Abstract
The purpose of this paper is to examine the effects of polydatin on cognitive function in rats self-administered with chronic ethanol levels. The levels of cyclin-dependent kinase 5 (Cdk5) were also determined. In the in vivo study, adult male Sprague-Dawley rats were used to establish an ethanol-administered rat model. Cognitive function was measured using the Morris water maze and the level of Cdk5 expression was measured to evaluate the effect of polydatin treatment. Cdk5 kinase activity and cell survival rate in primary hippocampal neuron cultures treated with ethanol or ethanol and polydatin were measured in the in vitro study. Polydatin reversed the performance impairments in chronic ethanol treated rats in Morris water maze test, and decreased unregulated Cdk5 expression. Moreover, polydatin increased cell survival rate, and decreased Cdk5 activity in the ethanol-treated primary culture of hippocampal neurons. The study results suggest that polydatin exhibits neuroprotective potential for ethanol induced neurotoxicity, both in vivo and in vitro, which is most likely related to its ability to target Cdk5 in neurons.
Keywords: Polydatin, chronic ethanol administration, Cdk5
Introduction
Alcoholism is a serious social problem and medical issue. Ethanol abuse related health, psychological and social problems have become a major public health challenge [1]. Polydatin (3,4’,5-trihydroxystibene-3-β-mono-d-glucoside), isolated from the P. Cuspidatum, has many pharmacological effects, including anti-inflammation and inducing the production of antioxidants [2]. It also conducts a neuroprotective effect on ischemia/reperfusion injury in the lungs, the heart and in shock treatment [3-8]. There are also some studies showing its neuroprotective effects, but the underlying mechanisms are not yet clear [9,10].
Cyclin-dependent kinase 5 (Cdk5) is a member of thecyclin-dependent kinase family. Cdk5 phosphorylates serine and threonine in the motif where the obligatory proline must be present [11]. Cdk5 is most abundant in the central nervous system and is activated by p35 and p39 [12]. Furthermore, Cdk5 has been reported to play a vital role in normal neuronal development and function, especially in learning and memory formation and drug addiction [13-18]. The purpose of this study was to investigate the effects of polydatin on cognitive function in rats self-administered with ethanol. Our results suggest that polydatin has a neuroprotective role in ethanol-induced toxicity in vivo and in vitro. This is most likely related to its ability to target Cdk5 in neurons.
Materials and methods
Polydatin preparation
Polydatin was extracted from the powder of Polygonum cuspidatum using a direct water extraction method. Briefly, the Polygonum cuspidatum powder was decocted, filtered and then concentrated in a container. The concentrated mixture was dissolved into 95% ethanol, which was filtered again to remove insoluble particles. The remaining ethanol phase was extracted 4 times by ethyl-acetate. All ethyl acetate extractions were combined together and evaporated under reduced pressure to form syrup. The syrup was then redissolved into diluted ethanol and filtered. The white needle-like crystal filtrate was polydatin.
Animals and Treatment
Male Sprague Dawley rats were purchased from the Animal Center at Zhengzhou University with initial body weights of 200 to 220 g. All procedures with animals were approved by the Animal Care Committee of the Xinxiang Medical University under the license 2005-0001. The animals were kept under a 12-h light-dark cycle (light on 6:00-18:00 h) and had free access to food and tap water. Rats were randomly assigned into four groups [n=10]: CNT: control; CEA: Chronic ethanol administered; ELP: Ethanol+low dose polydatin and EHP: Ethanol+high dose polydatin. Animals in the ethanol-groups were treated intragastrically with ethanol at 3.0 g/kg in a sucrose solution (1%, w/v) for 30 days whereas animals in the control group were treated intragastrically with the sucrose solution alone for 30 days [19]. The ELP and EHP groups were treated intragastrically with ethanol at 3.0 g/kg in a sucrose solution (plus polydatin either 12.5 or 50 mg/kg, respectively) for 30 days. During the study period, there was no significant difference in the body weight between ethanol-treated and control rats. Rats from each group were evaluated by the Morris water maze test followed by decapitation after 30 days of ethanol treatment.
Morris water maze testing
Spatial learning and memory of rats was assessed by the Morris water maze as previously described [20,21]. Briefly, rats are placed in a large circular pool of water and required to escape from water onto a hidden platform whose location can normally be identified only using spatial memory. The rats were given four trials per day and the average time to find the platform of the 4 trials (escape latency) was recorded by video tracking software. The shorter escape latency, the stronger spatial learning ability.
