Abstract
Purpose: KLF17 belongs to the Sp/KLF zinc-finger protein family as a regulator in tumor development. However, its expression and biologic function has remained unclear in EC. Methods: The esophageal carcinoma tissue samples and adjacent normal tissues were obtained from the Second Hospital of Hebei Medical University. Immunohistochemistry, Western blot, and transfection were applied to evaluate the expression and clinical significance of KLF17 in esophageal cancer. Results: In this study, we showed that KLF17 was overexpressed in esophageal normal samples compared to the cancer. Moreover, KLF17 was upregulated at lymph node non-metastatic cancer tissues when compared to metastatic cancer tissues. KLF17 overexpression decreased EC cell proliferation, migration and invasion ability. In contrast, the knockdown of KLF17 increased EC cell proliferation, migration and invasion ability. Conclusion: These results suggest that KLF17 inhibits tumor development and may serve as a potential therapeutic target.
Keywords: Esophageal cancer, KLF17, migration, invasion
Introduction
Esophageal cancer (EC) is a highly aggressive digestive tract tumor. Esophageal cancer is the sixth leading cause of cancer-related death worldwide and one of the five deadliest cancers in China [1,2]. Furthermore, the incidence of EC has been increasing in recent decades. Despite improvement in treatment of EC, the survival rates are still far from satisfactory. Therefore, identification of specific targets to prevention and treatment of cancer patients is important.
KLF17 is one member of the Sp/KLF zinc-finger protein family. This group plays a key role in various cell processes including tumor development [3,4]. KLF17 as a new tumor suppressor regulates the tumor progression [5]. KLF17 overexpression overcomes increased breast cancer cell invasion by DJ-1 [6]. KLF17 suppressed tumor growth and metastasis regulation of TGF-β/S mad signaling during cancer progression [7]. However, little is known about the significance of KLF17 expression in human esophageal cancer.
In the present study, we tried to evaluate the expression and clinical significance of KLF17 in esophageal cancer.
Materials and methods
Patients and tissue samples
The esophageal carcinoma tissue samples and adjacent normal tissues were obtained from the Second Hospital of Hebei Medical University. This study was approved by the ethical committees of the Second Hospital of Hebei Medical and informed content was obtained from all the patients.
Immunohistochemistry
Paraffin sections were deparaffinized with xylene and rehydrated in graded ethanol solutions. Activity of endogenous peroxidase was blocked with 3% H2O2 for 15 min at room temperature and then the sections were heated with 0.01 M citrate (pH = 6.0) at 95°C for 15 min in microwave for antigen retrieval. The anti-KLF17 rabbit polyclonal antibody (AbcamInc, dilution 1:200) was incubated for 2 h at room temperature. The sections were incubated with HRP-labeled anti-rabbit IgG secondary antibody.
Western blotting
Total proteins were extracted from frozen esophageal cancer tissues and cells using RIPA lysis buffer and quantified using Thermo Pierce BCA Protein Assay kit according to the manufacturer’s instructions. Equal quantities of protein were loaded and electrophoresed in a 10% SDS-PAGE gel, which was then transferred to nitrocellulose (NC) membrane. The membrane was incubated in 5% fat-free milk solution for 2 h at room temperature followed by incubation at 4°C with a rabbit anti-human KLF17 polyclonal antibody (Abcam, dilution 1:1000) or GAPDH antibody (Cell Signaling Technology, 1:1000 dilution). The membrane was washed three times for 10 min in PBS with 0.1% Tween-20 and then incubated for 1 h with HRP-conjugated bovine anti-rabbit (1:5,000 dilutions) secondary antibody (Boster Biological Technology, Wuhan, China) at room temperature. The immumoreactive proteins were then detected using ECL substrate following manufacturer’s recommendation. GAPDH was used as an endogenous protein for normalization.
Cell culture and transfection
The human esophageal carcinoma cell lines KYSE150 was purchased from the American Type Culture Collection (Manassas, VA). The cells were cultured in RPMI-1640 (Invitrogen, Carlsbad, CA) medium containing 10% fetal bovine serum (FBS, GIBCO), 100 IU/ml penicillin and 100 mg/ml streptomycin maintained at 37°C in humidified air containing 5% CO2.
For transfection, cells were cultured to 80% confluence and transfected with recombinant eukaryotic vector or siRNA using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s recommendation.
KLF17 recombinant eukaryotic vector plasmids were described in our previous study [8].
