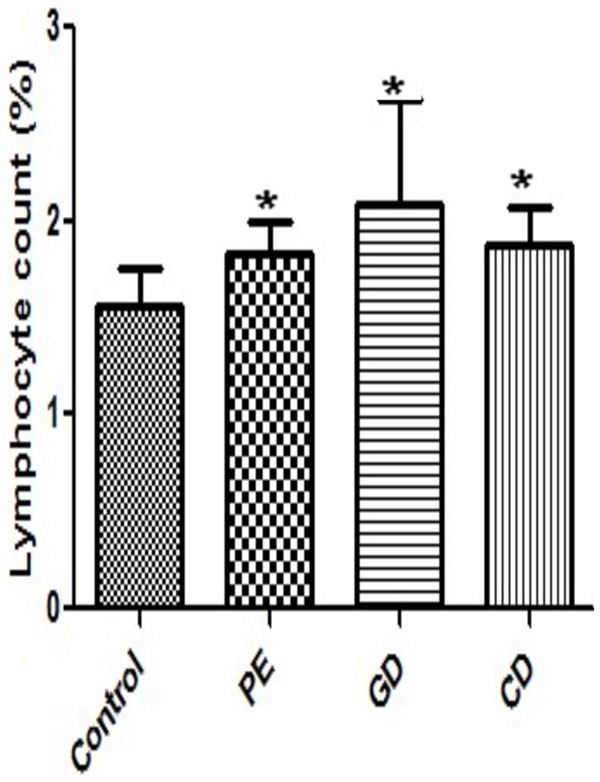
Percentage Lymphocyte cell counts in patients and healthy controls. Lymphocyte cell counts were determined by the blood count analyzer and calculated as % lymphocyte count. Data were analyzed by independent Student’s t-test and one way Anova to compare the three groups. *P<0.05, vs. control group. Healthy pregnant controls C; PE, Preeclampsia; GD, Gestational diabetes; CD, Chronic diabetes.
Increased IFN-γ, IL-17, IL-1β and HMGB1 in plasma from PE, CD and GD patients
Plasma IFN-γ, IL-17, IL-1β and HMGB1 were determined by ELISA. As shown in Figure 2A, HMGB1 concentrations were significantly higher in the PE, CD and GD patients compared to the control subjects (P<0.05). From Figure 2B, IL-17 concentrations were significantly higher in the PE, CD and GD patients compared to the control subjects (P<0.05). In Figure 2C. IFN-γ concentrations were significantly higher in the PE, CD and GD patients compared to the control subjects (P<0.05). Figure 2D shows that IL-1b concentrations were significantly higher in the PE, CD and GD patients compared to the control subjects (P<0.05). In addition, there were significant differences in IFN-γ and IL-1β concentrations between the PE and CD patients.
Figure 2.
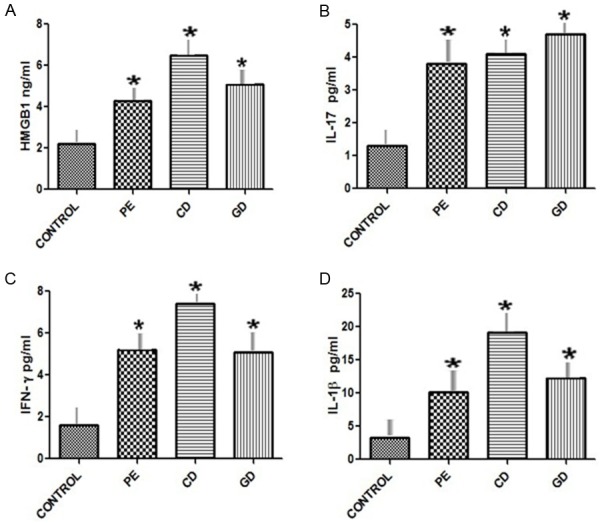
Plasma concentrations of cytokines in patients and healthy controls. Plasma concentrations of IFN-γ, IL-17, IL-1β and HMGB1 in Preeclamptic, Chronic diabetic, Gestational diabetic patients and healthy controls were determined by ELISA. Data were analyzed by independent Student’s t-test and one way Anova to compare the three groups. *P<0.05 vs. control group. ELISA, Enzyme linked immunosorbent assay; IFN, Interferon; IL, Interleukin. HMGB1: High Mobility Group Box 1. Healthy pregnant controls C; PE, Preeclampsia; GD, Gestational diabetes; CD, Chronic diabetes.