Immunofluorescence
Following behavioral testing, animals (n=6) were perfused with 50 mM cold PBS (pH 7.4) via injection into the ascending aorta. Animal brains were then frozen in isopentane, which was cooled by dry ice. Coronal sections (8-µm thick) were then obtained on a cryostat. Sections were fixed with 2% paraformaldehyde for 10 minutes and blocked with 0.3% Triton-X 100 in PBS and 2% BSA for 30 minutes. The primary antibody was incubated for overnight. After incubation, sections were washed with PBS. The secondary antibody incubation was performed with Alexa Fluor 488-labeled goat anti-rabbit antibodies. A BX41 (Olympus) microscope was used to acquire images.
Primary hippocampal neuronal culture
Hippocampal neuronal cell cultures were processed according to the proposals described previously [22]. Hippocampal neurons were cultivated from fetuses within 24 h of birth and cultured in neuron-defined medium. Fetuses were decapitated, and the hippocampus was dissected and collected under sterile conditions and kept in ice-cold Hank’s balanced salt solution, until incubation at 37°C for 15~30 min. The reaction was terminated by adding whole culture medium. Hippocampal tissue was dissociated to single cells by gentle trituration (10-20 times) using a 1 ml pipette. The cell suspension was centrifuged at 310× g for 3 min, and the resulting pellets were resuspended in whole culture medium and were seeded on 24-well tissue culture dishes coated with 100 μg/ml poly-L-lysine at a density of 6×105 cells/ml in 300 μl/well. Cultures were incubated in 5% CO2 and 95% air at 37°C and 100% humidity for 24 h. Then the culture medium was replaced with serum-free Neurobasal medium (NBM), supplemented with 2% (v/v) B-27. Cultures were subsequently fed every 4~5 days by half-changing the existing medium with fresh NBM and B-27. After a one-week incubation period, the neurons had matured and the dendritic network had been established. Cultures were maintained for 8-10 days prior to experimentation.
XTT Assay
Cells (10 μl of 5×105 cell/ml) were seeded into 96-well tissue culture plates. After 24 h when optimal population densities were reached, cells were assigned to groups according to different treatments: alcohol (25 mmol/ml), polydatin (12.5 μg/ml), polydatin (50 μg/ml), alcohol (25 mmol/ml) + polydatin (12.5 μg/ml), alcohol (25 mmol/ml) + polydatin (50 μg/ml), roscovitine (20 μmol/L) pretreatment for 24 hours + alcohol (25 mmol/ml). In addition, blank control groups were created. Cells were additionally cultured for 24 hours, then XTT/PMS (50 μl) was added to each well, and the solution was mixed and incubated for 2 hours at 37°C. The absorbance of the plate was measured with a spectrophotometer at a wavelength of 450 nm. The cell survival rate was calculated as Atreatment/Acontrol × 100%.
Cdk5 kinase activity assay
Cdk5 activity was measured based on previously published methods [23]. Lysis buffer containing a protease inhibitor tablet was used to lyse cells. An anti-Cdk5 antibody conjugated to agarose beads was used to immunoprecipitate Cdk5 from cell lysates. The immunoprecipitated samples were resuspended and 2 μg of histone H1 (type II-S) were used to incubate the samples in 20 ml of kinase assay buffer (10 min, 30°C). A scintillation counter was used to measure radioactivity in samples, as well as correct for basal activity.
Statistical analyses
Data were expressed as means ± standard deviations (SD) of three replicated determinations and then analyzed with SPSS 11.5 software (version 11.5, SPSS Inc., USA). One-way analysis of variance (ANOVA) and LSD multiple-range tests were used to determine the differences of means. P value less than 0.05 was considered to be statistically significant.
Results
Morris water maze
As shown in Figure 1, the time to find the platform (escape latency) in alcoholic rats was significantly longer than that in control rats (21.75±5.22 vs. 10.88±5.69). High dose polydatin significantly reversed the decrease in alternation performance in alcoholic rats (11.13±5.82). However, low dose polydatin was not obvious function.
Figure 1.
