Abstract
Objective: This study was to investigate the efficacy of olfactory ensheathing cell (OEC) transplantation on experimental autoimmune encephalomyelitis (EAE). Methods: EAE models were established by guinea pig spinal cord homogenate (GPSCH) immunization in Lewis rats. OECs were purified and cultured from the olfactory nerve layer of SD rats, and then transplanted to the EAE models through the vena caudalis (Group A) or into the lateral cerebral ventricle (Group B). Neurological function scores and body weights were daily recorded following transplantation, and histological analysis was performed to assess the pathological changes in EAE rats. Results: Cultured cells mainly exhibited bipolar or tripolar morphology, and the majority of these cells were positive for NGFR p75 staining. Neurological function scoring and the body weight measurement showed that, OEC transplantation could significantly improve the performance of EAE rats, and similar results were observed for the transplantation through the vena caudalis and into the lateral cerebral ventricle. Moreover, the transplanted OECs accumulated to the lesions in the brains of EAE rats, in spite of the different transplantation approaches. However, no significant differences in histopathology (HE and LFB staining) were observed between the OEC-transplanted groups and the control group. Conclusion: OEC transplantation could exert beneficial effects in the treatment of EAE, no matter which the cells were transplanted through the vena caudalis or into the lateral cerebral ventricle. Our findings might provide evidence for the clinical treatment of multiple sclerosis with cell transplantation.
Keywords: Multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE), olfactory ensheathing cells (OECs), cell transplantation
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS), characterized by neurological disturbance with complete or incomplete remission, eventually resulting in permanent impairments [1,2]. The pathogenesis of the disease is still unknown, which might be related to immunity, viral infection, and genetic and environmental factors [3-5]. Experimental autoimmune encephalomyelitis (EAE) is an inflammatory condition of the CNS, with similarities to MS, which has been widely used as a disease model [6-10].
In the pathogenesis of both MS and EAE, demyelination is regarded as one of the most characteristic pathological features. The neurological dysfunction is mainly caused by demyelination, as well as axonal damages. Therefore, numerous experimental and clinical studies of MS and EAE have focused on remyelination and axonal growth promotion, trying to alleviate the clinical symptoms and reduce the disease progression [11,12]. In recent years, transplantation of myelin-forming precursor cells or stem cells to the injury site is attracting a lot of attention for the treatment of CNS disorders [13,14]. However, due to the multifocal nature of MS and EAE and the restricted cell migration within demyelinated plaques, there are still serious challenges that the cell transplantation therapy needs to overcome.
Olfactory ensheathing cells (OECs) are the only specialized glial cells that exist in both the central and peripheral nervous systems [15-17]. OECs are mainly distributed in the olfactory bulb and olfactory mucosa, which support neuronal growth, promote axon extension, and myelinate axons [17-20]. Moreover, OECs can secrete a variety of neurotrophic factors, providing an environment for nerve regeneration and repair [21-24]. Furthermore, OECs possess strong migration ability, which all together make the cells a promising candidate for the cell transplantation treatment of demyelinating diseases like MS. In this study, the therapeutic effects of OEC transplantation through different approaches on EAE rats were investigated and analyzed.
Materials and methods
Isolation and purification of OECs
OECs were purified and cultured according to a previously published method with minor modifications [25]. Briefly, the olfactory nerve layer (ONL) was removed apart from the glomerular and the deeper layers of the olfactory bulb. The ONL tissues were digested with a solution containing 0.25% trypsin (Gibco, Grand Island, NY, USA) and 0.02% EDTA (Gibco) at 37°C for 15 min. The trypsinization was stopped by adding the complete medium consisting of 90% DME/F12 and 10% fetal bovine serum (FBS). After centrifugation at 1000 rpm for 5 min, the cells were re-suspended with fresh complete medium, and then cultured in a 37°C, 5% CO2 incubator. After purification for 18 h, these cells were cultured with complete medium in 37°C, 5% CO2 atmosphere for 11 d. Culture medium was changed every 2-3 d [26].
Immunofluorescent staining
Cultured cells were identified with immunofluorescent staining. These cells were fixed with 4% paraformaldehyde, and then incubated with rabbit anti-mouse anti-nerve growth factor receptor (NGFR) p75 antibody (1:100 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight. Subsequently, the cells were incubated with FITC-labeled second antibody in dark at room temperature for 15 min. The cells were counterstained with propidium iodide (PI), and then observed under a microscope.