IL-17 producing ILCs 3 not Th17 cells might be a potential danger factor for PE, CD and GD patients
Th17 cells were determined by flow cytometry and calculated as % PBMC. The level of individual Th17 cells was not significantly elevated in preeclampsia patients as compared to healthy pregnant controls (P > 0.05; Figure 3B). The proportion of Th17 cells in patients with gestational diabetes was not significantly higher compared to patients with chronic diabetes (2.30 ± 0.51 vs. 1.93 ± 0.34%; P > 0.05). Moreover, the proportion of Th17 cells in patients with preeclampsia and gestational diabetes showed no significant difference (1.80 ± 0.45% vs. 2.30 ± 0.51%; P=0.2430). There were no significant differences between the proportion of Th17 cells among the various study groups (P > 0.05).
Figure 3.
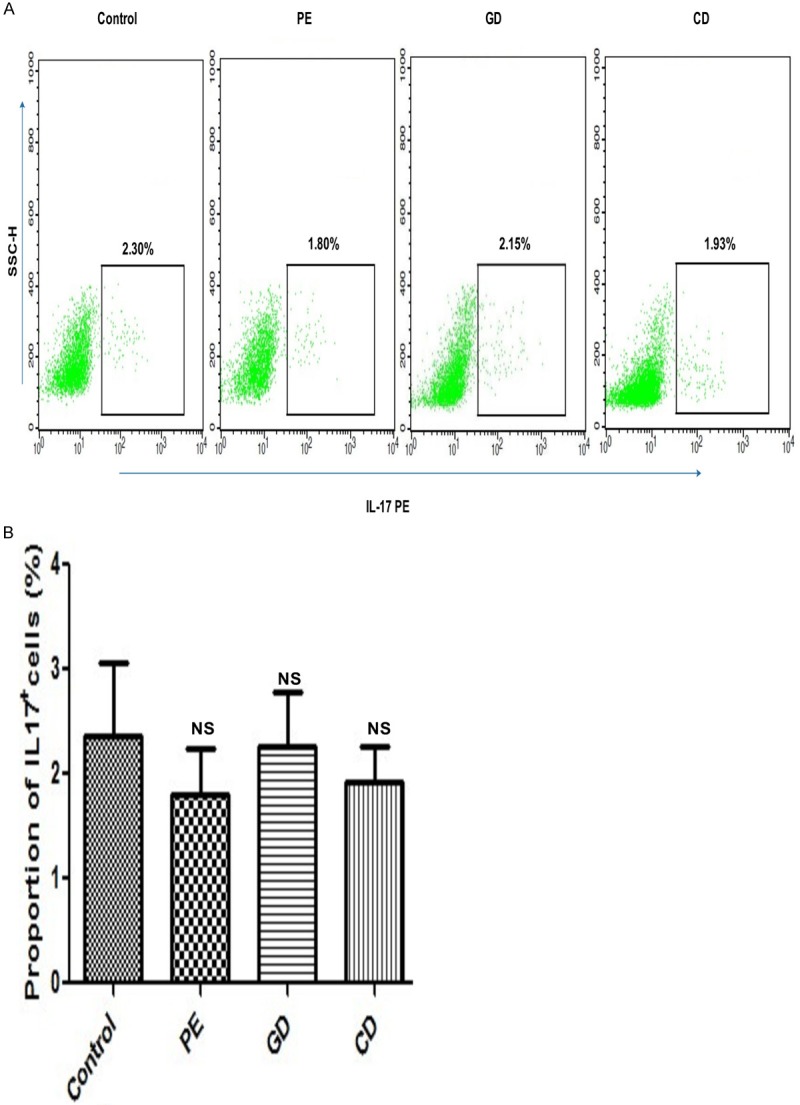
A. Representative diagram of flow cytometry analysis for circulating Th17 cells. Th17 cells from the study groups: Healthy pregnant controls C; PE, Preeclampsia; GD, Gestational diabetes; CD, Chronic diabetes; IL-17 PE: IL-interleukin phycoerythrin. B. Circulating Th17 cell frequencies. The frequency of Th17 cells in PBMC was analyzed by flow cytometry from the patients and healthy controls used in this experiment. Data were analyzed by independent Student’s t-test and one way Anova to compare the three groups. Data presented as mean ± SE. *P<0.05 vs. control group. Healthy pregnant controls C; PE, Preeclampsia; GD, Gestational diabetes; CD, Chronic diabetes; NS: Non significant.