Proliferation assay
MTT assay was used to analyze cell proliferation. Cells were seeded into 96-well plate at 5.0×103 cells/ml and cultured. At each time point, 10 µl MTT (5 mg/ml, Sigma) was added to each well, successive incubated for 4 h at 37°C. The supernatant was removed and 200 µl DMSO (Invitrogen) was added to dissolve the formazan crystals for 30 min. Spectrometric absorbance at a wavelength of 490 nm was measured on microplate reader. Each sample was tested in triplicate and all experiments were performed three times.
Monolayer wound healing assay
Cells were grown in 10% FBS medium on 60 mm plates. After the cells reached sub-confluence, the cells were wounded by scraping the monolayer and grown in medium for 24 h. The width of the wound was measured at different time points. The locations were visualized and photographed under a phase-contrast inverted microscope (40× objective, Leica, Solms, Germany).
Transwell invasion assay
Cell invasion assays were performed using 24-well transwells (8 mm pore size, Corning Life Sciences) coated with matrigel (1 mg/ml, BD Sciences). Cells (104/well) were seeded in the upper chambers of the wells in 200 μl FBS-free medium, and the lower chambers were filled with 500 μl 10% FBS medium to induce cell migration. Following incubation for 24 h, the cells on the filter surface were fixed with 4% formaldehyde, stained with 0.5% crystal violet, and examined under a microscope. Cells in at least six random microscopic fields (200×) were counted.
Statistical analysis
Data are expressed as mean ± SEM. The difference among groups was determined by ANOVA analysis and comparison between two groups was analyzed by the Student’s t-test using GraphPad Prism software version 4.0 (GraphPad Software, Inc., San Diego, CA). A value of P < 0.05 was considered statistically significant.
Results
Expression of KLF17 in EC tissue samples
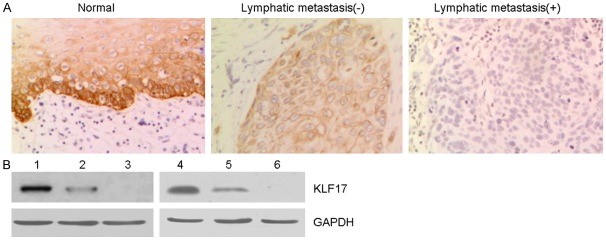
To assess the potential role of KLF17 in esophageal cancer (EC), we examined expression of RFT2 in 60 human EC samples and paired normal esophageal tissues using Immunohistochemistry. As shown in Figure 1, KLF17 was overexpressed in esophageal normal samples compared to the cancer tissues especially for lymph node metastatic cancer tissues. Although there was no significant difference for KLF17 expression levels grouped by age, gender, or, it was notable that KLF17 was upregulated at lymph node non-metastatic cancer tissues when compared to metastatic cancer tissues (Table 1), suggesting that KLF17 may be important during EC progression.
Figure 1.
The expressed of KLF17 in EC tissue samples. A. Immunohistochemical staining of KLF17 expression was observed in normal esophageal and cancer tissues (non-metastatic and metastatic esophageal cancer). (magnification ×200). B. Western blotting analysis of KLF17 expression in esophageal cancer tissues and normal tissues. GAPDH was used as the internal control.
Table 1.
Correlation of KLF17 expression with clinical and pathological features in 50 patients
| Features | Number (n=50) | KLF17 expression | P | |
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Gender | ||||
| Male | 28 | 21 | 7 | 0.3841 |
| Female | 22 | 14 | 8 | |
| Age | ||||
| <50 | 24 | 18 | 6 | 0.4585 |
| >50 | 26 | 17 | 9 | |
| Tumor size (cm) | ||||
| <5 | 15 | 11 | 4 | 0.7363 |
| >5 | 35 | 24 | 11 | |
| TNM stage | ||||
| I-II | 23 | 17 | 6 | 0.5773 |
| III-IV | 27 | 18 | 9 | |
| Lymphatic metastasis | ||||
| No | 22 | 12 | 10 | 0.0345 |
| Yes | 28 | 23 | 5 | |
Next we tested the expression of KLF17 protein in EC and normal tissues by western blotting analysis (Figure 1B). Overexpression of KLF17 was also observed in normal tissues and lest in metastatic cancer tissues. We confirm the results of Immunohistochemistry again. Taken together, these results indicated that KLF17 was low expressed in EC especially in metastatic tissues and may play an important role in the development of EC.