EAE induction
Female Lewis rats (6-8-w old) and guinea pigs (6-8-w old) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd (Beijing, China). To induce EAE, guinea pigs were first sacrificed to obtain spinal cord homogenate (GPSCH). Female Lewis rats were immunized with 0.4 mL antigen emulsion (GPSCH emulsified with incomplete Freund’s adjuvant, containing 4 mg/mL Mycobacterium tuberculosis strain H37Rv) [7,9]. At 0 h and 48 h after immunization with antigen emulsion, these rats were subjected to intravenous administration of 400 ng pertussis toxoid (Sigma-Aldrich, St. Louis, MO, USA). All animal experimental procedures were approved by the Animal Ethical Committee of the First Affiliated Hospital of Wenzhou Medical University.
Cell transplantation
OECs used for transplantation were pre-labeled with Hoechst 33342 (Sigma-Aldrich). Briefly, OECs were harvested and incubated with 10 µg/mL Hoechst 33342 at 37°C for 40 min. After washed with PBS, these cells were cultured with fresh complete medium until transplantation.
EAE rats were transplanted with on days 12-14 after immunization. For the vena caudalis injection (Group A), rats received 1 × 106 Hoechst 33342-labeled OECs in 1 mL PBS through the vena caudalis. For the intracerebroventricular injection (Group B), Hoechst 33342-labeled OECs were transplanted into the lateral cerebral ventricle of the rats. Group C was used as the control, where rats were subjected to intravenous injection of an equal volume of PBS through the vena caudalis. Neurological function scores and body weights were recorded daily according to a standard and validated scale (0 to 5) [27]. The onset and incidence of the disease, and the maximum scores, were recorded for each rat. Cumulative disease scores were calculated by summing up the daily-recorded neurologic function scores of each rat over the whole period of observation.
Histological analysis
At 2 w after cell transplantation, all rats were deeply anesthetized and killed. Brains and spinal cords were removed, and the tissues were divided into two parts. One part was used to investigate the distribution of OECs with immunofluorescence. The other part was used for the hematoxylin and eosin (HE) staining and the Luxol fast blue (LFB) staining to evaluate perivascular inflammatory infiltrates and demyelination, respectively. The tissues used for immunofluorescence was frozen in liquid nitrogen, and then cut into 10-µm sections. For HE and LFB staining, tissues were fixed in 4% paraformaldehyde overnight, and then embedded in paraffin. The tissues were then cut into 6-μm sections, and stained with HE and LFB, respectively. Inflammation and demyelination in EAE rats were scored according to the system described by Storch et al. [9]. For inflammation: 0, no inflammatory cells; 1, leptomeningeal and adjacent subpial cell infiltration; 2, mild perivascular cuffing; 3, extensive perivascular cuffing; 4, extensive perivascular cuffing and severe parenchymal cell infiltration. For demyelination: 0, no demyelination; 1, trace of perivascular or subpial demyelination; 2, focal demyelination; 3, demyelination involving a quarter of tissues examined, i.e., the spinal tract, brain stem, cerebellar white matter, or optic tract; 4, massive confluent demyelination involving half of the tissue; 5, extensive demyelination involving the entire tissues.
Statistical analysis
Data were expressed as mean ± SD. SPSS 19.0 software was used for statistical analysis. One-way ANOVA was performed to compare the differences between groups. P < 0.05 was considered statistically significant.
Results
OEC identification and labeling
Cultured cells were identified by immunofluorescent staining. Our results showed that the cultured cells mainly exhibited bipolar or tripolar morphology. Importantly, majority of these cells were positive staining for NGFR p75, a marker for OECs (Figure 1A). Before transplantation, OECs were pre-labeled with Hoechst 33342. Our results showed that 80-90% of the OECs could be successfully labeled. Moreover, the density of OECs could achieve 1 × 106 cells/mL. These results suggest that our cultured cells were mainly OECs with high purity, which were suitable for the following transplantation treatment.
Figure 1.