Discussion
Innate lymphoid cells (ILCs) have recently gained much attention as important mediators of tissue homeostasis and inflammation [12]. ILCs may contribute to intestinal inflammation through cytokine production, lymphocyte recruitment, and organization of the inflammatory tissue. ILCs are known to exhibit protective and pathologic functions either during the steady state, or following infection, inflammation or tissue damage [22]. However, the relevance of ILC populations in human health and disease especially in pregnancy associated diseases is currently poorly understood. Although a contribution of conventional αβ T cells to IL-17 and IL-22 cytokine production is well-established [23], the contribution of innate immune cell subsets is less well understood. Recent data in both mouse experimental models [24] and in human immune-mediated pathologies, such as Crohn’s disease [25], have established a potential role of innate lymphoid cells (ILCs) as key sources of IL-17 and IL-22 production in epithelial inflammatory disease. ILC3 have been shown to produce IL-17 in the gut of inflammatory bowel disease patients [16], suggesting a potential role in immune-mediated diseases such as colitis and psoriasis. Despite the growing interest about innate sources of these proinflammatory cytokines, the ILC populations and roles in pregnancy associated diseases remains to be elucidated. The NKp44+ ILC3 subset of ILCs, previously demonstrated to produce IL-17 and IL-22 [16], is increased in frequency in both the peripheral blood and skin of psoriasis patients.
Amidst the inconsistencies about the Th17 cell populations, recent data suggest that the proportion of Th17 cells might be decreased during pregnancy to prevent rejection [26,27]. On the other hand, works by two research groups have proven that Th17 levels might not change in preeclampsia, although Th1 type immunity is predominantly observed in preeclampsia [28,29]. This is again consistent with our findings. Our data found no statistical significant difference between the populations of Th17 cells among our study group. This therefore supports the fact that they have no association with the observed increase IL-17 cytokine. However, our data found statistically significant difference between the IL-17 levels among the study groups. This is consistent with findings from two independent groups which previously reported significantly higher levels of IL-17 in preeclamptic women than healthy pregnant women [30,31]. This therefore suggests that there could be another group of cells responsible for this observed increase of IL-17 levels detected in the preeclampsia and gestational diabetes. We confidently report that the IL-17 may be associated with the selective accumulation of a phenotypically distinct ILC population characterized by inflammatory cytokine expression. This implies that Th17 cells may not have promoted the increase in IL-17 levels observed in our study. ILCs have also been found to be the source of IL-17 in peripheral blood of Inflammatory Bowel Disease patients [25] as well as in the eye of experimental choroidal neovascularization disease [32]. Both studies showed that ILC3 were responsible for the increased IL-17 but not Th17 cells. Furthermore, it has been documented that ILC3 produce IL-17A following stimulation [33] and HMGB1/IFN-gamma axis have been reported to stimulate the ILC-3 to produce IL-17 [32]. Furthermore, ILC3 respond to IL-1β and IL-23 to produce IL-17 and IL-22 [22]. Our study again found increased HMGB1 and IFN gamma levels in preeclampstic, gestational and chronic diabetics. This suggests that their increased levels promoted the observed increased IL-17 among the study groups. In pregnancy associated diseases, increased IL-17 therefore means increased ILC3 cell population and available data suggest that dysregulation or expansion of proinflammatory ILC populations may directly promote disease through the production of cytokines IL-17, IL-22, TNF-α and IFN-γ [22]. Emerging evidence suggest that there is the possibility of shift from protective to pathologic ILC responses in disease models which may involve lineage plasticity, altered expression of transcriptional regulators, or engagement of alternative activation pathways [16,34]. Increased IL-17A is an inflammatory cytokine [35] and has been reported to play a major role in the pathogenesis of preterm delivery [36] and again high levels of IL-17 have been recorded in spontaneous abortion cases [37]. In humans, it has been suggested that IL-17 can increase the invasive capacity of JEG-3 cells (a trophoblast-like human choriocarcinoma cell line) and increase progesterone secretion significantly in in vitro coculture models [38]. This therefore means that dysregulated ILC3 in pregnant associated diseases may threaten the life of both the mother and fetus through excessive production of IL-17.