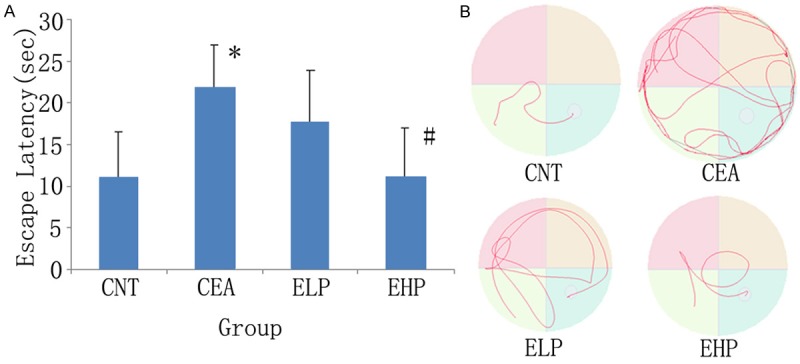
Effects of polydatin on spatial alternation performance in the Morris water maze in a chronic ethanol administered rat model. A. X-axis:groups; Y-axis: escape latency. Results are expressed as means ± SD. n=10. *P<0.01, vs control; #P<0.01 vs ethanol group. B. Typical swim-tacking path in the Morris water maze. CNT: Control group; CEA: Chronic ethanol administered group; ELP: Ethanol+low dose polydatin; EHP: Ethanol+high dose polydatin.
Cdk5 staining
Cdk5 expression levels in the hippocampus of rats were assessed using immunofluorescent staining (Figure 2). The Cdk5 in the control group showed concentrated staining in the cytosol, whereas the alcoholic rats showed more positive cells expressing Cdk5. By contrast, the groups treated with polydatin had much lower expression of Cdk5. This decreased expression was also dose dependent.
Figure 2.
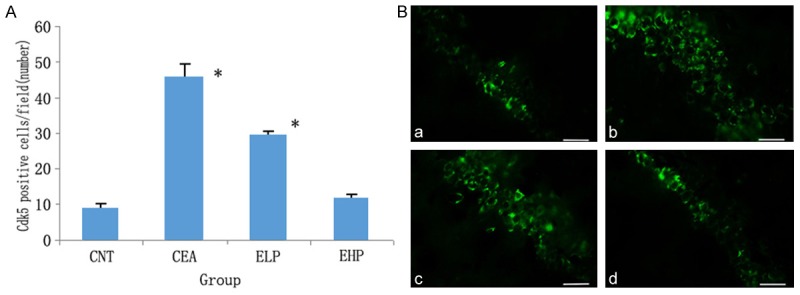
Cdk5 protein expression in the hippocampus by immunofluorescence (Alexa Fluor 488, 400×). A. Bar graph of Cdk5-positive cell number in the hippocampus by immunofluorescence (Cdk5-positive cell numbers perfield are shown), *P<0.01 vs control. B. Representative photomicrographs of Cdk5 (green) with immunofluorescent staining in the rat hippocampus of the control group (a), chronic ethanol administration group (b), ethanol+low dose polydatin (c) and ethanol+high dose polydatin (d). The scale bar represents 10 μm.
Viability of neurons
Primary hippocampal neuronal cell viability was measured using XTT Assay. Table 1 shows that cell survival rate of the hippocampal neurons decreased significantly in the ethanol treated culture as compared to normal cells, suggesting a neurotoxic effect of ethanol in the primary neuronal culture. However, no significant changes were seen in both 12.5 and 50 μg/ml polydatin treated cultures without ethanol pretreatment. By contrast, both 12.5 and 50 μg/ml polydatin treated cultures with ethanol pretreatment were not damaged as compared to ethanol treated cultures. Cell survival rate was significantly increased in the roscovitine pretreatment group as compared with the alcohol alone group, indicating that alcohol-induced neuronal toxicity is associated with Cdk5.
Table 1.
Effects of polydatin and ethanol on the cell survival rate of hippocampal cells
| Group | Cell viability (%) |
|---|---|
| Control | 100±2.95 |
| Ethanol | 88.7±4.11* |
| 12.5 | 101.2±2.36 |
| 50 | 102.6±5.15 |
| Ethanol+12.5 | 94.5±5.12# |
| Ethanol+50 | 99.3±4.37## |
| Roscovitine | 96.3±3.32# |
Control: control group; alcohol: 25 mmol/ml alcohol model group; 12.5:12.5 μg/ml polydatin group; 50:50 μg/ml polydatin group; alcohol +12.5:25 mmol/ml alcohol +12.5 μg/ml polydatin group; alcohol +50:25 mmol/ml alcohol +50 μg/ml polydatin group; roscovitine: 20 μmol/L roscovitine +25 mmol/ml alcohol group.