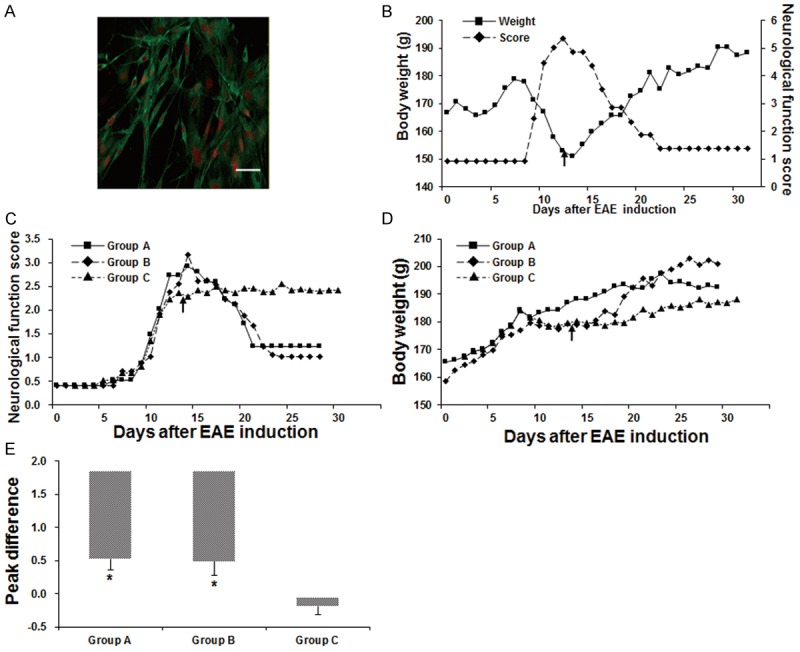
Effects of OEC transplantation on EAE rats. (A) Cultured OECs were identified by immunofluorescence staining with NGFR p75 (green) and PI (red). Scale bar, 50 μm. (B-E) Analysis of the neurological function scores and body weights of EAE rats. (B) A representative record of the neurological function scores and body weights of an EAE rat in Group A. (C, D) Comparison of the neurological function scores (C) and body weights (D) between Groups A-C. Arrows indicated the OEC transplantation time point. (E) The peak difference (PD) in the neurological function scores between pre-transplantation and post-transplantation in each group. Compared with the control group, *P < 0.05.
Effects of OEC transplantation on EAE rats
The effects of OEC transplantation on EAE rats were next investigated and compared between different transplantation methods (i.e., the vena caudalis injection, Group A, and the intracerebroventricular injection, Group B). Our observation showed that, starting from day 5 after cell transplantation, compared with the control group (Group C), the neurological function scores were dramatically lower for the EAE rats in both Groups A and B, indicating clinical improvement in these rats (Figure 1B-D). The symptoms of EAE rats in Group C did not show any alleviation over the whole observation period. However, in Group A, the EAE rats’ performance began to markedly improve on days 2-3 after OEC transplantation (Figure 1B-D). Particularly, one EAE rat model in Group A was completely paralyzed in the hind legs and tail, which could not crawl or walk before treatment. After cell transplantation, the rat was able to lurch around and swing the body just on day 2 after cell transplantation, and could climb up the cage walls on day 4 after cell transplantation. For this rat in Group A, the neurological function score began to decline, and the body weight began to rise, on day 3 after cell transplantation (Figure 1B). On the other hand, in Group B, the tail and hind limb weakness began to improve following the cell transplantation. The neurological function score declined and the body weight rebound on days 4-5 after cell transplantation (Figure 1C, 1D).
In addition, the peak differences (PD) in the neurological function scores of each rat between pre-transplantation and post-transplantation in Groups A and B were significantly higher than that in Group C (P < 0.01). However, no significant difference was observed between Group A and Group B (P > 0.05; Figure 1E). These results suggest that OEC transplantation could significantly improve the performance of EAE rats, and similar results were observed for the transplantation through the vena caudalis and into the lateral cerebral ventricle.
Distribution of OECs in brains of transplanted rats
To investigate the distribution of transplanted OECs in the brains of EAE rats, the cells were pre-labeled with Hoechst 33342, and the frozen sections of the brain tissues were detected with fluorescence microscopy. Our results showed that, in Group A, there were plenty of Hoechst-labeled cells around the inflammatory lesions (perivascular cuffing) and near the subpial region (Figure 2A, 2B). On the other hand, in Group B, implanted OECs migrated from the injection site to the lesions, gathering around the lesions (Figure 2C-E). These results suggest that transplanted OECs could accumulate to the lesions in the brains of EAE rats, in spite of different transplantation approaches.