Effects of KLF17 overexpression and knockdown on cell proliferation
To investigate the role of KLF17 in EC cells, we overexpressed KLF17 in KYSE150 cells which were transfected with KLF17 expression vector. Overexpression of KLF17 in KYSE150 cells was confirmed by immunoblotting (Figure 2A). We found that overexpression of KLF17 in KYSE150 cells inhibited the cell proliferation by MTT assay (Figure 2B). To further validate the proliferation-suppressing activity of KLF17, we silenced the expression of KLF17 in EC cells with the specific small interference RNA targeting to KLF17. The knockdown of KLF17 in KYSE150 cells were confirmed by Western blot (Figure 2C). Knockdown of KLF17 expression in KYSE150 cells also resulted in significant promotion of cell proliferation. Growth curve analysis demonstrated that growth of cells ablating KLF17 was significantly increased compared with control cells (Figure 2D). Together, these results indicated that KLF17 was required for cell proliferation in EC cells.
Figure 2.
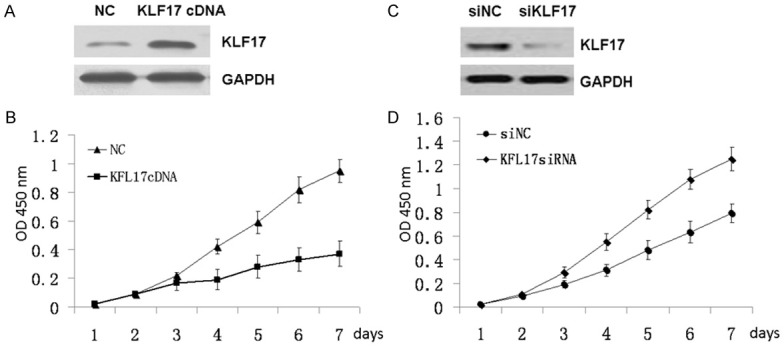
Effects of KLF17 overexpression and knockdown on cell proliferation. A. KLF17 overexpression in KYSE150 cells were confirmed by Western blot analysis. B. Cell proliferation of the KYSE150 cells transfected with KLF17cDNA and Control separately were analyzed by MTT assay. C. KLF17 knockdown in KYSE150 cells were confirmed by Western blot. D. Cell proliferation of the KYSE150 cells transfected with siKLF17 and Control separately were analyzed by MTT assay.
Effects of KLF17 overexpression and knockdown on cell migration and invasion
Because KLF17 is a regulator in tumor metastasis, next we tested the cell migration and invasion. Compared with the control group, KLF17-overexpressed cells displayed impeded migration in KYSE150 cells by wound healing assay. The migration decreased by approximately 50% (Figure 3A). Similarly, invasion of KYSE150 cells was reduced by % following overexpression of KLF17 by transwell assay (Figure 3C). Furthermore, we silenced the expression of KLF17 to assess the cell migration and invasion. Wound healing assay showed that the migration of cells down regulated KLF17 was significantly increased (Figure 3B). Transwell assay also showed that the cell invasion ability was enhanced upon KLF17 depletion (Figure 3D). EMT (epithelial-mesenchymal transition) has a critical role in metastasis. Next we tested the expression of EMT marker. The expression of epithelial markers E-cadherin and ZO-1 was increased, and the expression of mesenchymal markers fibronectin was decrease after overexpression of KLF17 in KYSE150 cells (Figure 3E). Subsequently knockdown of KLF17 led to the decrease of E-cadherin and ZO-1 and increase of fibronectin in KYSE150 cells. These suggest that KLF17 play an important role in EC cell migration and invasion.
Figure 3.