P<0.01, vs control group;
P<0.05, vs alcohol group;
P<0.01, vs alcohol group.
Cdk5 kinase activity
Since polydatin decreased the expression levels of Cdk5 in primary hippocampal neuronal cultures treated with ethanol, the Cdk5 kinase activity was further investigated. As shown in Figure 3, Cdk5 enzymatic activity of the ethanol treated hippocampal neurons was significantly increased as compared to that of the control group (3770.00±269.17 vs. 1892.77±167.33, P<0.01). This increased enzymatic activity was significantly decreased after treatment with polydatin, regardless of the dosage (2533.17±158.33, and 2247.91±177.12). Cdk5 kinase activity was significantly reduced in the roscovitine pretreatment group (2168.17±183.78) as compared with the alcohol alone group (P<0.01).
Figure 3.
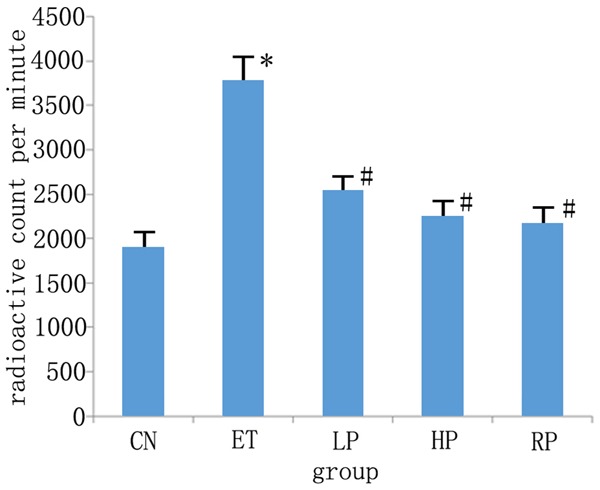
Effects of polydatin on Cdk5 kinase activity in primary hippocampal neuron cultures treated with or without ethanol. The vertical ordinate represents CPM (count per minute) value of reaction product. X-axis: groups; Y-axis: CPM values. Results are expressed as means ± SD. n=6. CN: Control group; ET: 25 mmol/ml ethanol treated group; LP: 25 mmol/ml ethanol +12.5 μg/ml polydatin group; HP: 25 mmol/ml ethanol +50 μg/ml polydatin group; RP: 20 μmol/L roscovitine +25 mmol/ml alcohol group. *P<0.01, vs control; #P<0.05 vs ethanol treated group.
Discussion
Alcohol-related toxicity and impairments to learning and memory can be attributed to ethanol damage in the central nervous system. The hippocampus, a brain structure crucial to learning and memory formation, demonstrates sensitivity to ethanol related neurotoxicity [24,25]. Previous studies have documented impaired hippocampal functions in both human alcoholism and alcoholic animal models [26]. In this study, the protective effects of polydatin were evaluated in both an animal model and primary hippocampal neuronal cultures.
The mechanisms of ethanol related damage to the central nervous system are very complicated. Most studies have focused on three major areas [27,28], such as ethanol-induced neuronal apoptosis, ethanol interference with neurotransmitters (including γ-amino butyric acid, 5-hydroxyltryptamine, dopamine, glutamate, the cholinergic system), and ethanol-related genes.