Figure 2.
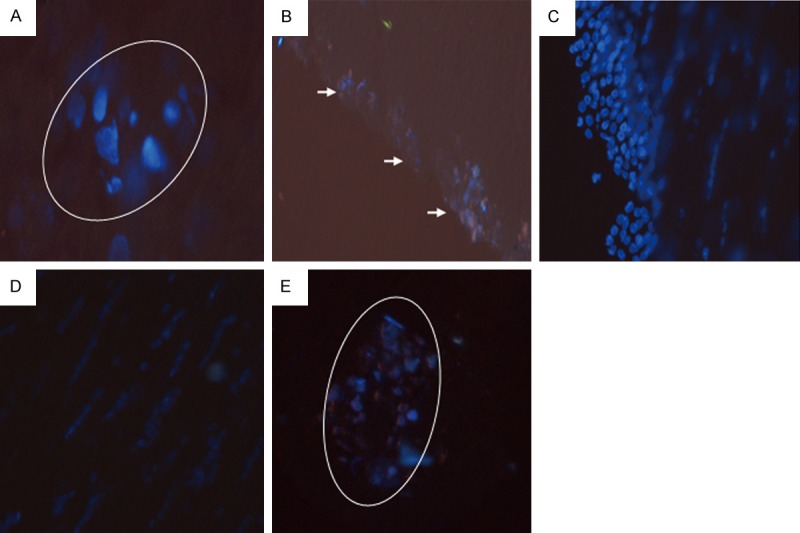
Distribution of OECs in the brains of transplanted rats. The OECs cells were pre-labeled with Hoechst 33342, and then transplanted into the EAE rats, into the lateral cerebral ventricle (Group A; A, B) or through the vena caudalis (Group B; C-E). The distribution of OECs in the frozen brain sections were detected with fluorescence microscopy. (A, B) In Group A, Hoechst-labeled cells accumulated around the inflammatory lesions (A: × 100; perivascular cuffing, indicated by the white circle) and near the subpial region (B: × 40; indicated by arrows). (C-E) In Group B, implanted OECs migrated from the injection site to the lesions (C, D: × 40), and some gathered around the lesions (E: × 40; indicated by the white circle).
Histopathological analysis of EAE rats transplanted with OECs
To investigate the histopathological changes in the EAE rats, the brain sections were subjected to HE and LFB staining, before and after OEC transplantation. HE staining showed significant inflammatory infiltration in all Groups A-C, with no significant differences between these groups. The inflammatory cells gathered around the lesions, and typical perivascular cuffing was observed in these EAE rats (typical results from Group A were shown in Figure 3A). On the other hand, LFB staining indicated substantial demyelination around the inflammatory lesions in all the three groups (typical results from Group B were shown in Figure 3B), without significant difference in the pathological scores (P > 0.05) (data not shown).
Figure 3.
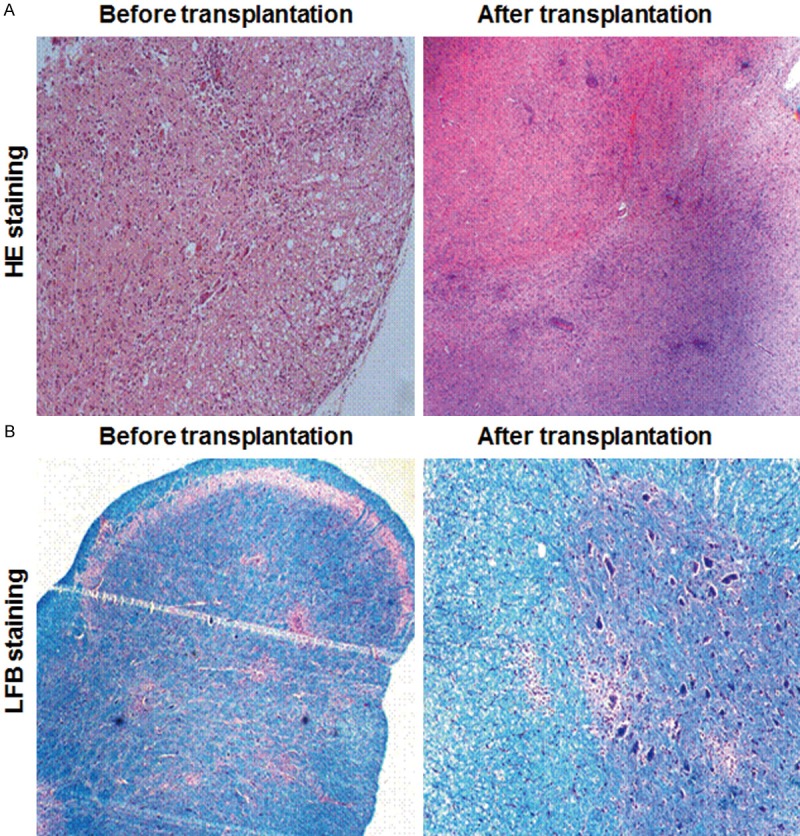
Histopathological analysis of EAE rats transplanted with OECs. The brain sections of EAE rats in Group A and B were subjected to HE (A) and LFB (B) staining, respectively, before and after OEC transplantation.