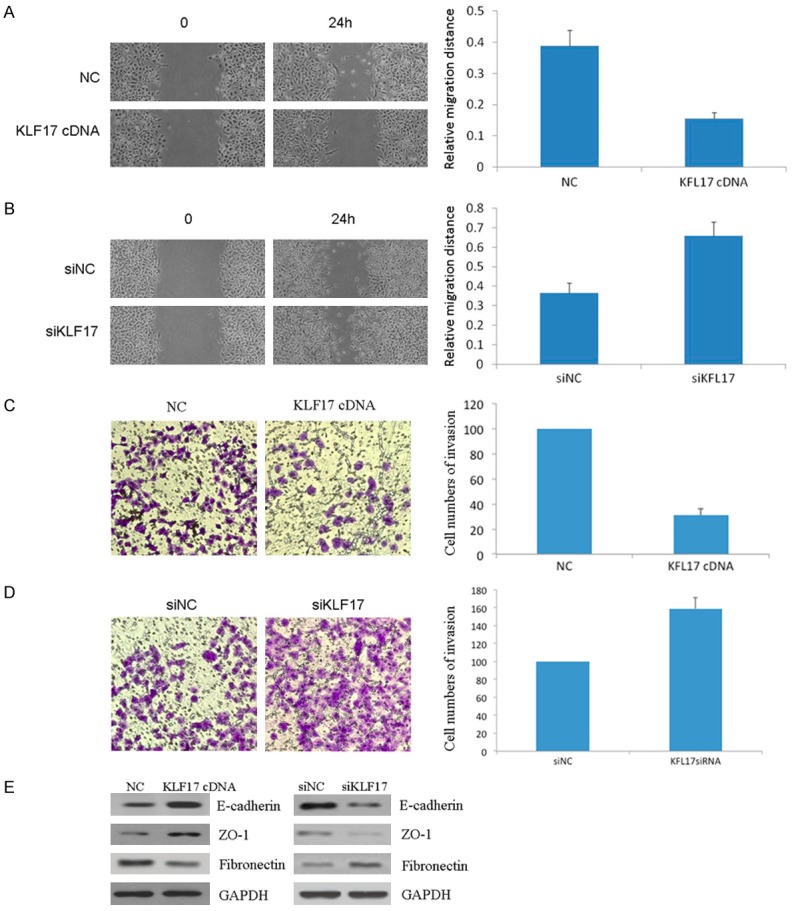
Effects of KLF17 overexpression and knockdown on cell invasion. A, B. The wound healing assay was performed to detect the cell motility. C, D. Transwell assay was used to detect the cell invasion. E. Epithelial-mesenchymal markers were analyzed by Western blot.
Discussion
Although KLF17 was identified as a new negative regulator of metastasis, its expression and biologic function was unknown in EC. The present study showed that the expression of KLF17 was reduced in esophageal cancer tissues. Moreover KLF17 protein expression was inversely correlated with lymph node metastasis in EC. These results suggested that KLF17 may play an important role in esophageal cancer development. It was also reported that KLF17 also was inversely related to tumor metastasis in other human cancer such as gastric cancer, lung adenocarcinoma, hepatocellular carcinoma [5,9-11]. Thus, combined with the previous studies the function of KLF17 in EC was worthy being further exploited.
Previous studies showed the function of KLF17 in tumor proliferation and metastasis. The reduced expression of KLF17 promoted the motility and proliferation ability of papillary thyroid carcinoma [12]. KLF17 depletion accelerates lungs cancer cells growth in response to chemotherapy [13]. Overexpression of KLF17 inhibited lung adenocarcinoma cell growth [14]. A novel in vivo screening approach has identified KLF17 as a key metastasis suppressor gene [5,15]. Our study also revealed that overexpression of KLF17 in EC cells inhibited the cell proliferation, and knockdown of KLF17 expression in EC cells also resulted in the promotion of cell proliferation. KLF17-overexpressed EC cells displayed impeded migration and invasion, however, knockdown of KLF17 increased the EC cell migration/invasion. EMT allows the cells which acquire migratory and invasive properties to migrate through the extracellular matrix [16]. EMT plays crucial roles in tumor cell metastasis [17-19]. Next we test the EMT marker and found that the expression of E-cadherin and ZO-1 was increased, and the expression of fibronectin and vimentin was decrease after overexpression of KLF17 in EC cells. Subsequently knockdown of KLF17 led to the decrease of E-cadherin and ZO-1 and increase of fibronectin and vimentin in EC cells.
In conclusion, our study indicated that KLF17 played an important role in EC tumorigenesis and development. Our results revealed an anti-tumorigenic effect of KLF17 in EC and could be an important chemotherapeutic target.