Cdk5 was first discovered by several independent research groups in 1992 [29]. However, unlike other Cdks, Cdk5 plays little role in the regulation of the cell cycle. Activated Cdk5 phosphorylates a spectrum of proteins at serine and threonine sites [29], most associated with the cytoskeleton and signaling or regulatory pathways [30]. During the development of the nervous system, Cdk5 plays a pivotal role in neuronal cell migration, cortical layering, synapse formation, synaptic plasticity, and learning and memory [31]. However, under certain pathophysiological conditions, the incorrect cleavage of p35 and the formation of protein complex Cdk5/p25 will inactivate Cdk5, leading to neuronal cell apoptosis or even cell death. Many studies have shown that this dysregulation of Cdk5 is involved in several neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis [32]. Recent findings have demonstrated that Cdk5 has an important novel role in learning and memory, especially during memory consolidation within the adult central nervous system. Fischer’s lab has confirmed the association between Cdk5 and learning and memory, that is, the injection of butyrolactone I (a Cdk5 inhibitor) into the hippocampus significantly impaired contextual fear conditioning [33]. Consistently, another group has shown that injection of roscovitine, another Cdk5 inhibitor, into the hippocampus could also profoundly reduce freezing behavior [14]. Tanaka et al. has demonstrated that specific genetic reconstitution of Cdk5 expression limited in neurons in Cdk5 null mice rescued the defects in the nervous system of this Cdk5 null phenotype [34]. Also, recent evidence has demonstrated that Cdk5 was closely related to drug addiction [35,18]. Our previous paper also revealed that Cdk5 was involved in ethanol exposure in animal models [36].
Based on the above-mentioned research, we investigated the neuroprotective effects of polydatin in a rat model of chronic self-administered ethanol and further explored the possible mechanism of this protection via Cdk5. Results showed that following chronic alcohol treatment, rats exhibited increased hippocampal Cdk5 expression in addition to memory impairment, indicating that Cdk5 may be involved in alcohol-induced learning and memory injuries. However, hippocampal Cdk5 expression was decreased and the learning and memory function of rats was protected following polydatin treatment. To further confirm whether Cdk5 is associated with the neuroprotection of polydatin, we cultured primary hippocampal neurons based on animal experiments. Neurons were pretreated with roscovitine, a selective inhibitor of Cdk5. Roscovitine is a purine-like substance and can occupy the ATP binding site of Cdk5 to inhibit Cdk5/p35 complex production, thereby inhibiting Cdk5 activity [37]. Results in the present study showed that the survival rate of roscovitine-pretreated neurons was significantly increased following alcohol treatment, indicating that inhibition of Cdk5 activation can inhibit alcohol-induced neurotoxicity. Interestingly, polydatin-pretreated neurons also presented significantly increased survival rate but decreased Cdk5 kinase activity, demonstrating a similar mechanism of action for polydatin as roscovitine. The findings indicate that inhibition of Cdk5 activation mediates suppressed alcoholinduced neurotoxicity by polydatin.
The neuroprotective mechanism of polydatin remains inconclusive. It may act as an antioxidative agent in the CNS, like many polyphenols. Some studies reported that polydatin may affect the function of NMDA receptor by rapidly inhibiting the glutamate induced Ca2+ toxicity in the hippocampus [38,39].
In conclusion, our study demonstrated for the first time that polydatin markedly improved the learning and memory deficits induced by chronic self-administered ethanol in rats. We also found that polydatin could decrease the expression and activity of Cdk5, consequently exerting protective effects. Polydatin may ameliorate the cognitive deficits by many other pathways, but further studies are required to clarify its mechanisms in detail.
Acknowledgements
This work was supported by National Nature Science Foundation (81171261); Fund for Talents With Innovation in Medical Science and Technology of Henan Province (3052); Henan key lab of Biological Psychiatry Open project (ZDSYS2011003) and Scientific Research Fund of Xinxiang Medical University (2013ZD112).
Disclosure of conflict of interest
None.