Discussion
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the CNS, characterized by the morphological hallmarks of inflammation, gliosis, demyelination, and loss of neuronal axons and oligodendrocytes [4,5]. In MS and EAE, inflammation can be completely prevented, while demyelination is still one of the major challenges for the disease treatment. Studies have shown that the complex mechanisms of demyelination and remyelination in the pathogenesis of MS and EAE are responsible for the pathological heterogeneity of the diseases [28,29]. Remyelination could help to preserve axons, restore conduction velocity, and ameliorate the symptoms of MS. Even though naturally occurring remyelination is impaired in MS lesions, remyelination could be achieved by either promoting endogenous repair mechanisms or providing exogenous myelinating cells via transplantation. Current therapies for MS are essentially promoting the CNS repair, including the application of growth factors, the intravenous administration of autoantibodies [3,30-32], and the transplantation of myelinating cells [3,31]. Many myelinating cells, such as Schwann cells, astrocytes, microglia, macrophages, and fibroblasts, have been used to repair injured spinal cords [33]. However, these cells were not as effective as olfactory ensheathing cells (OECs). It has been shown that OEC grafts could promote neural protection, axonal regeneration, and remyelination in the injured spinal cord [24,34]. The environment of the injury site in MS and EAS is really complex, containing reactive astrocytes, fibroblasts, immune cells, and various inhibitory factors and cytokines. OECs have been shown to express and secret cell surface molecules such as N-CAM, N-cadherin, and laminin, and produce growth factors like neuregulins and neurotrophins [21,35]. All these features of OECs make the cells an ideal candidate for the transplantation treatment of MS and EAE.
Our results showed that the clinical symptoms were dramatically alleviated in the EAE rats in Groups A and B, as indicated by the decreased neurological function scores and the changes in body weight. However, no significant differences were observed in the changes of body weight between the transplantation group and the control group. This phenomenon might be due to the experimental settings, where paralytic rats could also obtain the food and water even with paralyzed hind legs. The conclusion that implanted OECs could, at least partly, promote the recovery of EAE was mainly based on the clinical observation, rather than the histopathological findings, which may provide evidence for the clinical treatment of MS.
On the other hand, the EAE rats from Groups A and B showed persistent recovery, even though without significant differences in the peak difference between these groups. Moreover, fluorescence detection revealed Hoechst-labeled OECs around the lesions or near the subpial regions in the brains of EAE rats. Based on these results, considering the multifocal feature of the disease and the migration capability of OECs, vein transplantation of OECs might be a promising treatment method for MS and EAE. No significant differences in histopathology (HE and LFB staining) were observed between the OEC-transplanted groups and the control group, which might be due to the fact that OECs could not affect the inflammatory infiltration. Moreover, the limited post-transplantation observation period might be another reason for the phenomenon. In our study, the peak incidence of EAE was chose for the cell transplantation, and the efficacies of the treatment have been confirmed by our results.
In conclusion, our results showed that OEC transplantation could significantly improve the performance of EAE rats. Moreover, the transplanted OECs could accumulate to the lesions in the rat brains. Similar results were observed for transplantation through the vena caudalis and into the lateral cerebral ventricle. OEC transplantation could exert beneficial effects in the treatment of EAE, whether the cells were transplanted through the vena caudalis or into the lateral cerebral ventricle. Our findings might provide evidence for the clinical treatment of MS with cell transplantation.