In conclusion, we demonstrated a higher possibility that the increased plasma HMGB1 in pregnancy associated diseases can lead to ILCs differentiation and subsequently production of IL-17. Again the increased IL-17 in pregnancy associated diseases including preeclampsia and gestational diabetes may be produced by ILC3 and may pose a threat to fetus leading to spontaneous abortions if they are triggered to elicit uncontrolled inflammatory responses.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31270947, 81370084, 813170084 and 30972748), and Postdoctoral Foundation of China (2012M511705).
Disclosure of conflict of interest
None.
References
- 1.Parham P. NK Cells and Trophoblasts Partners in Pregnancy. J Exp Med. 2004;200:951–955. doi: 10.1084/jem.20041783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28:192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Baumwell S, Karumanchi SA. Pre-eclampsia: clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2006;106:c72–81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 4.Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341:1749–1756. doi: 10.1056/NEJM199912023412307. [DOI] [PubMed] [Google Scholar]
- 5.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 7.Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, Alexandre SM, Mattar R, Daher S. Cytokine levels in gestational diabetes mellitus: a systematic review of the literature. Am J Reprod Immunol. 2013;69:545–557. doi: 10.1111/aji.12088. [DOI] [PubMed] [Google Scholar]
- 8.Clark DA, Coulam CB, Daya S, Chaouat G. Unexplained sporadic and recurrent miscarrage in the new millennium: a critical analysis of immune mechanisms and treatments. Hum Reprod Update. 2001;7:501–511. doi: 10.1093/humupd/7.5.501. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima A, Ito M, Yoneda S, Shiozaki A, Hidaka T, Saito S. Short communication: Circulating and Decidual Th17 Cell Levels in Healthy Pregnancy. Am J Reprod Immunol. 2010;63:104–109. doi: 10.1111/j.1600-0897.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- 10.Sutton CE, Mielke LA, Mills KH. IL-17-producing γδT cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 11.Sedda S, Marafini I, Figliuzzi MM, Pallone F, Monteleone G. An Overview of the Role of Innate Lymphoid Cells in Gut Infections and Inflammation. Mediators Inflamm. 2014;2014:235460. doi: 10.1155/2014/235460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE. Innate lymphoid cells-a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 13.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Ann Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 14.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells-how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 15.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25-and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 16.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 17.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherkat R, Yazdani R, Hakemi MG, Homayouni V, Farahani R, Hosseini M, Rezaei A. Innate lymphoid cells and cytokines of the novel subtypes of helper T cells in asthma. Asia Pacific Allergy. 2014;4:212. doi: 10.5415/apallergy.2014.4.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace K, Dhillon P, Richards S, Weimer A, Martin JN Jr, LaMarca B. Interleukin 17 increases blood pressure during pregnancy: a link between autoimmunity and preeclampsia? FASEB J. 2011;25:1030.1010. [Google Scholar]
- 20.Activation of IL-17 during pregnancy; a link between autoimmunity and preeclampsia: The university of mississippi medical center. 2012 [Google Scholar]