References
- 1.de Rick A, Vanheule S, Verhaeghe P. Alcohol addiction and the attachment system: an empirical study of attachment style, alexithymia, and psychiatric disorders in alcoholic inpatients. Subst Use Misuse. 2009;44:99–114. doi: 10.1080/10826080802525744. [DOI] [PubMed] [Google Scholar]
- 2.Xiao K, Xuan L, Xu Y, Bai D. Stilbene glycoside sulfates from Polygonumcuspidatum. J Nat Prod. 2000;63:1373–1376. doi: 10.1021/np000086+. [DOI] [PubMed] [Google Scholar]
- 3.Miao Q, Wang S, Miao S, Wang J, Xie Y, Yang Q. Cardioprotective effect of polydatin against ischemia/reperfusion injury: roles of protein kinase C and mito K (ATP) activation. Phytomedicine. 2011;19:8–12. doi: 10.1016/j.phymed.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Zhang LP, Wei Y, Song SL, Cheng M, Zhang Y. Effect of polydatin on action potential in ventricular papillary muscle of rat and the underlying ionic mechanism. Sheng Li Xue Bao. 2011;63:48–54. [PubMed] [Google Scholar]
- 5.Zhang LP, Ma HJ, Bu HM, Wang ML, Li Q, Qi Z, Zhang Y. Polydatin attenuates ischemia/reperfusion-induced apoptosis in myocardium of the rat. Sheng Li Xue Bao. 2009;61:367–372. [PubMed] [Google Scholar]
- 6.Wang X, Song R, Bian HN, Brunk UT, Zhao M, Zhao KS. Polydatin, a natural polyphenol, protects arterial smooth muscle cells against mitochondrial dysfunction and lysosomal destabilization following hemorrhagic shock. Am J Physiol Regul Integr Comp Physiol. 2012;302:R805–814. doi: 10.1152/ajpregu.00350.2011. [DOI] [PubMed] [Google Scholar]
- 7.Zhao KS, Jin C, Huang X, Liu J, Yan WS, Huang Q, Kan W. The mechanism of Polydatin in shock treatment. Clin Hemorheol Microcirc. 2003;29:211–217. [PubMed] [Google Scholar]
- 8.Shiyu S, Zhiyu L, Mao Y, Lin B, Lijia W, Tianbao Z, Jie C, Tingyu L. Polydatin upregulates Clara cell secretory protein to suppress phospholipase A2 of lung induced by LPS in vivo and in vitro. BMC Cell Biol. 2011;12:31. doi: 10.1186/1471-2121-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li RP, Wang ZZ, Sun MX, Hou XL, Sun Y, Deng ZF, Xiao K. Polydatin protects learning and memory impairments in a rat model of vascular dementia. Phytomedicine. 2012;19:677–681. doi: 10.1016/j.phymed.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Ji H, Zhang X, Du Y, Liu H, Li S, Li L. Polydatin modulates inflammation by decreasing NF-κB activation and oxidative stress by increasing Gli1, Ptch1, SOD1 expression and ameliorates blood-brain barrier permeability for its neuroprotective effect in pMCAO rat brain. Brain Res Bull. 2012;87:50–59. doi: 10.1016/j.brainresbull.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Hawasli AH, Koovakkattu D, Hayashi K, Anderson AE, Powell CM, Sinton CM, Bibb JA, Cooper DC. Regulation of hippocampal and behavioral excitability by cyclin-dependent kinase 5. PLoS One. 2009;4:e5808. doi: 10.1371/journal.pone.0005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 13.Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Cyclin-dependent kinase 5 is required for ass ociative learning. J Neurosci. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J, Tsai LH. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10:1012–1019. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohshima T, Ogura H, Tomizawa K, Hayashi K, Suzuki H, Saito T, Kamei H, Nishi A, Bibb JA, Hisanaga S, Matsui H, Mikoshiba K. Impairment of hippocampal long-term depression and defective spatial learning and memory in p35 mice. J Neurochem. 2005;94:917–925. doi: 10.1111/j.1471-4159.2005.03233.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer-Alcón M, La Harpe R, Guimón J, García-Sevilla JA. Downregulation of neuronal cdk5/p35 in opioid addicts and opiate-treated rats:relation to neurofilament phosphorylation. Neuropsychopharmacology. 2003;28:947–955. doi: 10.1038/sj.npp.1300095. [DOI] [PubMed] [Google Scholar]
- 17.Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann N Y Acad Sci. 2004;1025:335–344. doi: 10.1196/annals.1316.041. [DOI] [PubMed] [Google Scholar]