Acknowledgements
This work was supported by Natural Science Foundation of Zhejiang Province (Grant No. LY13H090010).
Disclosure of conflict of interest
None.
References
- 1.Frisullo G, Nociti V, Iorio R, Patanella AK, Marti A, Caggiula M, Mirabella M, Tonali PA, Batocchi AP. IL17 and IFNgamma production by peripheral blood mononuclear cells from clinically isolated syndrome to secondary progressive multiple sclerosis. Cytokine. 2008;44:22–25. doi: 10.1016/j.cyto.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Duffy SS, Lees JG, Moalem-Taylor G. The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int. 2014;2014:285245. doi: 10.1155/2014/285245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stangel M, Hartung HP. Remyelinating strategies for the treatment of multiple sclerosis. Prog Neurobiol. 2002;68:361–376. doi: 10.1016/s0301-0082(02)00105-3. [DOI] [PubMed] [Google Scholar]
- 4.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 5.Pryce G, Baker D. Emerging properties of cannabinoid medicines in management of multiple sclerosis. Trends Neurosci. 2005;28:272–276. doi: 10.1016/j.tins.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Berger T, Weerth S, Kojima K, Linington C, Wekerle H, Lassmann H. Experimental autoimmune encephalomyelitis: the antigen specificity of T lymphocytes determines the topography of lesions in the central and peripheral nervous system. Lab Invest. 1997;76:355–364. [PubMed] [Google Scholar]
- 7.Gold R, Hartung HP, Toyka KV. Animal models for autoimmune demyelinating disorders of the nervous system. Mol Med Today. 2000;6:88–91. doi: 10.1016/s1357-4310(99)01639-1. [DOI] [PubMed] [Google Scholar]
- 8.Johns TG, Kerlero de Rosbo N, Menon KK, Abo S, Gonzales MF, Bernard CC. Myelin oligodendrocyte glycoprotein induces a demyelinating encephalomyelitis resembling multiple sclerosis. J Immunol. 1995;154:5536–5541. [PubMed] [Google Scholar]
- 9.Storch MK, Stefferl A, Brehm U, Weissert R, Wallstrom E, Kerschensteiner M, Olsson T, Linington C, Lassmann H. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681–694. doi: 10.1111/j.1750-3639.1998.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Feron F, Ho SM, Mackay-Sim A, Waite PM. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Res. 2001;889:344–357. doi: 10.1016/s0006-8993(00)03235-2. [DOI] [PubMed] [Google Scholar]
- 11.Aharoni R. Immunomodulation neuroprotection and remyelination - the fundamental therapeutic effects of glatiramer acetate: a critical review. J Autoimmun. 2014;54:81–92. doi: 10.1016/j.jaut.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Ellwardt E, Zipp F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol. 2014;262 Pt A:8–17. doi: 10.1016/j.expneurol.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Lassmann H. Stem cell and progenitor cell transplantation in multiple sclerosis: the discrepancy between neurobiological attraction and clinical feasibility. J Neurol Sci. 2005;233:83–86. doi: 10.1016/j.jns.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Pluchino S, Martino G. The therapeutic use of stem cells for myelin repair in autoimmune demyelinating disorders. J Neurol Sci. 2005;233:117–119. doi: 10.1016/j.jns.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Doucette JR. The glial cells in the nerve fiber layer of the rat olfactory bulb. Anat Rec. 1984;210:385–391. doi: 10.1002/ar.1092100214. [DOI] [PubMed] [Google Scholar]
- 16.Doucette R. PNS-CNS transitional zone of the first cranial nerve. J Comp Neurol. 1991;312:451–466. doi: 10.1002/cne.903120311. [DOI] [PubMed] [Google Scholar]
- 17.Doucette R. Glial influences on axonal growth in the primary olfactory system. Glia. 1990;3:433–449. doi: 10.1002/glia.440030602. [DOI] [PubMed] [Google Scholar]