- 21.Brown M, Hague W, Higgins J, Lowe S, McCowan L, Oats J, Peek M, Rowan J, Walters B. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Aust N Z J Obstet Gynaecol. 2000;40:139–155. doi: 10.1111/j.1479-828x.2000.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenberg GF. Regulation of intestinal health and disease by innate lymphoid cells. Int Immunol. 2014;26:501–7. doi: 10.1093/intimm/dxu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, Bruijnzeel-Koomen CA, Clark RA. CD8(+) T Cells in the Lesional Skin of Atopic Dermatitis and Psoriasis Patients Are an Important Source of IFN-γ, IL-13, IL-17, and IL-22. J Invest Dermatol. 2013;133:973–979. doi: 10.1038/jid.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Kooten C, Boonstra JG, Paape ME, Fossiez F, Banchereau J, Lebecque S, Bruijn J, De Fijter J, Van Es L, Daha M. Interleukin-17 activates human renal epithelial cells in vitro and is expressed during renal allograft rejection. J Am Soc Nephrol. 1998;9:1526–1534. doi: 10.1681/ASN.V981526. [DOI] [PubMed] [Google Scholar]
- 27.Tang JL, Subbotin VM, Antonysamy MA, Troutt AB, Rao AS, Thomson AW. Interleukin-17 antagonism inhibits acute but not chronic vascular rejection1. Transplantation. 2001;72:348–350. doi: 10.1097/00007890-200107270-00035. [DOI] [PubMed] [Google Scholar]
- 28.Saito S, Tsukaguchi N, Hasegawa T, Michimata T, Tsuda H, Narita N. Distribution of Th1, Th2, and Th0 and the Th1/Th2 cell ratios in human peripheral and endometrial T cells. Am J Reprod Immunol. 1999;42:240–245. doi: 10.1111/j.1600-0897.1999.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 29.Saito S, Umekage H, Sakamoto Y, Sakai M, Tanebe K, Sasaki Y, Morikawa H. Increased T-Helper-1-Type Immunity and Decreased T-Helper-2-Type Immunity in Patients with Preeclampsia. Am J Reprod Immunol. 1999;41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 30.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, de St Groth BF, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 31.Toldi G, Rigó J, Stenczer B, Vásárhelyi B, Molvarec A. Increased Prevalence of IL-17-Producing Peripheral Blood Lymphocytes in Pre-eclampsia. Am J Reprod Immunol. 2011;66:223–229. doi: 10.1111/j.1600-0897.2011.00987.x. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa E, Sonoda K-H, Shichita T, Morita R, Sekiya T, Kimura A, Oshima Y, Takeda A, Yoshimura T, Yoshida S. IL-23-Independent Induction of IL-17 from γδT Cells and Innate Lymphoid Cells Promotes Experimental Intraocular Neovascularization. J Immunol. 2013;190:1778–1787. doi: 10.4049/jimmunol.1202495. [DOI] [PubMed] [Google Scholar]
- 33.Hoorweg K, Peters CP, Cornelissen F, Aparicio-Domingo P, Papazian N, Kazemier G, Mjösberg JM, Spits H, Cupedo T. Functional differences between human NKp44-and NKp44+ RORC+ innate lymphoid cells. Front Immunol. 2012;3:72. doi: 10.3389/fimmu.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glatzer T, Killig M, Meisig J, Ommert I, Luetke-Eversloh M, Babic M, Paclik D, Blüthgen N, Seidl R, Seifarth C. RORγt+ innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity. 2013;38:1223–1235. doi: 10.1016/j.immuni.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int Immunol. 2009;21:489–9836. doi: 10.1093/intimm/dxp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, Yoneda S, Shiozaki A, Sumi S, Tsuneyama K. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84:75–85. doi: 10.1016/j.jri.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima A, Ito M, Shima T, Bac ND, Hidaka T, Saito S. Accumulation of IL-17-Positive Cells in Decidua of Inevitable Abortion Cases. Am J Reprod Immunol. 2010;64:4–11. doi: 10.1111/j.1600-0897.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- 38.Pongcharoen S, Niumsup P, Sanguansermsri D, Supalap K, Butkhamchot P. The Effect of Interleukin-17 on the Proliferation and Invasion of JEG-3 Human Choriocarcinoma Cells. Am J Reprod Immunol. 2006;55:291–300. doi: 10.1111/j.1600-0897.2006.00366.x. [DOI] [PubMed] [Google Scholar]