- 18.Xie WY, He Y, Yang YR, Li YF, Kang K, Xing BM, Wang Y. Disruption of Cdk5 associated phosphorylation of residue threonine-161 of the delta-opioid receptor: impaired receptor function and attenuated morphine antinociceptive tolerance. J Neurosci. 2009;29:3551–3564. doi: 10.1523/JNEUROSCI.0415-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard LA, Miksys S, Hoffmann E, Mash D, Tyndale RF. Brain CYP2E1 is induced by nicotine and ethanol in rat and is higher in smokers and alcoholics. Br J Pharmacol. 2003;138:1376–1386. doi: 10.1038/sj.bjp.0705146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Liu C, Zhang X, Han J. Acupuncture improved cognitive impairment caused by multi-infarct dementia in rats. Physiol Behav. 2005;86:434–41. doi: 10.1016/j.physbeh.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Guo M, Zhang G, Xu X, Li Q. Neuroprotection of nicotiflorin in permanent focal cerebral ischemia and in neuronal cultures. Biol Pharm Bull. 2006;29:1868–1872. doi: 10.1248/bpb.29.1868. [DOI] [PubMed] [Google Scholar]
- 23.Pareek TK, Lam E, Zheng X, Askew D, Kulkarni AB, Chance MR, Huang AY, Cooke KR, Letterio JJ. Cyclin-dependent kinase 5 activity is required for T cell activation and induction of experimental autoimmune encephalomyelitis. J Exp Med. 2010;207:2507–2519. doi: 10.1084/jem.20100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown TG, Ouimet MC, Nadeau L, Gianoulakis C, Lepage M, Tremblay J, Dongier M. From the brain to bad behaviour and back again: neurocognitive and psychobiological mechanisms of driving while impaired by alcohol. Drug Alcohol Rev. 2009;28:406–418. doi: 10.1111/j.1465-3362.2009.00053.x. [DOI] [PubMed] [Google Scholar]
- 25.Burke CJ, Tobler PN, Baddeley M, Schultz W. Neural mechanisms of observational learning. Proc Natl Acad Sci U S A. 2010;107:14431–14436. doi: 10.1073/pnas.1003111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33:945–969. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen F, Lawrence AJ, Liang JH. Advanced in search for central neurotransmitters involved in alcoholism and addiction. Chin J Drug Depend. 2007;16:5–12. [Google Scholar]
- 28.de Licona HK, Karacay B, Mahoney J, McDonald E, Luang T, Bonthius DJ. A single exposure to alcohol during brain development induces microencephaly and neuronal losses in genetically susceptible mice, but not in wild type mice. Neurotoxicology. 2009;30:459–470. doi: 10.1016/j.neuro.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Dhariwala FA, Rajadhyaksha MS. An unusual member of the Cdk family: Cdk5. Cell Mol Neurobiol. 2008;28:351–369. doi: 10.1007/s10571-007-9242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhavan R, Greer PL, Morabito MA, Orlando LR, Tsai LH. The cyclin-dependent kinase 5 activators p35 and p39 interact with the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II and alpha-actinin-1 in a calcium-dependent manner. J Neurosci. 2002;22:7879–7891. doi: 10.1523/JNEUROSCI.22-18-07879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes JP, Agostinho P. Cdk5: multitasking between physiological and pathological conditions. Prog Neurobiol. 2011;94:49–63. doi: 10.1016/j.pneurobio.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Cheung ZH, Ip NY. Cdk5: a multifaceted kinase in neurodegenerative diseases. Trends Cell Biol. 2012;22:169–175. doi: 10.1016/j.tcb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Regulation of contextual fear conditioning by baseline and inducible septo-hippocampal cyclin-dependent kinase 5. Neuropharmacology. 2003;44:1089–1099. doi: 10.1016/s0028-3908(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Veeranna , Ohshima T, Rajan P, Amin ND, Cho A, Sreenath T, Pant HC, Brady RO, Kulkarni AB. Neuronal cyclin-dependent kinase 5 activity is critical for survival. J Neurosci. 2001;21:550–558. doi: 10.1523/JNEUROSCI.21-02-00550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clay SW, Allen J, Parran T. A review of addiction. Postgrad Med. 2008;120:E01–7. doi: 10.3810/pgm.2008.07.1802. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Xu CY, Jiang HB, Hao W, Zhang RL. (Effect of alcohol exposure during pregnancy on learning and memory and expression of cdk5 in the hippocampus of infant rats) Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2012;28:13–4. [PubMed] [Google Scholar]
- 37.Benson C, White J, De Bono J, O’Donnell A, Raynaud F, Cruickshank C, McGrath H, Walton M, Workman P, Kaye S, Cassidy J, Gianella-Borradori A, Judson I, Twelves C. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; RRoscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. doi: 10.1038/sj.bjc.6603509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng XJ, Sun SG, Mei YW. The research of polydafin protection on cerebral ischemia neurous. Modern Rehabilitation. 2001;5:52–53. [Google Scholar]
- 39.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induced cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]