- 18.Devon R, Doucette R. Olfactory ensheathing cells myelinate dorsal root ganglion neurites. Brain Res. 1992;589:175–179. doi: 10.1016/0006-8993(92)91182-e. [DOI] [PubMed] [Google Scholar]
- 19.Ramon-Cueto A, Nieto-Sampedro M. Glial cells from adult rat olfactory bulb: immunocytochemical properties of pure cultures of ensheathing cells. Neuroscience. 1992;47:213–220. doi: 10.1016/0306-4522(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 20.Goodman MN, Silver J, Jacobberger JW. Establishment and neurite outgrowth properties of neonatal and adult rat olfactory bulb glial cell lines. Brain Res. 1993;619:199–213. doi: 10.1016/0006-8993(93)91613-w. [DOI] [PubMed] [Google Scholar]
- 21.Miragall F, Kadmon G, Schachner M. Expression of L1 and N-CAM cell adhesion molecules during development of the mouse olfactory system. Dev Biol. 1989;135:272–286. doi: 10.1016/0012-1606(89)90179-6. [DOI] [PubMed] [Google Scholar]
- 22.Scotti AL, Hoffmann MC, Nitsch C. The neurite growth promoting protease nexin 1 in glial cells of the olfactory bulb of the gerbil: an ultrastructural study. Cell Tissue Res. 1994;278:409–413. doi: 10.1007/BF00414183. [DOI] [PubMed] [Google Scholar]
- 23.Ubink R, Halasz N, Zhang X, Dagerlind A, Hökfelt T. Neuropeptide tyrosine is expressed in ensheathing cells around the olfactory nerves in the rat olfactory bulb. Neuroscience. 1994;60:709–726. doi: 10.1016/0306-4522(94)90499-5. [DOI] [PubMed] [Google Scholar]
- 24.Ramon-Cueto A, Valverde F. Olfactory bulb ensheathing glia: a unique cell type with axonal growth-promoting properties. Glia. 1995;14:163–173. doi: 10.1002/glia.440140302. [DOI] [PubMed] [Google Scholar]
- 25.Nash HH, Borke RC, Anders JJ. New method of purification for establishing primary cultures of ensheathing cells from the adult olfactory bulb. Glia. 2001;34:81–87. doi: 10.1002/glia.1043. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Zhang X. The purification methods of olfactory ensheathing cells for transplantation. Chin J Neuroimmunol Neurol. 2008;16:325–329. [Google Scholar]
- 27.Kono DH, Urban JL, Horvath SJ, Ando DG, Saavedra RA, Hood L. Two minor determinants of myelin basic protein induce experimental allergic encephalomyelitis in SJL/J mice. J Exp Med. 1988;168:213–227. doi: 10.1084/jem.168.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR. Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia. 2014;62:452–467. doi: 10.1002/glia.22616. [DOI] [PubMed] [Google Scholar]
- 29.Olsen JA, Akirav EM. Remyelination in multiple sclerosis: cellular mechanisms and novel therapeutic approaches. J Neurosci Res. 2015;93:687–696. doi: 10.1002/jnr.23493. [DOI] [PubMed] [Google Scholar]
- 30.Wingerchuk DM. Current evidence and therapeutic strategies for multiple sclerosis. Semin Neurol. 2008;28:56–68. doi: 10.1055/s-2007-1019128. [DOI] [PubMed] [Google Scholar]
- 31.Payne N, Siatskas C, Bernard CC. The promise of stem cell and regenerative therapies for multiple sclerosis. J Autoimmun. 2008;31:288–294. doi: 10.1016/j.jaut.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Munger KL, Reindl M, O’Reilly EJ, Levin LI, Berger T, Ascherio A. Myelin oligodendrocyte glycoprotein antibodies and multiple sclerosis in healthy young adults. Neurology. 2008;71:1142–1146. doi: 10.1212/01.wnl.0000316195.52001.e1. [DOI] [PubMed] [Google Scholar]
- 33.Duncan ID. Replacing cells in multiple sclerosis. J Neurol Sci. 2008;265:89–92. doi: 10.1016/j.jns.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 34.Ramon-Cueto A. Olfactory ensheathing glia transplantation into the injured spinal cord. Prog Brain Res. 2000;128:265–272. doi: 10.1016/S0079-6123(00)28024-2. [DOI] [PubMed] [Google Scholar]
- 35.Ramon-Cueto A, Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp Neurol. 1994;127:232–244. doi: 10.1006/exnr.1994.1099. [DOI] [PubMed] [Google Scholar]