Acknowledgements
This work was supported by the Natural Science Foundation of Hebei Province (H2014206375).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Zhang S, Zeng H, Fan Y, Qiao Y, Zhou Q. Esophageal cancer incidence and mortality in China, 2010. Thoracic Cancer. 2014;5:343–348. doi: 10.1111/1759-7714.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Zhang W, Liu X, Zhang X, He J, Feng Q, et al. Prognosis of esophageal squamous cell carcinoma patients with preoperative radiotherapy: Comparison of different cancer staging systems. Thoracic Cancer. 2014;5:204–210. doi: 10.1111/1759-7714.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumireddy K, Li A, Gimotty PA, Klein-Szanto AJ, Showe LC, Katsaros D, Coukos G, Zhang L, Huang Q. KLF17 is a negative regulator of epithelial-mesenchymal transition and metastasis in breast cancer. Nat Cell Biol. 2009;11:1297–1304. doi: 10.1038/ncb1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ismail IA, Kang HS, Lee HJ, Kim JK, Hong SH. DJ-1 upregulates breast cancer cell invasion by repressing KLF17 expression. Br J Cancer. 2014;110:1298–306. doi: 10.1038/bjc.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali A, Zhang P, Liangfang Y, Wenshe S, Wang H, Lin X, Dai Y, Feng XH, Moses R, Wang D, Li X, Xiao J. KLF17 empowers TGF-β/Smad signaling by targeting Smad3-dependent pathway to suppress tumor growth and metastasis during cancer progression. Cell Death Dis. 2015;6:e1681. doi: 10.1038/cddis.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Qin X, Liu B, Sun L, Zhang X, Li Z, Shan B, You J, Zhou Q. Dickkopf-1 is involved in invasive growth of esophageal cancer cells. J Mol Histol. 2011;42:491–8. doi: 10.1007/s10735-011-9347-1. [DOI] [PubMed] [Google Scholar]
- 9.Peng JJ, Wu B, Xiao XB, Shao YS, Feng Y, Yin MX. Reduced Krüppel-like factor 17 (KLF17) expression correlates with poor survival in patients with gastric cancer. Arch Med Res. 2014;45:394–9. doi: 10.1016/j.arcmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Cai XD, Zhou YB, Huang LX, Zeng QL, Zhang LJ, Wang QQ, Li SL, Feng JQ, Han AJ. Reduced expression of Krüppel-like factor 17 is related to tumor growth and poor prognosis in lung adenocarcinoma. Biochem Biophys Res Commun. 2012;418:67–73. doi: 10.1016/j.bbrc.2011.12.129. [DOI] [PubMed] [Google Scholar]
- 11.Liu FY, Deng YL, Li Y, Zeng D, Zhou ZZ, Tian DA, Liu M. Down-regulated KLF17 expression is associated with tumor invasion and poor prognosis in hepatocellular carcinoma. Med Oncol. 2013;30:425. doi: 10.1007/s12032-012-0425-3. [DOI] [PubMed] [Google Scholar]
- 12.Ye WC, Gao L, Huang J, Fang XM, Xie G. Suppressed Krüppel-like factor 17 expression induces tumor proliferation, metastasis and a poor prognosis in papillary thyroid carcinoma. Mol Med Rep. 2014;10:2087–92. doi: 10.3892/mmr.2014.2429. [DOI] [PubMed] [Google Scholar]
- 13.Ali A, ZeeshanBhatti M, Saboor Shah A, Duong HQ, Mohammad Alkreathy H, Faisal Mohammad S, Ali R, Ahmad A. Tumor suppressive p53 signaling empowers metastatic inhibitor KLF17-dependent transcription to overcome tumorigenesis in non-small cell lung cancer. J Biol Chem. 2015;290:21336–51. doi: 10.1074/jbc.M114.635730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai XD, Zhou YB, Huang LX, Zeng QL, Zhang LJ, Wang QQ, Li SL, Feng JQ, Han AJ. Reduced expression of Krüppel-like factor 17 is related to tumor growth and poor prognosis in lung adenocarcinoma. Biochem Biophys Res Commun. 2012;418:67–73. doi: 10.1016/j.bbrc.2011.12.129. [DOI] [PubMed] [Google Scholar]
- 15.Iwanicki MP, Brugge JS. Transcriptional regulation of metastatic [Id] entity by KLF17. Genome Biol. 2009;10:244. doi: 10.1186/gb-2009-10-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–49. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72:4883–9. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 19.Shrivastava SR, Shrivastava PS, Ramasamy J. Negating the impact of radiation in development of cancers. Asia-Pac J Oncol Nurs. 2015;2:52–53. doi: 10.4103/2347-5625.143763. [DOI] [PMC free article] [PubMed] [Google Scholar]